Intravitreal slow-release dexamethasone alleviates traumatic proliferative vitreoretinopathy by inhibiting persistent inflammation and Müller cell gliosis in rabbits
2023-02-11YiMingZhaoRongShaSunFangDuanFangYuWangYuJieLiXiaoBingQianJieTingZengYaoYangXiaoFengLin
Yi-Ming Zhao, Rong-Sha Sun, Fang Duan, Fang-Yu Wang, Yu-Jie Li, Xiao-Bing Qian,Jie-Ting Zeng, Yao Yang, Xiao-Feng Lin
1State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat‐sen University, Guangdong Provincial Key Laboratory of Ophthalmology and Visual Science, Guangzhou 510060, Guangdong Province, China
2Xi’an People’s Hospital (Xi’an Fourth Hospital), Shaanxi Eye Hospital, Xi’an 710004, Shaanxi Province, China
Abstract
● KEYWORDS: ocular trauma; proliferative vitreoretinopathy;
INTRODUCTION
Traumatic proliferative vitreoretinopathy (PVR) is a severe complication after ocular injury; it has a high incidence of occurring in 40%–60% of the patients with open globe injury[1]. Inflammation and proliferation are involved in the mechanism of PVR development[2]. The release of enormous inflammatory cytokines contributes to a mitogenic environment, resulting in the proliferation of relevant cells,such as retinal pigment epithelium cells, glial cells, and fibroblasts[3‐4]. Excessive proliferation progress to tissue remodeling, scar formation, tractional retinal detachment,failure of retinal reattachment post‐surgery, and irreversible loss of visual acuity[5‐6].
Post‐traumatic inflammation is usually intense and prolonged.In an early clinical retrospective study, the intraocular inflammatory response was observed for longer than 5wk in patients with traumatic PVR, and it was regarded as an important risk factor for PVR development[7]. However,previous experimental studies on post‐traumatic inflammation didn’t mimic the protracted process virtually due to the short observation period of post‐injury inflammation, typically 1‐30d[8‐9]. Therefore, it is needed for further research on the progression of traumatic PVR.
Following ocular trauma, gliotic changes in the retina have an essential role in PVR pathologic process associated with retinal stiffness and shortening[10‐13]; and the primary participating cellular components are Müller glial cells[14‐15].Glial fibrillary acid protein (GFAP) is considered a sensitive biomarker for reactive Müller gliosis and correlates positively with PVR severity[16]. Müller cells can be triggered by various inflammatory cytokines and subsequently release more cytokines and growth factors into the local retinal environment to promote intraretinal glial proliferation and tissue insults[17].Tremendous advancements in surgical techniques for PVR management have been achieved[18‐19], but persistent inflammation and proliferation compromise surgical outcomes. Additionally, protracted inflammation might cause poor vision and retinal shortening[20‐21], even after surgical intervention for PVR. Determining the underlying causes and preventing prolonged inflammation are crucial for achieving optimal patient outcomes. Triamcinolone as a long‐acting glucocorticoid may be speculated to inhibit traumatic PVR[22];however, it has some adverse effects, such as intraocular hypertension and secondary cataract[23‐24]. Currently, a slow‐release dexamethasone agent (Ozurdex®, Allergan, Irvine, CA,USA) is available to inhibit inflammation for up to 6mo; and it has been approved by the Food and Drug Administration for the clinical treatment of macular edema following noninfectious uveitis, retinal vein occlusion, and diabetic retinopathy[25‐26]. However, whether this management provides a benefit for traumatic PVR has not been studied.
This study investigated the process of inflammation over a 6‐week period and evaluated the therapeutic effects of Ozurdex on PVR and Müller cell gliosis in a rabbit model of penetrating ocular trauma. Additionally, we preliminarily explored the possible inflammatory mechanism in the traumatic PVR model.
MATERIALS AND METHODS
Ethical ApprovalAnimal care and experimental procedures were performed strictly in accordance with the Association for Research in Vision and Ophthalmology resolution. All animal studies were approved by the Institutional Animal Care and Use Committee of Zhongshan Ophthalmic Center, Guangzhou,China (No.O2021018).
