Effects of N-acetylcysteine on growth,viability and reactive oxygen species levels in small antral follicles cultured in vitro
2023-02-17EfigniaCordeiroBiancaSilvaLaPaulinoPedroBarrosoLaryssaBarrozoMigueldeLimaNetoJosSilva
Efigênia B.Cordeiro,Bianca R.Silva,Laís R.F.M.Paulino,Pedro A.A.Barroso,Laryssa G.Barrozo,Miguel F.de Lima Neto,José R.V.Silva
Laboratory of Biotechnology and Physiology of Reproduction,Federal University of Ceara,Av.Comandante Maurocélio Rocha Ponte 100,62041-040,Sobral,CE,Brazil
ABSTRACT Objective: To investigate the effects of different concentrations of N-acetylcysteine on follicular growth and morphology,as well as on viability,levels of reactive oxygen species (ROS) and meiotic progression of oocytes from in vitro cultured bovine early antral follicles.Methods: Isolated early antral follicles (about 500 μm) were cultured in TCM-199+ alone or supplemented with 1.0,5.0 or 25.0 mM N-acetylcysteine at 38.5 ℃ with 5% CO2 for 8 days.Follicle diameters were evaluated at day 0,4 and 8 of culture.At the end of culture,the levels of ROS,chromatin configuration and viability (calcein-AM and ethidium homodimer-1 staining) were investigated in the cumulus-oocyte complexes.Comparisons of follicle diameters between treatments were performed.Data on percentages of morphologically normal follicles,growth rates and chromatin configuration in different treatments were compared.Results: An increase in follicular diameters after culture in all treatments was observed,except for follicles cultured with 25.0 mM N-acetylcysteine.Fluorescence microscopy showed that oocytes cultured in all treatments were stained positively with calcein-AM,and that 5.0 mM N-acetylcysteine reduced fluorescence for ethidium homodimer-1.Intracellular levels of ROS in oocytes from follicles cultured with 1.0 mM N-acetylcysteine showed a significant reduction compared to other treatments.The presence of N-acetylcysteine in culture medium did not influence the rates of oocyte at the germinal vesicle stage.Conclusions: N-acetylcysteine at concentrations of 1.0 and 5.0 mM reduces ROS levels and staining for ethidium homodimer-1 in in vitro cultured follicles,respectively,while 25.0 mM N-acetylcysteine decreases follicular growth and the percentages of continuously growing follicles.
KEYWORDS: Antioxidant;N-acetylcysteine;Antral follicles;Bovine;Reactive oxygen species
Significance
Thein vitrodevelopment of early antral follicles up to maturation opens new perspectives to use of their oocytes forin vitrofertilization.However,oxidative stress duringin vitroculture of these follicles can result in accumulation of reactive oxygen species (ROS) and consequently oocyte degeneration.To minimize the damages caused by oxidative stress,the results of this study show that supplementation of culture medium with 1.0 mMN-acetylcysteine reduces ROS levels and improves oocyte viability in cultured early antral follicles.Thus,the presence of this substance in culture media has great potential to improvein vitroculture systems for follicles and oocytes,which can bring positive impacts for assisted reproduction technologies.
1.Introduction
The growth of preantral and early antral follicle is a key step of follicular development associated with intense transcriptional activity and acquisition of oocyte competence[1].Somein vitrostudies report that oocytes from small antral follicles,between 1 and 2 mm,have significantly reduced competence when compared to those from larger antral follicles (>3 mm),which have the ability to complete nuclear maturation[2,3].Several molecular events and the bidirectional communication between the oocyte and surrounding granulosa cells by transzonal projections coordinate the development of early antral follicles up to ovulation[4].
In the last decades,in vitroculture of ovarian follicles has contributed to a better understanding of the roles of hormones and growth factor during follicular development[5,6],butin vitrogrowth of follicles up to maturation was still not reported for human and domestic animals[7,6].In domestic animals,thein vitrodevelopment of secondary follicles during 18 days of culture is generally associated with increased degeneration rate at early antral follicle stages[8].Under normal physiological conditions,the cells produce variable levels of reactive oxygen species (ROS),but their excess can cause oxidative stress and compromise cell development[9,10].This is one of the main factors associated with the low quality ofin vitrocultured ovarian follicles[6,11].Therefore,adding substances with antioxidant activity in the culture medium can prevent or mitigate these damages[12].
