Mechanistic investigation on Hg0 capture over MnOx adsorbents: effects of the synthesis methods
2023-02-06DongYEYongjinHUZhichangJIANGXinLIUHainingWANG
Dong YE, Yongjin HU, Zhichang JIANG, Xin LIU, Haining WANG
Regular paper
Mechanistic investigation on Hg0capture over MnOadsorbents: effects of the synthesis methods
Dong YE, Yongjin HU, Zhichang JIANG, Xin LIU, Haining WANG
College of Quality & Safety Engineering, China Jiliang University, Hangzhou 310018, China
This study demonstrated the impacts of the synthesis methods on the textural structures, chemical properties, and Hg0capture capability of the MnOsystem. Compared with the samples synthesized using the precipitation (PR) and hydrothermal (HT) methods, the adsorbent prepared via the sol-gel (SG) technique gave the best performance. At 150 ℃, ca. 90% Hg0removal efficiency was reached after 7.5 h for MnOprepared by the SG method, ca. 40% higher than that of the other two methods. The specific surface area of the adsorbent synthesized via the SG technique (23 m2/g) was almost double that of the adsorbent prepared by the HT method (12 m2/g) and three times that of the one prepared by the PR method (7 m2/g). The presence of plentiful acid sites from the SG method facilitated the physisorption of Hg0, making more Hg0available to be oxidized to HgO by the redox sites and thus giving the adsorbent prepared by the SG method the highest Hg0removal efficiency. The strong oxidative ability accelerated the oxidation of the physically adsorbed Hg0to HgO, which explained the higher Hg0removal efficiency of the sample prepared using the HT method than that of the one synthesized by the PR technique. During the whole Hg0removal cycles, chemisorption dominated, with the initial adsorption stage and the external mass-transfer process playing important roles.
Synthesis methods; Hg0capture; MnO; Acidity; Oxidative ability
1 Introduction
Mercury poses a great threat to the environment and human health owing to its high toxicity and long residence (Liu H et al., 2020; Ye et al., 2022d). The consumption of fossil fuels accounts for ca. 35% of the totally produced mercury and around 25% of the global emissions come from anthropogenic sources in China (Xu et al., 2015). Faced with such a severe environmental problem, a national standard GB13223-2011 (MEP, 2011) was issued to illustrate the emission limit of mercury and the Chinese government also approved the "Minimata convention" in 2013, demonstrating that great efforts should be made to effectively reduce the emission of mercury (Wang Z et al., 2020).
In coal-fired flue gas, the emitted mercury can be classified into three categories, namely elemental mercury (Hg0), oxidized mercury (Hg2+), and particulate-bounded mercury (Hgp) (Ye et al., 2022b). These last two species can be easily captured by existing air pollution control devices because of the satisfactory solubility of Hg2+and the strong adhesion of Hgpto the fly ash surfaces, whereas its insolubility and high volatility make Hg0hard to remove. Thus, improving Hg0removal performance is recognized as the key step in reducing mercury emissions (Ye et al., 2021).
To date, adsorption has been shown to be an effective and mature technology to remove Hg0from the flue gas (Chalkidis et al., 2019a). The performance of the adsorbents, the key component in this technique, always dictates the Hg0removal efficiency of the whole system. Several adsorbents, including carbon- and zeolite-based systems, do have satisfactory Hg0capture capability in a certain temperature window (Liu DJ et al., 2020b; Liu H et al., 2020). As a typical transition metal oxide, MnOhas been proven to be suitable for Hg0removal due to the merits of its acidity, its plentiful surface oxygen vacancies, and its superior oxygen migration ability (Yang et al., 2019). Prepared MnOnanotubes, nanorods, and nanowires can effectively capture Hg0from simulated flue gas (Chalkidis et al., 2019b).
It is acknowledged that apart from the formula, the preparation method also significantly affects the activity of the adsorbent material (Ye et al., 2022a). It is reported that precipitation (PR), hydrothermal (HT), and sol-gel (SG) methods are recognized as the most commonly used techniques for its preparation (Gao et al., 2010; Liu et al., 2013). Each of the methods has its own merits and drawbacks. However, there has been no systematic analysis and comparison of the impacts of the synthesis conditions on the physicochemical properties and Hg0removal activity of MnO, and it is those which will be extensively explored in this study. By this means, structure–performance relationships of the adsorbents can be established, and through exploring the detailed Hg0capture pathways, the active sites and the key factors involved in the Hg0removal cycles can be revealed. Knowledge of such effects can help to increase adsorbent Hg0capture capability to its maximum extent and lay a solid foundation for the effective reduction of mercury emission and the practical application of the adsorbents.
2 Experimental
2.1 Preparation of the adsorbents
MnOprepared using the SG method (denoted as Mn-SG): Mn(NO3)2and citric acid with the molar ratio of 1/2 were dissolved in 30-mL deionized water. After stirring for 3 h, a beaker of transparent sol was obtained and then placed in an oven at 80 ℃. Until the foam-like product was formed, the powder was dried in an oven (DHG-9256a, Shanghai Zollo Instrument Co., Ltd., China) at 110 ℃ for 8 h and finally calcined in a muffle furnace (SGM·M8/10, Guangzhou Hang xin Scientific Instrument Co., Ltd., China) at 500 ℃ for 5 h.
MnOsynthesized via the PR method (denoted as Mn-PR): Certain amount of Mn(NO3)2was dissolved in 30-mL H2O, which was then slowly added into the excess ammonia solution under vigorous stirring. After 3 h, the produced paste was separated by centrifuge (TG20-WS, Shanghai Chuan Hong Yiqi Co., Ltd., China) and then washed with H2O. After drying at 110 ℃ in an oven (DHG-9256a, Shanghai Zollo Instrument Co., Ltd., China) for 8 h, the product was calcined in a muffle furnace (SGM·M8/10, Guangzhou Hangxin Scientific Instrument Co., Ltd., China) at 500 ℃ for 5 h.
MnOprepared using the HT method (denoted as Mn-HT): The calculated amount of Mn(NO3)2was dissolved in 30-mL deionized water, which was then dropwise added into 40-mL 5-mol/L NaOH solution. After stirring for 3 h, the mixture was transferred to a 100-mL Teflon autoclave. The HT process at 120 ℃ in an oven (DHG-9256a, Shanghai Zollo Instrument Co., Ltd., China) lasted for 16 h. Then the brownish paste was washed six times, followed by drying in an oven (DHG-9256a, Shanghai Zollo Instrument Co., Ltd., China) at 110 ℃ for 8 h. Finally, the powder was calcined in a muffle furnace (SGM·M8/10, Guangzhou Hangxin Scientific Instrument Co., Ltd., China) at 500 ℃ for 5 h.
2.2 Reaction systems
The Hg0capture capability of the serial samples was evaluated in a fixed-bed micro-reactor (PXJF-6-2A, Tianjin Golden Eagle Technology Co., Ltd., China). In each 7.5-h test, a 0.1-g sample was used. The simulated flue gas consisted of 5% O2, 200-μg/m3Hg0, and N2in balance. The total flow rate was 600 mL/min, corresponding to a gas hourly space velocity of 360 L/(g·h). Hg0vapour was generated through a mercury permeation tube (QMG-6-6) placed in a U-shaped quartz tube. The temperature of the water bath was maintained at 32 ℃. The inlet and outlet Hg0concentrations were recorded using an online mercury analyzer (RA-915M, LUMEX Ltd., Russia). Adsorbent Hg0removal efficiency () was determined using the following equation:
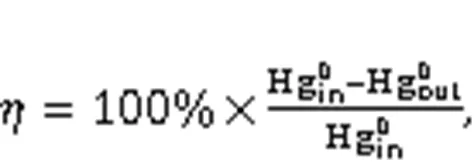
2.3 Characterizations
X-ray diffraction (XRD) data in the scanning range of 10o–90owere collected on Rigaku apparatus (Rigaku, D/max-2200, Japan) using the radiation source of Cu Kα. Transmission electron microscope (TEM) images were acquired on FEI Tecnai F20 (FEI, USA), which clearly showed the morphology of the investigated samples.