AnimalsOverall, 165 pigmented rabbits (male and female,8–12 weeks old, weighing 2–3 kg) were used in this study. The animals were acclimated to the animal facility for 7d prior to any manipulation. They were maintained in a dedicated facility with a temperature of 23°C and a 12‐hour light/dark cycle,they hadad libitumaccess to food and water. The fundus of each eye was examined, in detail, before traumatic PVR model induction, and rabbit eyes with a fundus abnormality were excluded.
Platelet-Rich Plasma Preparation and Traumatic PVR Model InductionThe preparation protocol of platelet‐rich plasma (PRP) was implemented as described previously with slight modifications[27]. The PRP concentration in this study was 1400–1800×109/L. Animals were randomly divided into the normal control, PVR (PVR induction+sham injection),and Dex (PVR induction+intravitreal Ozurdex injection)groups. The traumatic PVR model was modified in this study according to a previously described procedure[28]. Rabbits were anesthetized with an intramuscular injection of ketamine hydrochloride (30 mg/kg) and 2% xylazine hydrochloride(3.5 mg/kg). The pupils of the right eyes were dilated with topical 1% tropicamide. After sterilization with a 5% iodine solution, 8‐mm, circular, full‐thickness incisions were made 2.5 mm behind the limbus in the supertemporal area of each experimental eye, avoiding the lens and retina. The prolapsed vitreous was resected, and then scleral wounds were closed with interrupted 7‐0 vicryl sutures. Then, 0.2 mL of PRP was injected into the mid‐vitreousviathe space between the scleral sutures. Finally, Ozurdex was applied to the eyes in the Dex group, following the previous method of Ozurdex injection in rabbit eyes[29‐30]; rabbits in the PVR group received a sham injection. Tobramycin ointment was used once daily, post‐injury, for 7d.
Anatomical and Histological EvaluationsAt 1‐week intervals for 6wk (postoperative days 7, 14, 21, 28, 35, and 42),rabbits were euthanized under deep anesthesia and the eyeballs were enucleated. As intravitreal injection of PRP hampered an intuitive clinical observation, a detailed fundus examination of enucleated eyes was performed under dissecting microscopy;the eyes were scored according to the classification of Cardilloet al[31]. After an anatomical observation, globes were embedded in paraffin, sectioned (5‐μm‐thick) into retinal slices, and stained with hematoxylin‐eosin (H&E) for histological examination using a light microscope (Carl Zeiss,Oberkochen, Germany). PVR severity was graded as described by Ozeret al[32].
ImmunofluorescenceCryosections were blocked with 5% bovine serum albumin containing 0.3% Triton‐X100 at room temperature for 1h and then incubated with primary antibodies against alpha‐smooth muscle actin (αSMA, 1:200,MA5‐11547, Thermo Scientific, Waltham, MA, USA) and GFAP conjugated with Alexa Fluor 488 (1:50, 53‐9892‐82,Thermo Scientific) overnight at 4°C, followed by corresponding secondary antibodies for 2h at room temperature. Immunostaining was captured using a laser scanning confocal microscope(Carl Zeiss, Oberkochen, Germany) and analyzed by Image J software (National Institutes of Health, Bethesda, MD, USA).
Western Blot AnalysisThe total protein was obtained from the retinas (n=3) of each group and was homogenized with a lysis buffer (KeyGen Biotech, Nanjing, China) containing phosphatase and protease inhibitors. The protein concentration was measured using PierceTMBCA Protein Assay Kit (Thermo Scientific). Equal amounts of protein from each sample were subjected to 4%–12% gels and then transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore,Merck, Kenilworth, NJ, USA). After being blocked, the PVDF membranes were incubated with primary antibodies against αSMA (1:200, MA5‐11547, Thermo Scientific), GFAP (1:800,14‐9892‐80, Thermo Scientific), nod‐like receptor family pyrin domain containing‐3 (NLRP3, 1:500, MA5‐32255, Thermo Scientific), phosphorylated nuclear factor‐kappa B (p‐NF‐κB,1:500, ab86299, Abcam), NF‐κB (1:500, ab16502, Abcam),and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH,1:1000, NB600‐502, Novus) overnight at 4℃. On the next day, the membranes were incubated with secondary antibodies(1:5000, ab6802, and 1:5000, ab6789, both from Abcam) for 1h at room temperature. Protein bands were imaged using the ChemiDoc Touch Imaging System (Bio‐Rad, Hercules, CA,USA), and then band intensities were quantified using Image J software.