N-acetylcysteine (NAC) is a precursor of cysteine and reduced glutathione that has an important role in the control of oxidative stress[13].Fabbriet al[14] reported that NAC and follicle-stimulating hormone (FSH) improved preantral follicle growth and viability in cultured ovarian tissues.Furthermore,NAC increased the content of glutathione and reduced ROS levels in mice cumulus-oocyte complexes (COCs) maturedin vitro[15].In porcine COCs,NAC also reduced the levels of ROS,and increased cumulus cell expansion duringin vitromaturation[16].However,the effects of NAC during thein vitrogrowth of bovine early antral follicles are still unknown.Studying early antral follicle growthin vitrocan contribute to the elucidation of the mechanisms that regulate their growth,and consequently enable the use of their oocytes inin vitromaturation protocols.
This study aims to investigate the effects of different concentrations of NAC on the growth and morphology of early antral follicles and on the viability,ROS levels and meiotic progression of oocytes from small antral follicles culturedin vitro.
2.Materials and methods
2.1.Source of ovaries
Ovaries (n=150) from adult cows were collected from a local slaughterhouse immediately after slaughter.The ovaries were washed in 70% ethanol,followed by two rinses in tissue culture medium 199 (TCM-199) buffered with 2-hydroxyethyl (HEPES) and supplemented with penicillin (100 IU) and streptomycin (0.1 mg/mL).The ovaries were transported within 1 h to the laboratory in TCM-199 at 4 ℃.
2.2.Follicle isolation and in vitro culture
In the laboratory,the ovarian cortex (1-2 mm) was fragmented with a sterile scalpel blade and placed in TCM-199 medium supplemented with HEPES.All procedures were aseptically performed in a safe laminar flow station.Antral follicles of approximately 500 μm in diameter were manually dissected from the strips of cortical tissues using 26-gauge needles under a stereomicroscope (SMZ 645 Nikon,Tokyo,Japan).After isolation,follicles with a visible oocyte surrounded by granulosa cells,an intact basement membrane and the presence of an antral cavity were selected for culture.The follicles were individually cultured in 150 μL of culture medium under mineral oil in Petri dishes (60 mm × 15 mm,Corning,USA).The control culture medium,called TCM-199+,consisted of TCM-199 (pH 7.2-7.4) supplemented with FSH (50 μg/mL),insulin (50 μg/mL),transferrin (50 μg/mL) and selenium (50 μg/mL) (ITS),0.015 mg/mL of bovine serum albumin (BSA),penicillin (100 IU),streptomycin (0.1 mg/mL),glutamine (50 μg/mL),and hypoxanthine (50 μg/mL)[17,18].For the treatments,the follicles were randomly cultured in TCM-199+alone or supplemented with 1.0,5.0 or 25.0 mM NAC[19,20].Follicles were cultured at 38.5 ℃ with 5% CO2in air for 8 days[19].At day 4 of culture,75 μL of medium was replaced by fresh medium.
2.3.Assessment of follicular morphology and growth
The morphological evaluation of follicles was performed with the aid of a stereomicroscope (SMZ 645 Nikon,Tokyo,Japan) at days 0,4 and 8.Morphologically normal,follicles had spherical oocyte surrounded by homogeneous granulosa cells,intact basement membrane and external stromal-thecal layer.Follicles with dark oocytes and cumulus cells were considered degenerated.In addition,perpendicular measurements were performed on normal follicles at days 0,4 and 8.
2.4.Assessment of oocyte viability by fluorescence microscopy
After culture,COCs were extruded with the aid of 25 G needles and incubated with 100 μL of TCM-199 containing 4 mM calcein-AM and 2 mM ethidium-1 homodimer (Molecular Probes,Invitrogen,Karlsruhe,Germany) at 37 ℃ for 15 min.Then,they were washed three times in TCM-199 and examined under fluorescence microscope (Nikon,Eclipse,TS 100.,Japan).Oocytes and cumulus cells were considered viable if cytoplasm was stained positively with calcein-AM (green) and the chromatin not labeled with ethidium homodimer-1 (red)[21].