N2adsorption experiments were conducted on QuantachromeAutosorb-1 apparatus. Adsorbent specific surface area and pore size distribution were determined based on N2adsorption-desorption isotherms using the Brunauer-Emmett-Teller (BET) equation and the Barrett-Joyner-Halenda (BJH) method, respectively. The degas temperature was set at 300 ℃.
H2-temperature programmed reduction (H2-TPR) profiles were obtained on Micromeritics apparatus (ChemiSorb 2720 TPx, Micromeritics, USA). A sample of 100 mg was held at 300 ℃ in Ar for 1 h and then cooled to 50 ℃. Under 5% H2/Ar atmosphere, the sample was finally heated to 800 ℃ with a ramping rate of 10 ℃/min. H2consumption was analyzed using a thermal conductivity detector (TCD).
NH3-temperature programmed desorption (NH3-TPD) profiles were recorded on Micromeritics appa ratus (ChemiSorb 2720 TPx, Micromeritics, USA). Briefly, 0.1 g sample was held at 300 ℃ in Ar for 1 h, followed by lowering the temperature to 50 ℃. Given saturation with NH3and purging by N2, the sample was finally heated to 800 ℃ in N2. The outlet NH3signal was analyzed using the TCD.
X-ray photoelectron spectroscopy (XPS) analysis was conducted on an ESCALab220i-XL electron spectrometer. All the binding energies were calibrated with a definite C 1s line of 284.6 eV. Raman spectra of the investigated samples were recorded by micro-Raman spectroscopy (Renisaw, InVia).
Hg0temperature-programmed desorption (Hg0-TPD) was carried out in a fixed-bed micro-reactor (PXJF-6-2A, Tianjin Golden Eagle Technology Co., Ltd., China). As with the procedures of the NH3-TPD characterization, the desorption starts from 150 to 450 ℃ in N2as the pretreatment, Hg0saturation, and N2purging stages end. The outlet signal of Hg0was collected using an online mercury analyzer (RA-915M, LUMEX Ltd., Russia).
3 Results and discussion
3.1 Performance of the adsorbents
The Hg0capture capabilities of the serial adsorbents are illustrated in Fig. 1. Among these three samples, the Mn-SG method exhibits the highest activity, followed by Mn-HT and Mn-PR. At 150 ℃, ca. 90% Hg0removal efficiency is achieved by Mn-SG, ca. 40% higher than that of the other two samples. As the temperature rises, a downward trend in the Hg0removal efficiency is observed for Mn-SG and Mn-PR. By contrast, Mn-HT presents a volcano-shaped activity profile with adsorbent Hg0removal efficiency reaching a peak value of ca. 60% at 200 ℃. This result indicates that there must exist some variation in the physicochemical properties of these three samples, which br ings about different adsorbent Hg0abatement activities.
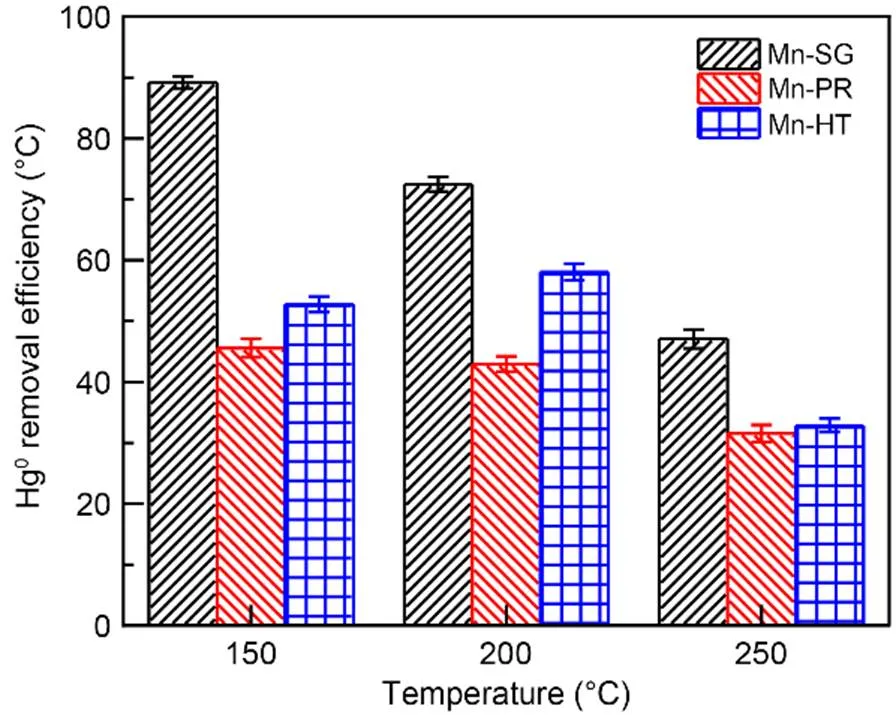
Fig. 1 Hg0 capture capability of the serial adsorbents
3.2 Textural structures of the adsorbents
The micro morphology of these three investigated adsorbents is illustrated in Fig. 2. It can be seen that all the samples present a morphology of irregular particles fused together, suggesting that morphology is not the main explanation for the variations in the Hg0capture capability of the adsorbents. Table 1 summarizes the N2adsorption results of the samples. It is observed that the specific surface area of the Mn-SG adsorbent is almost double that of the Mn-HT adsorbent and three times that of the precipitation one. This demonstrates that an increased number of surface-active sites were available for Hg0capture and contributed to the elevated Hg0removal performance of Mn-SG.
XRD patterns are shown in Fig. 3a, and can be used to express the crystal information from the samples. In both the Mn-PR and Mn-HT samples, the peaks are those of Mn2O3, whereas the bands indexed to Mn3O4crystallites are present only for Mn-SG. Moreover, a decrease in the peak intensity occurs for Mn-SG. This result demonstrates that the growth of the crystals is suppressed and a relatively high specific surface area is in turn obtained, as is evidenced by the N2adsorption results in Table 1. The local structures of these three samples are further explored through Raman spectroscopy and the related spectra are shown in Fig. 3b. It can be seen that all the samples exhibit three peaks in the wavenumber range of 200–900 cm-1. Peaks at ca. 308, 354, and 643 cm-1correspond to the out-of-plane bending modes of MnO, asymmetric stretch of bridge oxygen species (Mn-O-Mn), and symmetric stretch of MnOgroups, respectively (Ramesh et al., 2008).