Quantitative Real-Time Polymerase Chain ReactionTotal RNA was collected from rabbit retinas (n=3) and isolated with the Tissue RNA Purification Kit Plus (ES Science, Shanghai,China). Complementary DNA was synthesized using the HiScript III RT SuperMix kit (Vazyme, Nanjing, China).Primer sequences are presented in Table 1. Gene expression levels were calculated using the LightCycler 480 system(Roche, Basel, Switzerland). The polymerase chain reaction procedure was performed at 95℃ for 30s (pre‐incubation),followed by 40‐cycle amplification at 95°C for 10s and annealing at 60℃ for 30s. Data were calculated as the fold expression after normalization to GAPDH.
Cytokine Antibody ArraysRabbit inflammation antibody arrays (QAL‐CYT‐1, Raybio, RayBiotech, Norcross, GA,USA) were used to detect inflammatory cytokines in vitreous samples (n=3) per the manufacturer’s instructions. The signals were visualized using cyanine 3‐conjugate streptavidin and detected using a SureScan Dx Microarray Scanner (Agilent,Santa Clara, CA, USA). GenePix Pro 6.0 software (Axon Instruments, Seattle, WA, USA) was used to analyze the data.

Table 1 Primer sequences for quantitative real-time polymerase chain reaction
Intraocular Pressure Monitoring After Ozurdex InjectionThe intraocular pressure (IOP) was monitored with a tonometer(Icare, TONOVET, Helsinki, Finland) preoperatively on postoperative days 1 and 3, and then every other week at the same time of day to minimize the influence of circadian variation.
Statistical AnalysisAll data are presented as mean±standard errors of the mean (SEM). The data were analyzed using one‐way analysis of variance, followed by Tukey’s multiple comparisons (GraphPad Prism, version 8.0, GraphPad Software, San Diego, CA, USA). AP‐value<0.05 was considered statistically significant.
RESULTS
Ozurdex Mitigated PVR Severity After Ocular InjuryAfter modeling, fundus evaluations of radial sections of enucleated eyes in the PVR group indicated severe proliferation and tractional retinal detachment. As shown in Figure 1A, at post‐injury week 1, proliferation started from the area of the vitreous base near the wound and extended to the posterior retina in PVR eyes. Over time, proliferative membranes pulled the retina with medullary ray elevation and focal retinal detachment. Total tractional retinal detachment was observed in all eyes of the PVR group by 6wk post‐injury.In contrast, the Dex group showed no evidence of retinal detachment, with a lower anatomical stage than the eyes in the PVR group (P<0.001; Figure 1B).
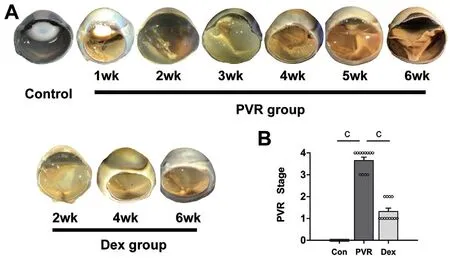
Figure 1 Comparison of anatomical PVR stages after trauma in the normal control, PVR, and Dex groups A: Gross photographs of the enucleated globes; B: Statistical analysis of PVR stages at 6wk post-injury. Data are presented as mean±SEM. cP<0.001. Con:Normal control; PVR: Proliferative vitreoretinopathy; Dex: PVR induction+intravitreal Ozurdex injection.