2.5.Evaluation of ROS levels in COCs from cultured follicles
Oocytes were washed in 0.1% polyvinyl alcohol in phosphatebuffered saline (PBS-PVA) and incubated with 6-carboxy-2,7-dichlorodihydrofluorescein diacetate (H2DCFDA,Molecular Probes®,Eugene,OR) at 38.5 ℃ for 30 min,in the dark.Then,oocytes were washed with PBS-PVA and placed on glass slides with ProLong®Gold (Molecular Probes,Eugene,OR).The slides were evaluated under an epifluorescence microscope (Nikon,TS100) at a wavelength of 460 nm.The fluorescence intensity of ROS saturation was analyzed individually using Image J software (version 1.46;National Institutes of Health,Bethesda,MD).The relative fluorescence intensity was considered directly proportional to the ROS concentration.
“Well, now, you are grown up,” said the old dowager, her grandmother; “so you must let me adorn38 you like your other sisters;” and she placed a wreath of white lilies in her hair, and every flower leaf was half a pearl
2.6.Evaluation of chromatin configuration
To assess chromatin,cumulus cells were removed by vortexing and the oocytes fixed in 4% paraformaldehyde for 15 min and transferred to 0.1% Triton X-100.The chromatin configuration was evaluated after adding 10 μg/mL of Hoechst 33342 on an inverted epifluorescence microscope (Nikon,TS100).Oocytes were classified according to the stages of nuclear maturation,i.e.,as germinal vesicle (GV),germinal vesicle breakdown (GVBD) or degenerated.
2.7.Statistical analysis
Statistical analyses were performed using GraphPad Prism software,version 9.0 (https://www.graphpad.com/scientific-software/prism/).Data of follicular diameter and the levels of ROS,calcein and ethidium homodimer-1 staining were initially subjected to normal distribution analysis using D'Agostinho &Pearson Test.After passing the normality test,comparisons between treatments were performed by analysis of variance (ANOVA) and Tukey test.These data are expressed as mean±SD.For data that did not pass normality test,like those of follicular growth in the different periods (days 0 to 4 or days 4 to 8),comparisons were performed by Kruskall-Wallis test.They were presented as median [interquartile range (IQR)].Data of percentages of morphologically normal follicles,growth rates and chromatin configuration were compared usingChi-square or Fisher's exact tests.Differences were considered significant whenP<0.05.
2.8.Ethics statement
This study was approved and carried out in accordance with the rules and guidelines of the Ethics and Animal Welfare Committee of the Federal University of Ceara (number 02/21).
3.Results
3.1.Effects of NAC on follicular morphology and growth
A progressive and significant increase in follicular diameters was observed with the increase of culture period from 0 to 4 and 8 days in all treatments,except for follicles cultured with 25.0 mM NAC that did not have significant growth between days 4 and 8 (Table 1).After 8 days of culture,follicles cultured with 25 mM had significantly smaller diameters compared to follicles cultured with TCM-199+(Table 1).
Table 2 shows follicular growth in the different periods of culture (from day 0 to day 4 and from day 4 to day 8).Different from follicles cultured with 1.0 or 5.0 mM NAC,25.0 mM NAC significantly reduced follicular growth rate between the periods of 4 and 8 days of culture (Table 2).

Table 1.Diameters of early antral follicles day 0,4 and 8 after in vitro culture in TCM-199+ alone or supplemented with different concentrations of N-acetylcysteine.

Table 2.Follicular growth in the different periods (days 0 to 4 or days 4 to 8) after culture in vitro TCM-199+ alone or supplemented with different concentrations of N-acetylcysteine.

Table 3.Percentage of continuously growing follicles after 4 and 8 days of in vitro culture in TCM-199+ alone or supplemented with different concentrations of N-acetylcysteine.

Table 4.Percentages of oocytes at germinal vesicle and germinal vesicle breakdown stages and with degenerated chromatin after culture of antral follicles in TCM199+ alone or supplemented with different concentrations of N-acetylcysteine.
The presence of 25 mM NAC in culture medium significantly reduced percentage of growing follicles after 4 and 8 days,when compared to other treatments.In presence of 25.0 mM NAC,the percentages of growing follicles were reduced,when compared with growing follicles in the first 4 days of culture (Table 3).