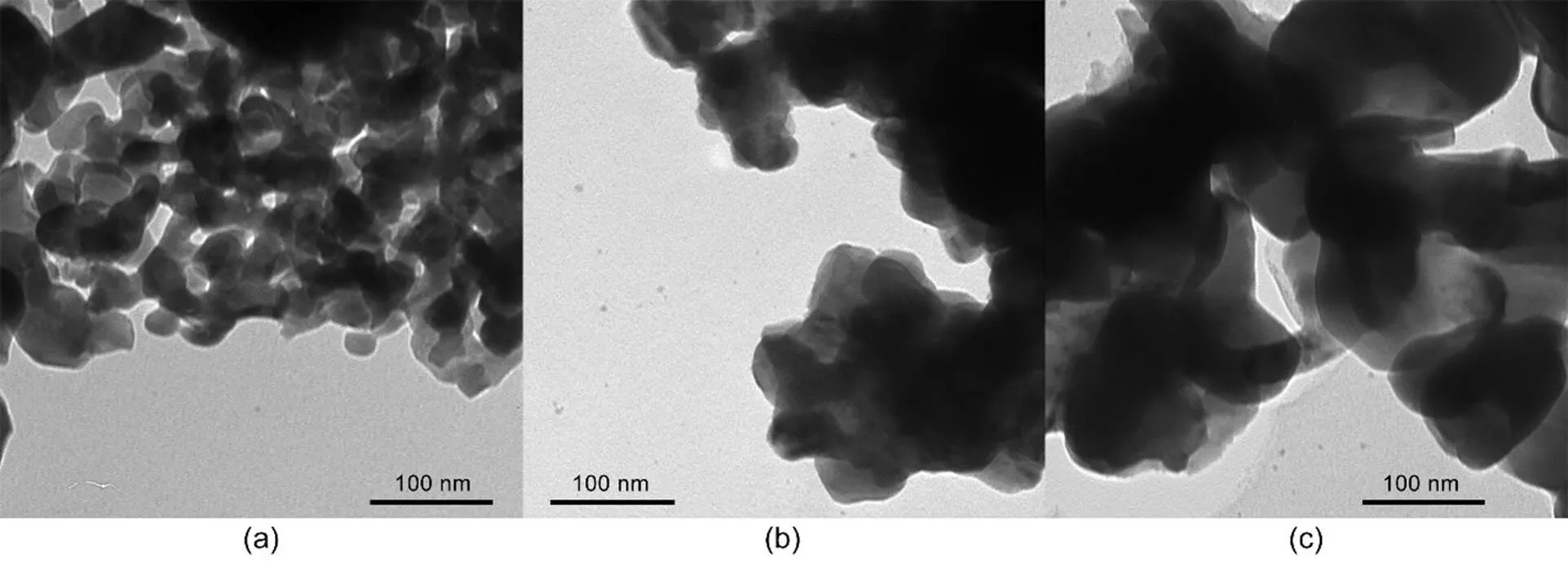
Fig. 2 TEM images of Mn-SG (a), Mn-PR (b), and Mn-HT (c)
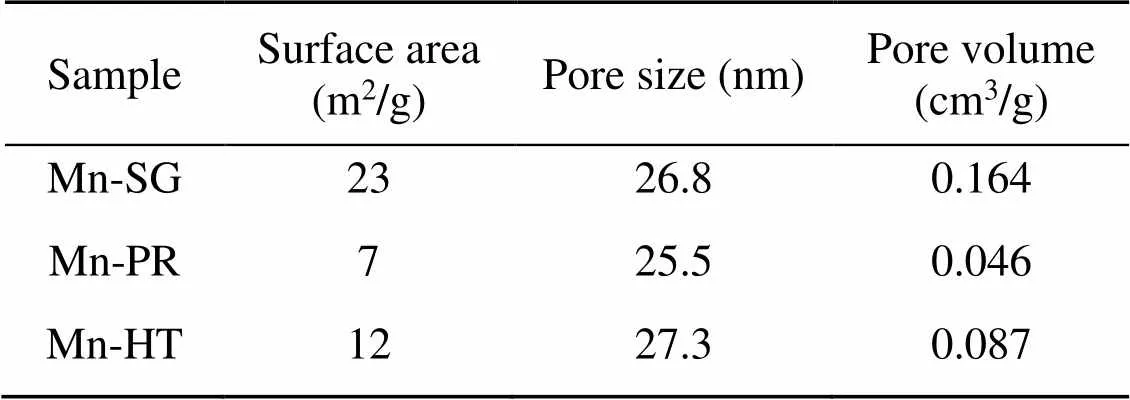
Table 1 N2 adsorption results of the adsorbents
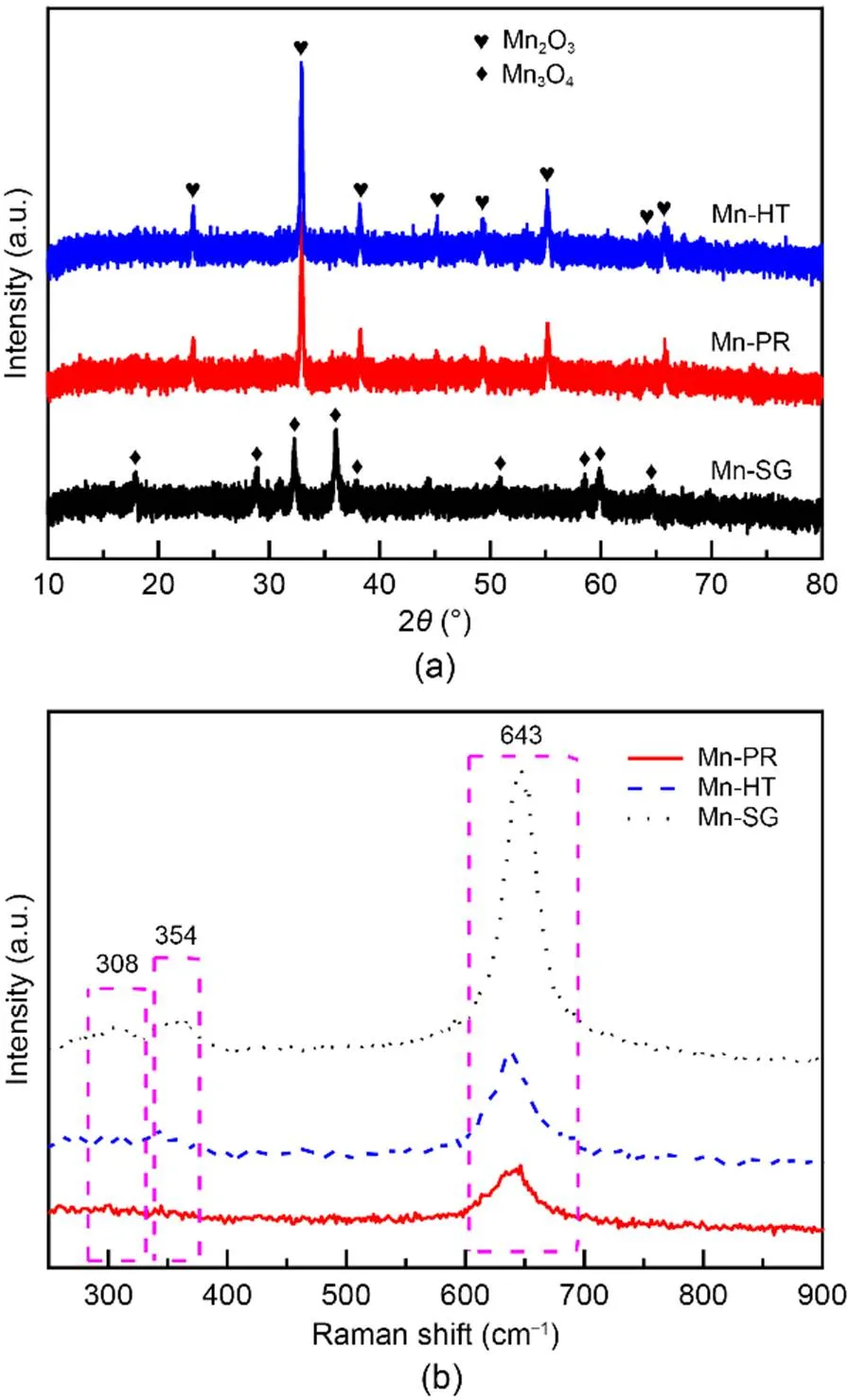
Fig. 3 XRD patterns (a) and Raman spectra (b) of the adsorbents
3.3 Chemical properties of the adsorbents
The NH3-TPD method was employed to study the acidity of the serial adsorbents and the NH3adsorption quantity can be used to elucidate the number of surface acid sites. As is illustrated in Fig. 4, Mn-SG possesses the largest number of surface acid sites, followed by Mn-PR and Mn-HT. That would contribute to the physisorption of Hg0and partially explains the highest Hg0removal efficiency of Mn-SG among these three samples. It also indicates that, after acidity, some other properties, such as oxidative ability, play a part in the Hg0capture capability of the adsorbents.