H&E staining in PVR retinas at week 6 post‐injury showed proliferative membranes, inner retinal edema, and full‐thickness retinal scars near the wound (Figure 2A–2D).Compared with PVR eyes, Ozurdex‐injected eyes had a statistically significant lower histological grade (P<0.001,Figure 2E). The proliferated cells per field were counted in proliferative tissues, revealing a significantly lower number of proliferated cells in the Dex group than in the PVR group(P<0.001, Figure 2F). A significant increase in overall retinal thickness was observed at 6wk after injury (Figure 2G).Further analysis of each retinal layer in the central retina showed that the thickness of the inner limiting membrane to inner plexiform layer (IML‐IPL) was significantly increased in the Dex group compared with the normal control group,but it was decreased in the inner nuclear layer (INL) and outer nuclear layer (ONL). Ozurdex treatment significantly alleviated traumatic PVR‐induced changes on thickness of the central retina compared with the PVR group, with significant differences in overall retinal thickness (P=0.034, Figure 2G),IML‐IPL (P=0.04, Figure 2H), INL (P<0.001, Figure 2I), and ONL (P<0.001, Figure 2J).
Ozurdex Inhibited Fibrous Proliferation in the Traumatic PVR Model To confirm the efficacy of Ozurdex on fibrous proliferation after injury, we assessed the expression of αSMA.Immunofluorescence images showed that the abnormal staining of αSMA was mainly localized in the tissue exhibiting anterior PVR (Figure 3A), corresponding to the H&E figures.Western blot analysis demonstrated that expression of αSMA protein was increased significantly at week 4 in PVR eyes(Figure 3B, 3C). After Ozurdex treatment, a smaller αSMA staining area percent was observed in the eyes assessed by immunofluorescence analysis (P<0.001, Figure 3G); moreover,Ozurdex significantly suppressed αSMA protein expression according to Western blot analysis (P=0.0097, Figure 3I).Consistent with the Western blot analysis data, penetrating injury induced elevated αSMA mRNA expression (Figure 3D),which was markedly reduced by Ozurdex treatment (P=0.019,Figure 3J).
Ozurdex Attenuated Müller Cell Gliosis in the Traumatic PVR ModelOn day 28 post‐injury, immunofluorescence images showed that GFAP expression significantly increased whether in the central, mid‐peripheral, or peripheral retina in the PVR eyes. GFAP staining extended into the subretinal space through the external limiting membrane (Figure 4A,white arrows). In the peripheral retina near the wound, the retinal structure collapsed due to the formation of the full‐thickness retinal glial scars. After Ozurdex treatment, GFAP‐positive area percents were lower whether in the central, mid‐peripheral or peripheral retina (allP<0.001, Figure 4B–4D)on day 28 post‐injury. Additionally, Ozurdex inhibited GFAP protein levels analyzed by Western blot (P<0.001, Figure 4H–4I). GFAP mRNA transcript levels were highest on day 21, and Ozurdex treatment significantly reduced GFAP mRNA expression (P=0.002, Figure 4J).
Ozurdex Reduced the Release of Inflammatory Cytokines After TraumaInflammatory cytokines interleukin (IL)‐1β,IL‐8, and tumor necrosis factor‐alpha (TNF‐α) were detected during the study. The secreted protein levels of IL‐1β, IL‐8, and TNF‐α were significantly upregulated on day 3 post‐injury in PVR eyes (Figure 5A–5D). The mRNA levels of IL‐1β, IL‐8,and TNF‐α also increased significantly, peaking on day 3;the expressions of IL‐1β and TNF‐α increased again on day 21 (Figure 5H–5J). IL‐1β and IL‐8 had lower levels both of protein and mRNA at the peak point in the Dex group than in the PVR group (Figure 5E, 5F, 5K, 5L). The TNF‐α level was also lower in the eyes of the Dex group; however, the difference in protein expression was not statistically significant(P>0.05, Figure 5G).