3.2.Viability of oocytes from cultured follicles
Oocytes from follicles cutured with 5.0 mM NAC showed a significant reduction in fluorescence intensity for ethidium homodimer-1 when compared to those cultured in the control medium.In each treatment,significant lower fluorescence for ethidium-1 homodimer in relation to calcein-AM was observed in oocytes from follicles cultured with 5.0 and 25.0 mM NAC (Figure 1 and 2).However,when comparing the fluorescence intensity of calcein-AM in oocytes from follicles cultured in the different treatments,there was no significant difference.

Figure 1.Oocytes from bovine antral follicles cultured for 8 days after staining with calcein-AM (green) and ethidium homodimer-1 (red).Oocytes from all treatments are mainly stained with calcein-AM.Oocytes from antral follicles cultured in TCM-199+ alone (A,E) or supplemented with 1.0 (B,F),5.0 (C,G) or 25.0 mM N-acetylcysteine (D,H).

Figure 2.Fluorescence intensity staining shows that oocytes cultured with 5.0 mM N-acetylcysteine (NAC) have lower fluorescence intensity for ethidium homodimer-1 (grey columns) than those cultured in the control medium.Within each treatment,lower fluorescence for ethidium-1 homodimer in relation to calcein-AM is observed in oocytes cultured with 5.0 and 25.0 mM NAC.No differences are observed in fluorescence intensity of calcein-AM in oocytes cultured in the different treatments.Data are expressed as mean±SD.The same letter A represents no significant differences.Lowercase letters (a,b) represent statistically significant differences in fluorescence intensity for ethidium homodimer-1 (grey columns) between different treatments (P<0.05).*Represents the significant difference in fluorescence intensity between calcein-AM and ethidium homodimer-1 in each treatment.
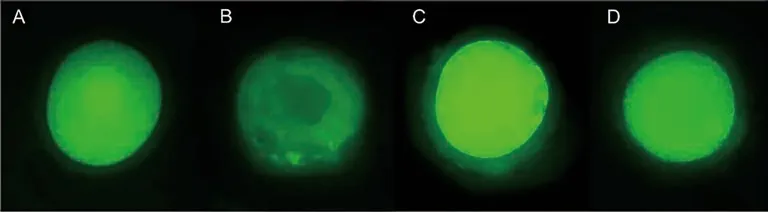
Figure 3.Oocytes stained with 6-carboxy-2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) for detection of reactive oxygen species.Oocytes cultured in TCM-199+ alone (A),or with 5.0 (C) or 25.0 mM (D) N-acetylcysteine are more intensely stained than those cultured with of 1.0 mM N-acetylcysteine (B).

Figure 4.Fluorescence intensity stained with 6-carboxy-2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) for detection of reactive oxygen species in oocytes from follicles cultured in TCM-199+ alone or supplemented with different concentrations of N-acetylcysteine (NAC).Lowercase letters (a,b,c) represent significant differences between treatments.Data are expressed as mean±SD.
3.3.Evaluation of ROS levels in oocytes from cultured follicles
Oocytes from follicles cultured with 1.0 mM NAC had significantly lower levels of ROS than those cultured in the control medium alone or with 5.0 and 25.0 mM NAC (Figure 3 and 4).
3.4.Evaluation of chromatin configuration in oocytes from cultured antral follicles
4.Discussion
This study shows that 1.0 mM NAC reduces the levels of ROS in oocytes from early antral follicles culturedin vitro.It is known that the accumulation of ROS is one of the limiting factors duringin vitroculture,causing oxidative stress and various harmful effects to cells[22,23].Likewise,Sunet al[24] showed that 1.0 mM NAC attenuated ROS levels duringin vitrooocyte maturation.In addition,many other studies have also demonstrated the antioxidant effects of NAC[24-27].The antioxidant potential of NAC is mainly due to its ability to easily penetrate the cell membrane and be deacetylated producing cysteine,for the intracellular synthesis of glutathione[28,29].