H2-TPR profiles of the samples are presented in Fig. 5a and demonstrate their redox properties. As reported in the literature, the peak centered at ca. 320 ℃ can be ascribed to the transformation of MnO2to Mn2O3, whereas the reduction of Mn2O3to Mn3O4explains the presence of the peak at ca. 395 ℃; the formation of MnO gives rise to the band located in the higher temperature region (Yang et al., 2015; Jia et al., 2016; Liu et al., 2018). By calculating the integrated areas of these three peaks, it seems that Mn-HT posse sses the largest proportion of Mn4+, followed by Mn-PR and Mn-SG. Thus, Mn-HT exhibits an enhanced oxidative ability compared with the other two samples, which can explain its higher Hg0removal efficiency compared with that of Mn-PR. However, the superior Hg0capture capability of Mn-SG demonstrates that oxidizability is not the unique factor for deciding the performance of an adsorbent and that acidity must also be a factor and will play a role.
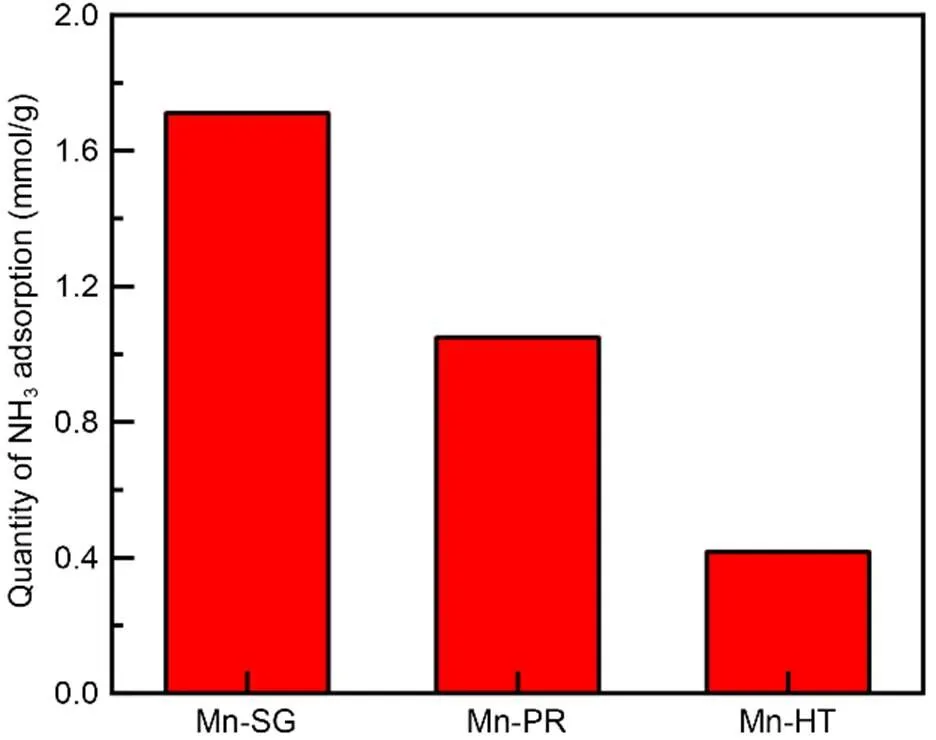
Fig. 4 NH3-TPD results of the adsorbents
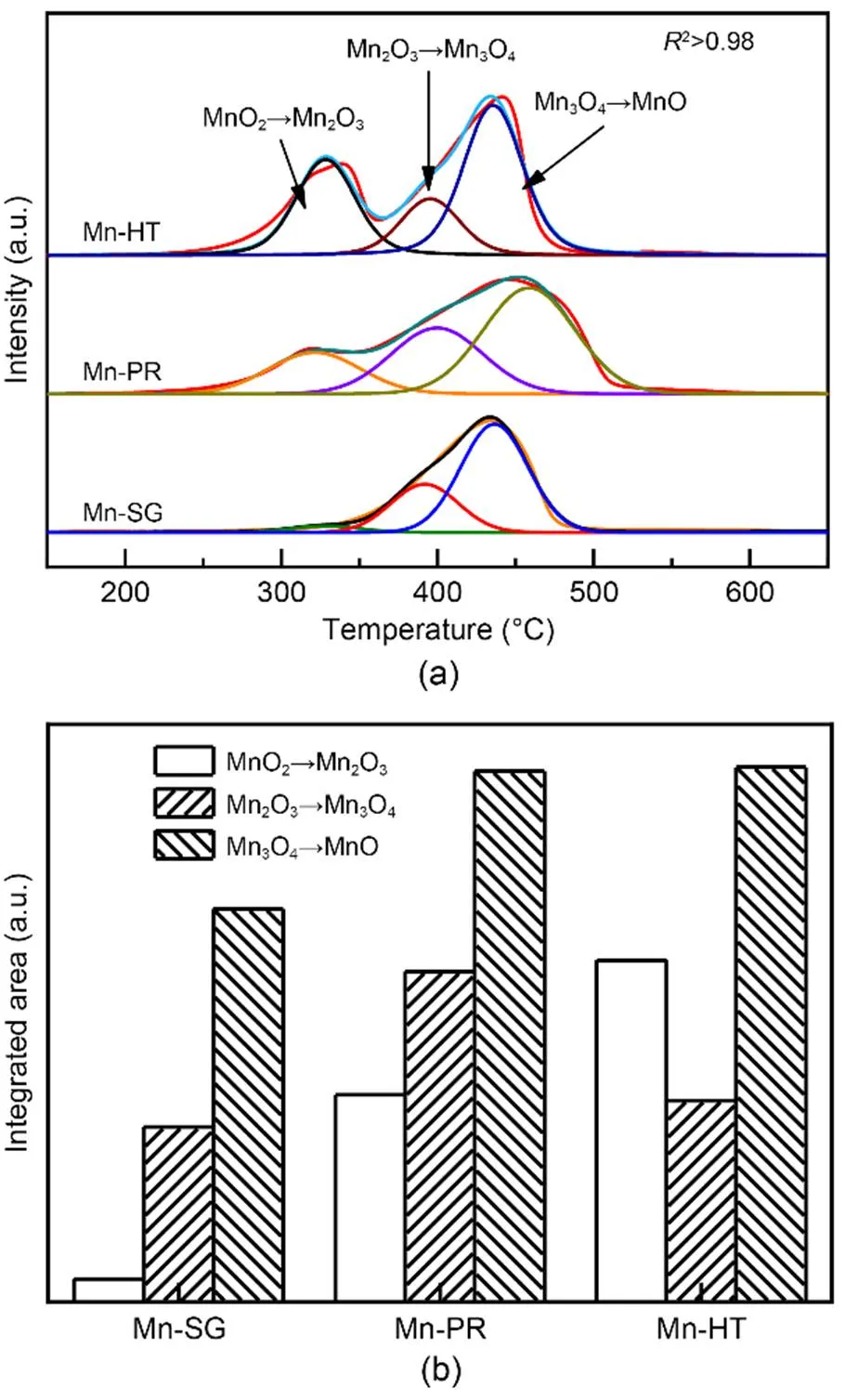
Fig. 5 H2-TPR profiles (a) and semi-quantitative calculation results (b) of the adsorbents
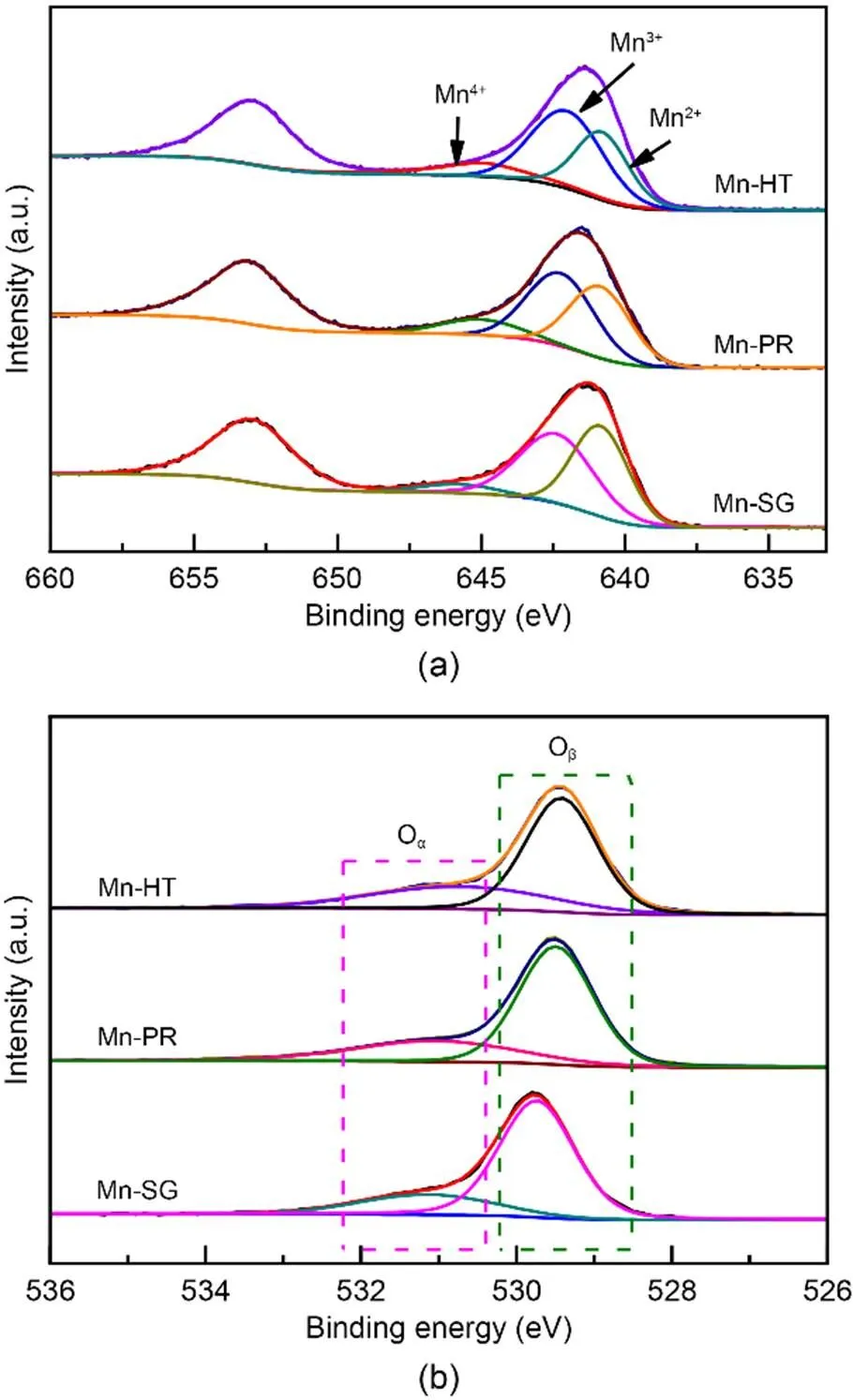
Fig. 6 XPS spectra: (a) Mn 2p; (b) O 1s
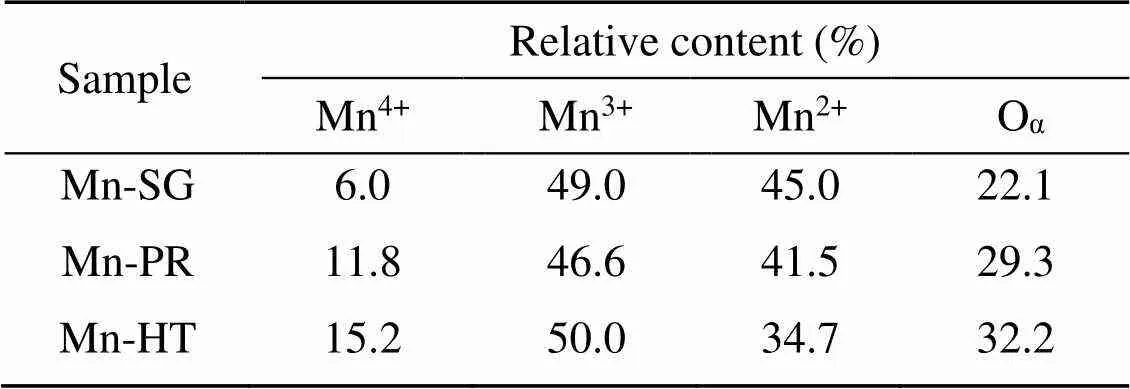
Table 2 XPS data of the adsorbents
3.4 Hg0 removal mechanisms