Ozurdex Suppressed NF-κB/NLRP3 Signaling ActivationNLRP3 protein level was upregulated, peaking on day 1 post‐injury and rebounding to another peak on day 14 (Figure 6A–6B). After treatment, a significantly lower NLRP3 protein level was detected in the Dex group than in the PVR group(P=0.005, Figure 6E). Additionally, the expression of NLRP3 mRNA increased and peaked on day 3 post‐injury (Figure 5C).Consistent with the effect of Ozurdex on NLRP3 protein, there was also a significant reduction in mRNA transcripts after treatment (P=0.015, Figure 6F). Moreover, the p‐NF‐κB level was upregulated post‐injury, which was obviously inhibited by Ozurdex treatment on day 1 (P=0.004, Figure 6G).
IOP Assessment After Ozurdex InjectionAfter modeling,the average IOP value in PVR eyes was lower than that in the normal controls. The IOP values in Ozurdex‐injected eyes were within the normal range, although it was higher than that of PVR eyes (10.45±0.32vs8.583±0.34 mm Hg,P>0.05;Figure 7).

Figure 2 Assessment of retinal structure and proliferation by hematoxylin-eosin staining A: Overview photography (0.8× magnification)of the three groups. Dotted boxes represent proliferative tissue. Long arrows in PVR group represent tractional retinal detachment (scale bar=2000 μm). B: 4× magnification of dotted boxes from Figure 2A (scale bar=400 μm). r: Retina; p: Proliferative vitreoretinopathy; c: Ciliary body. C: 40× magnification of Figure 2A (scale bar=40 μm). D: Variation in the central retina, mid-peripheral retina, and peripheral retina at 40× magnification. The single arrow represents the epiretinal membrane; double black arrows represent subretinal membranes; blue star represents intraretinal edema (scale bar=50 μm). E: Statistical analysis of histological grades at 6wk post-injury. F: Comparison of the proliferative cell number per field at 6wk post-injury. G: Quantitative analysis of retinal thickness in the central retina, mid-peripheral retina,and peripheral retina. H–J: Quantitative analysis of the IML-IPL, INL, and ONL thicknesses in the central retina, mid-peripheral retina, and peripheral retina. Data are presented as mean±SEM. aP<0.05, bP<0.01, cP<0.001, ns: Not significant. Con: Normal control; PVR: Proliferative vitreoretinopathy; Dex: PVR induction+intravitreal Ozurdex injection. IML-IRL: Inner limiting membrane to inner plexiform layer; INL: Inner nuclear layer; ONL: Outer nuclear layer; RPE: Retinal pigment epithelium.
DISCUSSION
PVR is often the primary reason for poor visual clinical prognoses and even loss of normal ocular appearance after trauma. At present, prevention and intervention of traumatic PVR remain the most challenging tasks. In this study, a traumatic PVR model was established in pigmented rabbits,and a long‐term (6wk) assessment of the pathological process, inflammatory response, and cellular proliferation was conducted. Intravitreal Ozurdex treatment significantly alleviated post‐traumatic inflammation, Müller cell gliosis, and PVR severity in this animal model.

Figure 3 Immunofluorescence, quantitative real-time polymerase chain reaction, and Western blot analyses of αSMA levels A: The epithelial-mesenchymal transition of RPE cells was stained positively with αSMA (red), and the intraretinal change was stained with GFAP(green) to indicate the detached retina. Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI, blue). Scale bar=2000 μm. B, C: Western blot analysis of αSMA in PVR eyes compared with normal controls. D: Changes in αSMA mRNA levels over time. E: Tile scan of αSMA staining in the proliferative sections near the wound of the PVR group and Dex group (scale bar=100 μm). r: Retina; p: Proliferative vitreoretinopathy;c: Ciliary body. F: 40× amplification of proliferative tissues from E (scale bar=50 μm). G: Comparison of the αSMA-positive area percents among the three groups. H, I: Representative Western blot images and quantification graph for αSMA. J: Comparison of the αSMA mRNA levels among the three groups. Data are presented as mean±SEM. aP<0.05, bP<0.01, cP<0.001. Con: Normal control; PVR: Proliferative vitreoretinopathy; Dex:PVR induction+intravitreal Ozurdex injection; αSMA: Alpha-smooth muscle actin; GFAP: Glial fibrillary acid protein; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; DAPI: 4’,6-diamidino-2-phenylindole.