The presence of 5.0 mM NAC in culture reduced the fluorescence intensity for ethidium homodimer-1 in oocytes,showing that NAC helps maintain oocyte membrane integrity during culture.When there is loss of integrity,the ethidium homodimer-1 can easily enter the cell,and due to the high affinity with nucleic acids,it binds to the DNA emitting red fluorescence[30],allowing verifying the physical and chemical changes in the cell membrane.In contrast,calcein can passively cross the cell membrane,and within cells it is converted by intracellular esterases to a lipid-insoluble polar product (calcein) that is retained by cells with intact membranes,producing intense green fluorescence[31],thus allowing the verification of cell viability.In caprine granulosa cells,5.0 and 10.0 mM NAC reduced the genotoxicity caused in response to exposure to methoxychlor[17],while in fibroblasts,5.0 and 10.0 mM NAC reduced the production of ROS and the toxicity induced by 2-hydroxyethyl methacrylate,consequently reducing cell death and restoring mitochondrial activity[32].
In our study,25.0 mM NAC reduced follicular growth and the percentages of continuously growing follicles.Recently,Sunet al[24] reported that NAC at concentrations above 10.0 mM causes a reduction in the pH of the culture medium and is harmful to oocyte growth duringin vitromaturation.The reduction in the pH of the medium may explain the lower follicular growth of early antral follicles cultured with 25 mM NAC.Clinical studies have shown that the plasma concentration of NAC ranges from 300 to 900 mg/L,which is equivalent to 1.8-5.5 mM[33].Furthermore,previous studies have suggested that NAC at higher concentrations may have a prooxidant effect[34].Spronget al[35] showed that high doses of NAC (550 and 950 mg/kg in 48 h) in rats increase oxidative stress and toxicity induced by lipopolysaccharide,but low doses of NAC are highly effective against lipopolysaccharide toxicity.Moraeset al[36] also observed that the increase in the plasma concentration of NAC,as a result of its association with dapsone,potentiated the adverse effect of depsone in rats.
Regarding to chromatin configuration,NAC did not influence the percentages of oocytes that remained in the GV stage.The experimental model used with the culture of the intact antral follicle maintains communication between the oocyte and granulosa cells,allowing the supply of follicular components to the oocyte and the conservation of high levels of cyclic adenosine monophosphate (cAMP),consequently preventing the spontaneous resumption of meiosis[36,37],and guaranteeing the time necessary for the oocyte to acquire the developmental competence to proceed with the following stages of its growth[38].In addition,evidence suggest that the control of meiotic resumption or arrest can be influenced by ROS levels,and the presence of antioxidants in the culture medium is very important to inhibit the spontaneous resumption of meiosis underin vitroculture conditions[23].
In conclusion,supplementation of culture medium with 1.0 mM NAC reduces ROS levels,wihte the presence of 5.0 mM NAC reduces the fluorescence intensity for ethidium homodimer-1.NAC at concentration of 25 mM,however,reduces follicular growth and percentages of continuously growing follicles duringin vitroculture of bovine early antral follicles.In future studies,in vitroculture of early antral follicles for long term periods is still needed to promote complete oocyte cytoplasmic maturation.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Funding
This research was supported by grants from the National Council for Scientific and Technological Development (CNPq,Brazil,grant number 308737/2018-0).
Authors’ contributions
Efigênia B.Cordeiro performedin vitroculture of bovine early antral follicles and statistical analysis of data.Bianca R.Silva isolated the follicles and measured their diameters before and after culture.Laís R.F.M.Paulino isolated the follicles and stained them with fluorescent markers.Pedro A.A.Barroso collected the ovaries in slaughterhouses and preparedin vitroculture media.Laryssa G.Barrozo contributed to the acquisition and analysis of data.Miguel F.de Lima Neto evaluated follicular viability after staining with fluorescent markers.José R.V.Silva designed the work and critically revised the manuscript.
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- The fate of surplus embryos in the setting of assisted reproductive technology: A scoping review
- Embryo quality and chromosomal abnormality in embryos from couples undergoing assisted reproductive technology using preimplantation genetic screening
- A novel herbal combination ameliorates ovarian dysfunction and regulates altered biochemical parameters in rats with letrozole-induced polycystic ovary syndrome
- Impact of chronological ageing on semen parameters in southern Indian men visiting infertility centre: A retrospective study
- Bacteriospermia among smallholder artificial insemination boars in the Philippines and potential associated factors
- Serum anti-leukemia inhibitory factor antibody and recurrent pregnancy loss in Iranian women