Among these three investigated samples, it can be seen that Mn-SG exhibits the best Hg0removal performance between 150 and 250 ℃. It is thus reasonable to select this sample for the investigation of the Hg0adsorption mechanisms, results of which can help discovery of the important factors during the Hg0removal cycles and thus the development of new adsorbents with superior Hg0capture capabilities.
As an important step during Hg0capture, the mass-transfer process affects the adsorbent Hg0rem oval efficiency to a considerable extent. According to the literature, the mass transfer process mainly consists of an external mass transfer, a global mass transfer, and an internal mass transfer, which can be expre ssed by the following four equations (Fulazzaky et al., 2013):
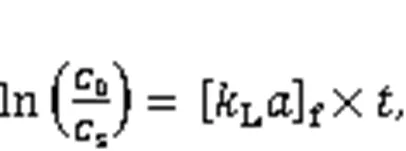
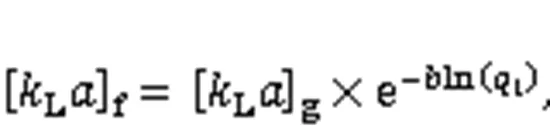

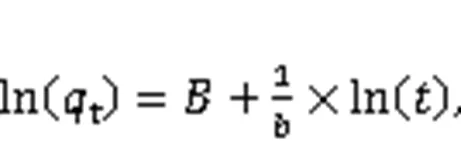
where0is the inlet concentration of Hg0(μg/m3),sis the outlet concentration of Hg0(μg/m3),is the Hg0capture accumulation time,is the surface of interfacial liquid-solid (m-1),is the adsorbate-adsorbent affinity parameter (g·h/mg),tis the Hg0cumulative quantity (mg/g),is the potential mass transfer index corresponding to the driving force of mass transfer (mg/g),Lis the mass transfer coefficient (m/h), [L]fis the external mass transfer factor (h-1), [L]gis the global mass transfer factor (h-1), and [L]dis the internal mass transfer factor (h-1).