Previous studies led to controversy regarding the role of Ozurdex in inhibiting PVR. In a prospective randomized controlled clinical trial, Banerjeeet al[33]found that Ozurdex did not improve the primary anatomic success rate in PVR eyes (Grade C) undergoing vitrectomy surgery with silicone oil. In an experimental PVR model induced by delivering RPE cells into the vitreous cavity, Kuoet al[30]found that Ozurdex suppressed the TNF‐α level, but did not inhibit PVR development at the end of observation on day 28 post‐injury.Wuet al[22]applied a sustained‐release triamcinolone film onto ruptured sclera in a traumatic PVR model and found that the severity of PVR was suppressed at the end of 30d. However,the effect of Ozurdex on traumatic PVR is unknown. Here, 42d after‐Ozurdex injection, the anatomical stage of traumatic PVR was significantly decreased compared with the PVR group(Dex group, 1.33vsPVR group, 3.67), and the PVR grade in the histological examination was also reduced (Dex group,1.33vsPVR group, 2.50). These findings suggest a significant effect of Ozurdex on inhibiting the severity of traumatic PVR to 42d post‐injury.

Figure 4 Comparison of immunofluorescence, quantitative real-time polymerase chain reaction, and Western blot analyses of GFAP levels in the normal control, PVR, and Dex groups A: Immunofluorescence in different sections of the retina in the three groups. The inner retinal change was stained with GFAP, demonstrating the activation of retinal glial cells, especially Müller glial cells. In the PVR group, GFAP staining extended to the subretinal space (white arrows). There is limited staining within the inner retina in the Ozurdex-injected retina and no evidence of subretinal glial scars. Bar scale=50 μm. B–D: The GFAP staining area percentages in different sections from various groups. E, F: Western blot analysis of GFAP expression over time in PVR eyes. H, I: Comparison of the GFAP protein levels on days 28 post-injury by Western blot analysis. G, J: Changes in GFAP mRNA levels over time and comparison with Ozurdex treatment on day 21. Data are presented as mean±SEM.aP<0.05, bP<0.01, cP<0.001. GCL: Ganglion cell layer; INL: Inner nuclear layer; ONL: Outer nuclear layer; Con: Normal control; PVR: Proliferative vitreoretinopathy; Dex: PVR induction+intravitreal Ozurdex injection; αSMA: Alpha-smooth muscle actin; GFAP: Glial fibrillary acid protein;GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; DAPI: 4’,6-diamidino-2-phenylindole.
Generally, intense inflammation and retinal injury always occur following ocular trauma. Cardilloet al[7]reviewed data from 71 patients with traumatic PVR and found that persistent intraocular inflammation was an important independent risk factor for traumatic PVR. Khanumet al[8]discovered that TNF‐α and IL‐6 mRNA levels were upregulated at 24, 48, and 72h after injury in a traumatic PVR model. Chenet al[9]found that the TNF‐α mRNA level significantly increased during the observation period in a traumatic PVR model. However,the observation periods in these studies were relatively short.Therefore, we performed an observation on post‐traumatic inflammation for a long time, up to 6wk. We found that IL‐1β and TNF‐α were the major inflammatory cytokines.Their protein levels were significantly upregulated on day 3,and their mRNA expression levels also increased and peaked on day 3, with a second peak on day 21 post‐injury. After treatment with Ozurdex, mRNA and protein levels of IL‐1β were significantly suppressed; Ozurdex also inhibited the TNF‐α level, but the difference was not significant.However, Kuoet al[30]discovered that Ozurdex inhibited TNF‐α in an experimental PVR model involving intravitreal RPE cell injection. This difference might be related to the way PVR was induced. In our study, penetrating ocular trauma induced an immediate and intense inflammation closely linked to injury and Müller cell gliosis; intravitreally delivering RPE cells in the aforementioned study induced inflammation that mainly contributed to fibrous proliferation. In summary, we confirmed the chronic inflammation after ocular trauma and highlighted the crucial role of IL‐1β in this traumatic PVR model.