Fig. 7a illustrates the linear fitting results of the experimental data. The correlation coefficients (2) at 150, 200, and 250 ℃ were calculated to exceed 0.998 (Table 3). Thus, it is reasonable to use the indexand parameterto scrutinize the mass transfer potential and adsorbate-adsorbent affinity for Hg0capture. Given an ongoing adsorption process, the absolute values of [L]f, [L]d, and [L]gcontinuously decrease and almost reach zero after 240 min, suggesting that the mass transfer process mainly influences the initial Hg0adsorption stage and that, after a period of time, its impact can be neglected. Considering that Hg0is a basic molecule, the physically adsorbed Hg0in the initial Hg0capture stage would occupy parts of the adsorbent surface acid sites (Zhao et al., 2020). That is to say, the accumulation of Hg0would lead to a decreased surface acidity and thus create a repulsion force between the adsorbate and the adsorbent. As a result, a negative value of [L]dis obtained.
The adsorption kinetics of Hg0over the adsorbe nts were explained through the fitting of four models, namely the pseudo-first-order (Eq. (6)), the pseudo-second-order (Eq. (7)), the Webber-Morris (Eq. (8)), and the Elovich (Eq. (9)) models, to the experimen tal data (Chen et al., 2015). Among them, both the pseudo-second-order model and the Elovich model explain the chemisorption process, while the external mass transfer process and the internal mass transfer process can be expressed by the pseudo-first-order model and the Webber-Morris model (Yang et al., 2021). In addition, the Elovich model can also illustrate the initial Hg0adsorption stage.
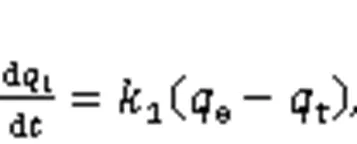
2
, (7)
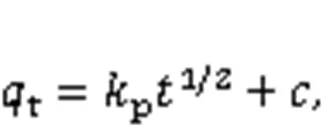
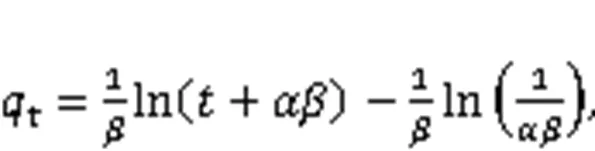
where1,2,e,p,,, andare attributed to the pseudo-first-order rate constant (min-1), the pseudo-second-order rate constant (μg/(g·min)), the adsorbent equilibrium adsorption capacity (μg/g), the intraparticle diffusion coefficient (μg/(g·min1/2)), the constant related to the boundary conditions (μg/g), the initial adsorption rate constant (μg/(g·min1/2)), and the desorption rate constant (g/μg), respectively.
As presented in Fig. 8 and Table 4, the pseudo-first-order, the pseudo-second-order, and the Elovich models can describe the Hg0capture process on Mn-SG effectively. As reported in the literature, the initial adsorption stage and the external mass transfer process play important roles, and that, to some extent, supports the conclusions drawn from Fig. 7 (Shi et al., 2016; Liu DJ et al., 2020a).
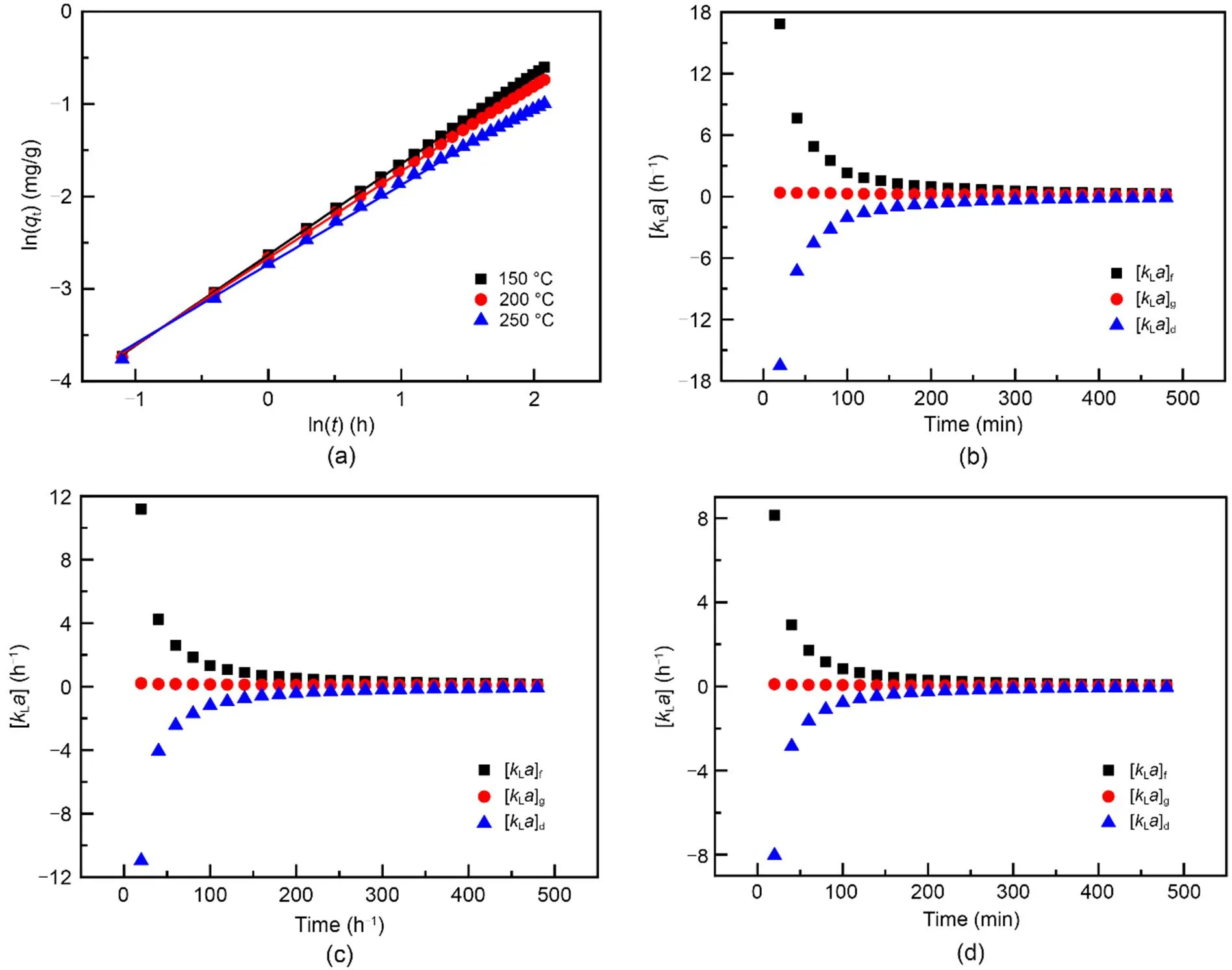
Fig. 7 Linear regression analysis (a) and curves of [kLa]f, [kLa]d, and [kLa]g versus time at 150 ℃ (b), 200 ℃ (c), and 250 ℃ (d)
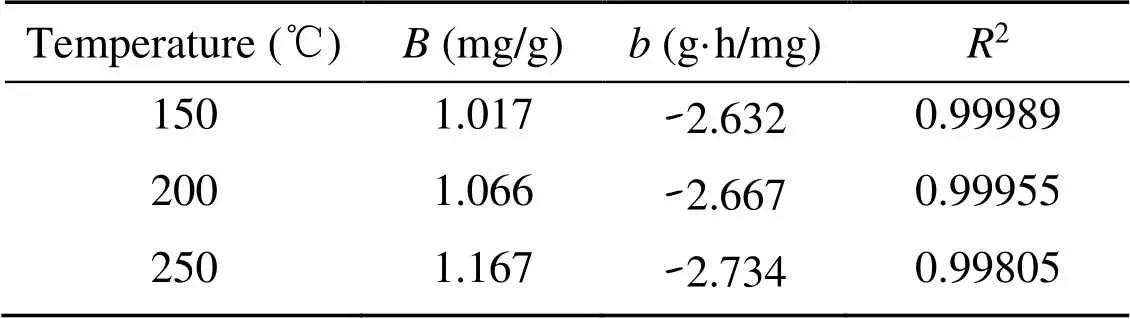
Table 3 Linear regression results
XPS was conducted to investigate the variations in the valence state of the adsorbent surface atoms after the adsorption of Hg0, to help elucidate the possible Hg0capture mechanisms. According to Fig. 9 and Table 5, the decrease in the relative contents of Mn4+, Mn3+, and Oαindicates that these species function as the active sites to directly participate in the removal of Hg0. The photoelectron peaks centered at ca. 101 and 105 eV in Fig. 9c and the Hg0-TPD profile in Fig. 9d demonstrate that the adsorbed mercury species are present in the form of HgO (Yang et al., 2011a). Thus, the Hg0capture pathways over Mn-SG can be summarized as follows (Eqs. (10)–(13) and Fig. 10) (Wang HN et al., 2020):
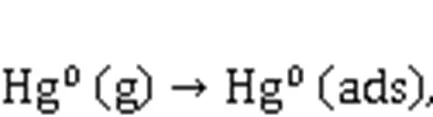
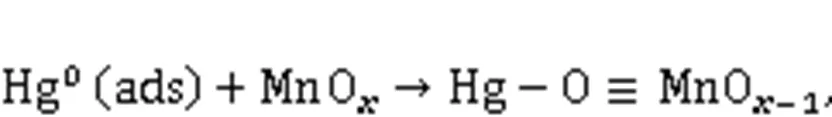
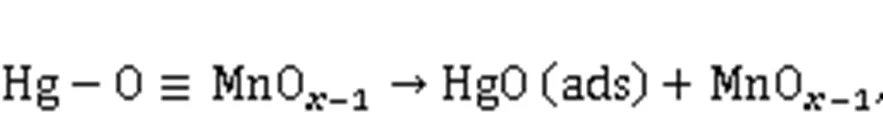
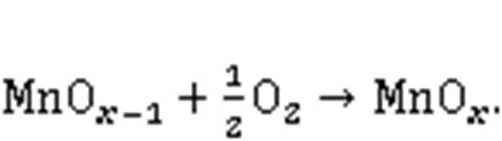
3.5 Discussion
Based on our previous study, the capture of Hg0can be considered as a dual-site reaction: adsorbent surface acid sites are primarily responsible for the physisorption of Hg0, whereas the redox sites participate in the oxidation of the physically adsorbed Hg0to HgO (Ye et al., 2022a, 2022c). In the case of Mn-SG, abundant surface acid sites are available to physically adsorb Hg0, which provides sufficient reactant for the next oxidation step and hence enables a large portion of the Hg0to be efficiently oxidized to HgO by the sites with oxidative ability. Only a small part of the gaseous Hg0is free from capture by the adsorbent and thus a relatively high Hg0removal efficiency is obser ved. While for the other two samples, even though an enhanced oxidative ability is obtained, yet the relative low amount of surface acid sites interferes with the physisorption of Hg0. Therefore, only a limited