Figure 5 Comparison of inflammatory cytokines levels in the control group, PVR group, and Dex group A–D: The average cytokine concentrations in the vitreous by cytokine antibody array analysis, from baseline to week 6; E–G: Comparisons of inflammatory cytokines IL-1β,IL-8, and TNF-α protein levels on day 3; H–J: Changes in IL-1β, IL-8, and TNF-α mRNA levels in the total retina over time. K–M: Comparisons of inflammatory cytokines IL-1β, IL-8, and TNF-α mRNA levels. Data are presented as mean±SEM. aP<0.05, bP<0.01, cP<0.001. Con: Normal control;PVR: Proliferative vitreoretinopathy; Dex: PVR induction+intravitreal Ozurdex injection; IL: Interleukin; TNF-α: Tumor necrosis factor-alpha.

Figure 6 Expression of NLRP3 and phosphorylated NF-κB A, B: Western blot analysis of NLRP3 in PVR eyes post-injury over time; C: Bar graphs show the relative expression levels of NLRP3 after PVR induction in the quantitative real-time polymerase chain reaction analysis; D:Representative bands of NLRP3, p-NF-κB, and NF-κB proteins were assessed by Western blot analysis after treatment; E: Bar graphs show the efficacy of Ozurdex in suppressing NLRP3 protein levels on day 1; F: Bar graphs show the efficacy of Ozurdex in suppressing NLRP3 mRNA levels on day 3; G: Bar graphs show the efficacy of Ozurdex in suppressing NF-κB activation on day 1. Data are presented as mean±SEM. aP<0.05,bP<0.01, cP<0.001. NLRP3: Nod-like receptor family pyrin domain containing-3; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; NF-κB:Nuclear factor-kappa B; p-NF-κB: Phosphorylated nuclear factor-kappa B.

Figure 7 Boxplot of IOP variations from day 1 to week 6 The thick line within the colored boxes represents the median IOP, and the dots within the boxes represent the average values. The limits of the boxes represent IQR, and the whiskers indicate the lowest to the highest values within 1.5 times the IQR excluding dot outliers (gray dot beyond the whiskers in the P-5W box). Con: Normal control;PVR: Proliferative vitreoretinopathy; Dex: PVR induction+intravitreal Ozurdex injection; IOP: Intraocular pressure; IQR: Interquartile range.
As known, IL‐1β is a secreted protein; its activation and release need a key mediator, namely NLRP3, to cleave pro‐IL‐1β into the mature form for secretion into the local environment[34].The NLRP3/IL‐1β inflammatory axis is closely associated with various chronic inflammatory diseases, such as Alzheimer’s disease[35], atherogenesis[36], diabetic retinopathy[37‐38],glaucoma[39‐40], age‐related macular degeneration[41], and uveitis[42]. Nevertheless, the role of NLRP3 in post‐traumatic chronic inflammation and PVR development is unclear. Thus,we investigated NLRP3 expression in this animal model. The results showed that NLRP3 had an expression pattern similar to IL‐1β but expressed earlier than IL‐1β. The NF‐κB signal,which binds to the promoter of NLRP3 and facilitates NLRP3 transcription, is a priming step in NLRP3 inflammasome activation[38,43]. Similarly, in the current study, we found that NF‐κB was activated on day 1 post‐injury, consistent with the time point of NLRP3 expression. After Ozurdex treatment,NF‐κB phosphorylation and NLRP3 expression decreased simultaneously. These findings indicated that the NF‐κB/NLRP3 signaling pathway participated in regulating the inflammatory response of traumatic PVR. Ozurdex alleviated post‐traumatic inflammation and PVR severity, presumablyviathe NF‐κB/NLRP3/IL‐1β inflammatory axis. However, its precise mechanism merits further investigation.Müller cell gliosis also plays an important role in the development of PVR[15,44‐45]. Xiaoet al[13]reported that Müller cells were activated on day 3 after retinal detachment induced by infusing sodium hyaluronate into the subretinal space through a tiny retinotomy. However, in the case of traumatic PVR, the status and extent of Müller cell reaction have not yet been fully elucidated. In the present study,GFAP was upregulated on day 3 post‐injury and remained elevated through week 6, suggesting that Müller cells were persistently activated during the progression of traumatic PVR.Additionally, Müller glia has been reported to be an important source of the inflammatory cytokines present in the gliotic retina during PVR[46]. El‐Azabet al[47]reported that expression of IL‐1β co‐localized with Müller cells in neuronal degenerated retina induced by intravitreal injection of N‐methyl‐D‐aspartate in mice. We consider that persistent Müller cell gliosis observed in our study was closely associated with persistent post‐injury inflammation. Of note, we observed glial scars across the whole‐thickness retina in the peripheral retina near the wound. Moreover, we found a few scattered small glial scars far from the incision, even in the central retina. These findings demonstrated the extensive, persistent, and severe Müller cell gliosis in the traumatic PVR model. Müller cell gliosis is characteristic of intraretinal proliferation, which is the most severe type of PVR, and massive Müller cell gliosis leads to scarring of the retina and retinal shortening[14,48‐49]. Given the evidence that Müller cell gliosis is closely related to PVR progression, such massive gliosis observed in our study was in accordance with the final outcome of INL and ONL thinning and tractional retinal detachment. Müller cell activation and inflammatory cytokines release interact as both stimulus and effector[50‐51], thereby facilitating PVR progression and further retina damage in this animal model.
Our findings confirmed the beneficial effect of Ozurdex on traumatic PVR by inhibiting the chronic inflammation response and Müller cell gliosis in rabbits after penetrating ocular injury. NF‐κB/NLRP3 signaling may play an important role in regulating persistent inflammation of the traumatic PVR model. However, the present study has some limitations. First,this animal study might not totally mimic the actual conditions or symptoms of human diseases. Second, the inflammatory mechanism of traumatic PVR and excessive Müller cell gliosis was only investigated to a limited extent and requires further research.
ACKNOWLEDGEMENTS
Authors’ contributions:Conceptualization (Zhao YM and Lin XF); Data curation (Zhao YM); Formal analysis, (Zhao YM, Sun RS, and Wang FY); Funding acquisition (Yang Y and Lin XF); Investigation (Zhao YM, Sun RS, Li YJ, Qian XB, and Zeng JT); Methodology (Zhao YM and Yang Y);Project administration (Duan F and Yang Y); Resources (Yang Y and Lin XF); Supervision (Duan F, Yang Y, and Lin XF);Validation (Sun RS and Yang Y); Visualization (Zhao YM and Sun RS); Original draft (Zhao YM); Review & editing (Zhao YM, Yang Y, and Lin XF).
Foundations:Supported by National Natural Science Foundation of China (No.81974135; No.81900851).
Conflicts of Interest: Zhao YM,None;Sun RS, None;Duan F,None;Wang FY, None;Li YJ,None;Qian XB, None;Zeng JT,None;Yang Y, None;Lin XF, None.
杂志排行
International Journal of Ophthalmology的其它文章
- Instructions for Authors
- Morphological and functional changes in the macular area in diabetic macular edema after a single intravitreal injection of aflibercept
- Macular vascularisation changes analysed using OCT angiography after successful rhegmatogenous retinal detachment repair
- Comparison of success rate and intraocular pressure spikes between selective laser trabeculoplasty and micropulse laser trabeculoplasty in African American and Hispanic patients
- Efficacy of custom-made soft keratoconus lenses on corneal aberrations and photic phenomena in patients with keratoconus: a corneal topography imaging based study
- Clinical observation of recombinant human nerve growth factor in the treatment of neurotrophic keratitis