amount of Hg0can be involved in the following reaction step and most Hg0just passes directly through the adsorbent bed (Ye et al., 2022a). That is to say, that on this occasion, redox sites on Mn-HT and Mn-PR might be superfluous for the oxidation of Hg0and the lack of acid sites constitutes the main barrier to high Hg0removal efficiency. Opposite reasons can be used to explain the better Hg0capture capability of Mn-HT than that of Mn-PR. Besides, the optimized textural structures are somehow responsible for promoting diffusion of the reactant, which also partially explains the Hg0abatement performance order of Mn-SG>Mn-HT>Mn-PR (Chen et al., 2014; Ye et al., 2022c).
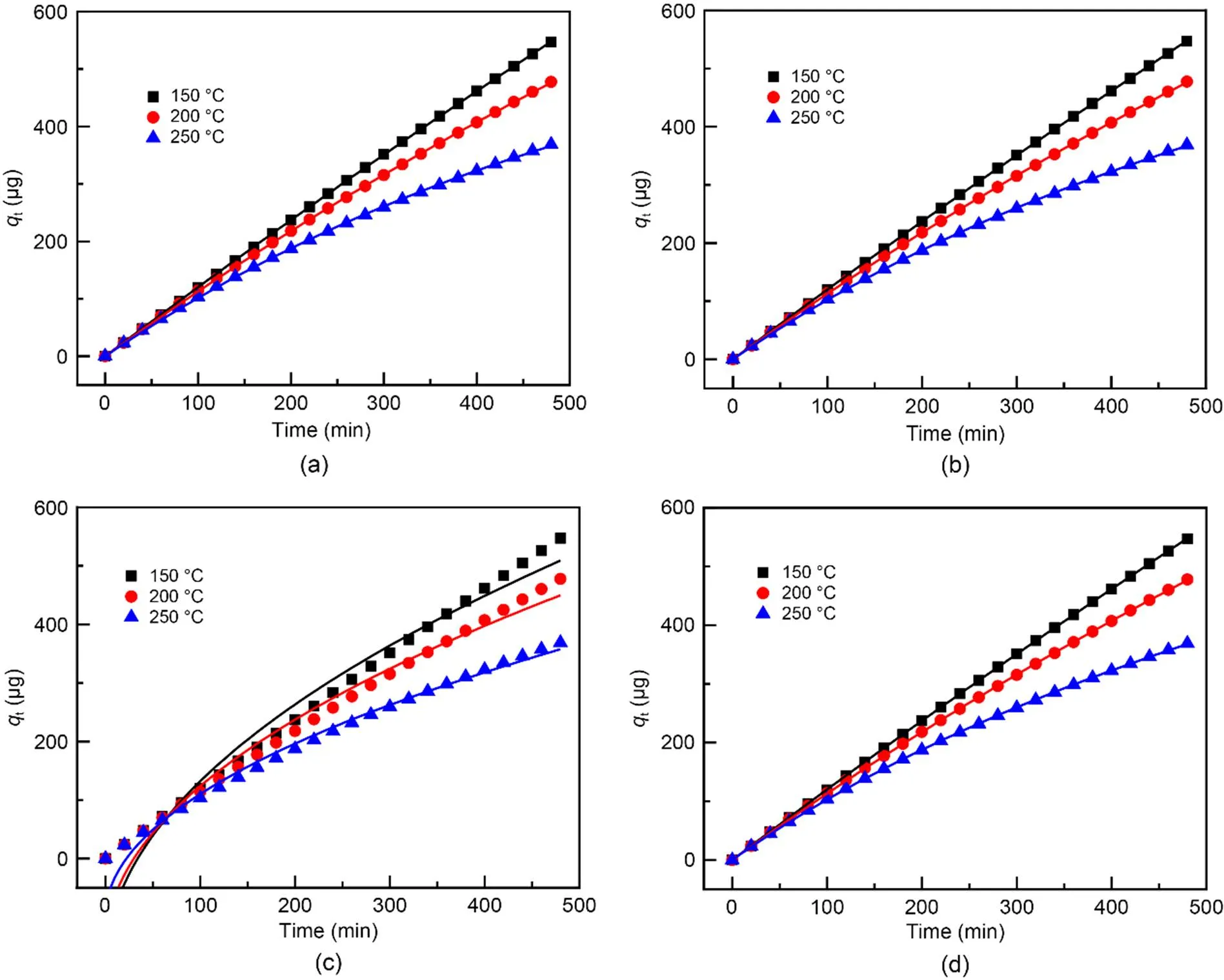
Fig. 8 Curve fitting of the experimental data by the pseudo-first-order (a), pseudo-second-order (b), Weber-Morris (c), and Elovich (d) models
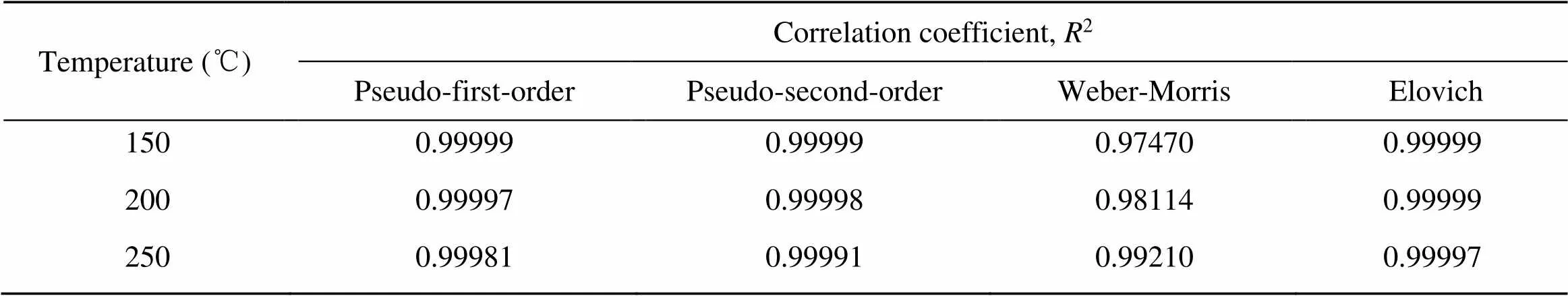
Table 4 Correlation coefficients (R2) of the pseudo-first-order, pseudo-first-order, Weber-Morris, and Elovich models
As the temperature rises, the differences between the positive effect of the enhanced Hg0oxidation step and the inhibitory impact of the weakened Hg0physisorption process mainly determine the trend of the adsorbent Hg0capture capability (Ye et al., 2022b). In other words, provided that the promotional impact of the accelerated Hg0oxidation stage is dominant during heating, adsorbent Hg0removal efficiency will be in turn elevated and vice versa. In the case of Mn-SG and Mn-PR, the suppressed Hg0physisorption process constitutes the main barrier to an increase in the Hg0removal efficiency at higher temperatures and thus there is a downtrend in the adsorbent Hg0capture capability (Yang et al., 2011b, 2011c). By contrast, the positive effect of the facilitated Hg0oxidation stage can overpower the above-mentioned negative impact for Mn-HT, thereby bringing out an elevated Hg0removal efficiency at 200 ℃. With a further increase in temperature, this inhibitory effect would dominate and consequently adsorbent Hg0removal efficiency would decline.
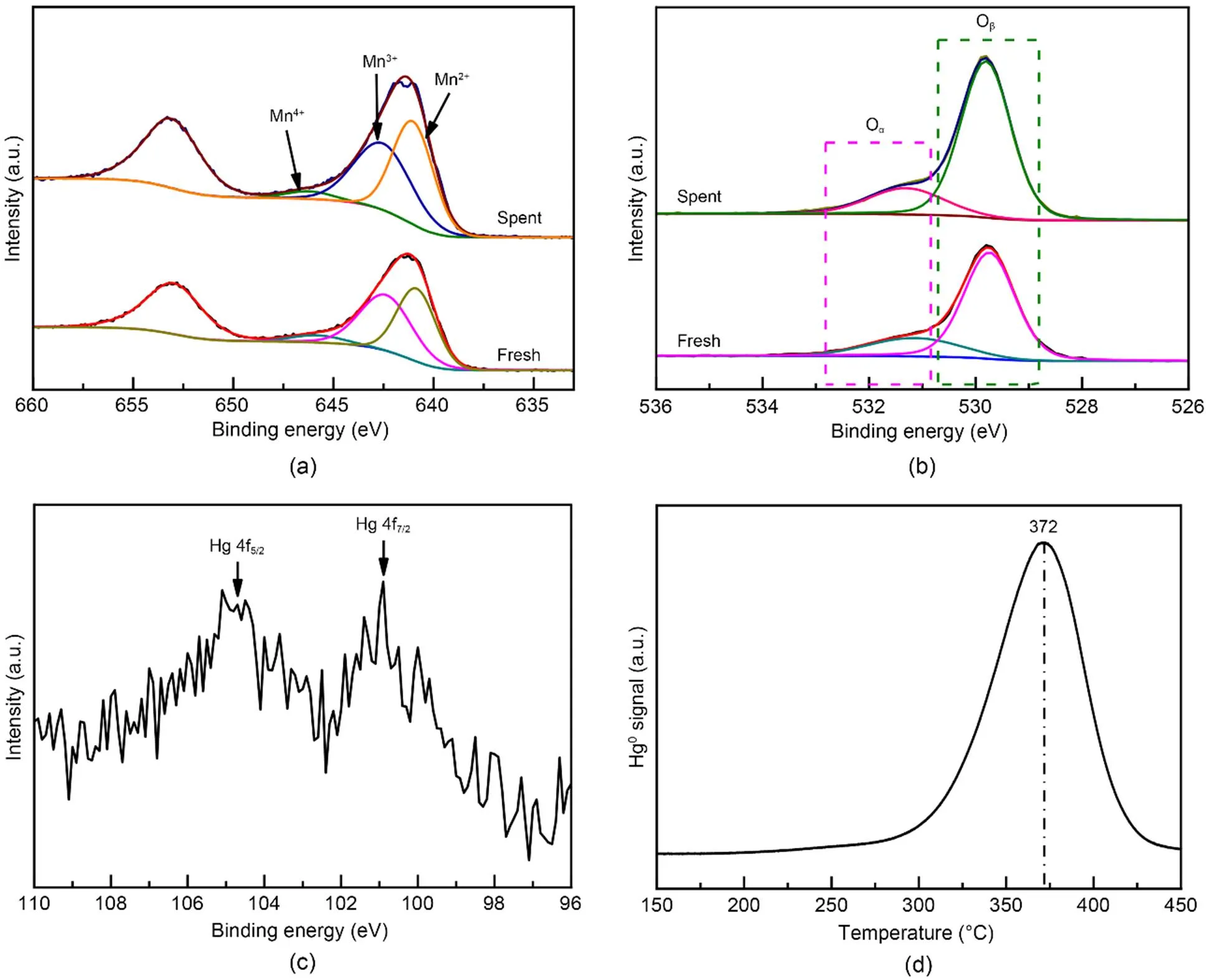
Fig. 9 XPS spectra of the fresh and spent samples: (a) Mn 2p; (b) O 1s; (c) Hg 4f; (d) Hg0-TPD profile
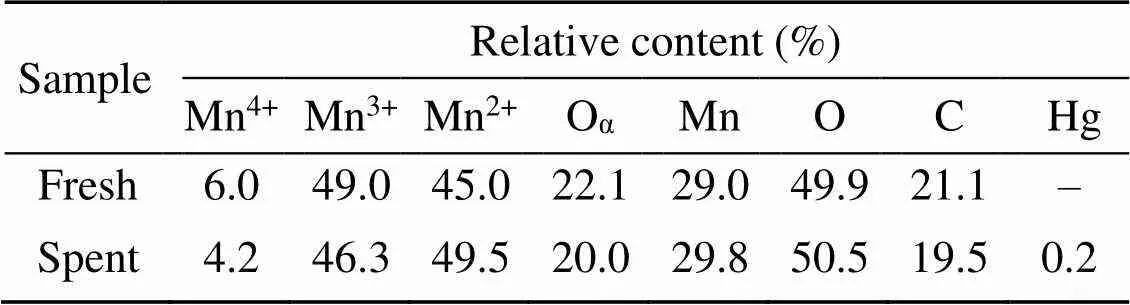
Table 5 XPS data of the fresh and spent samples
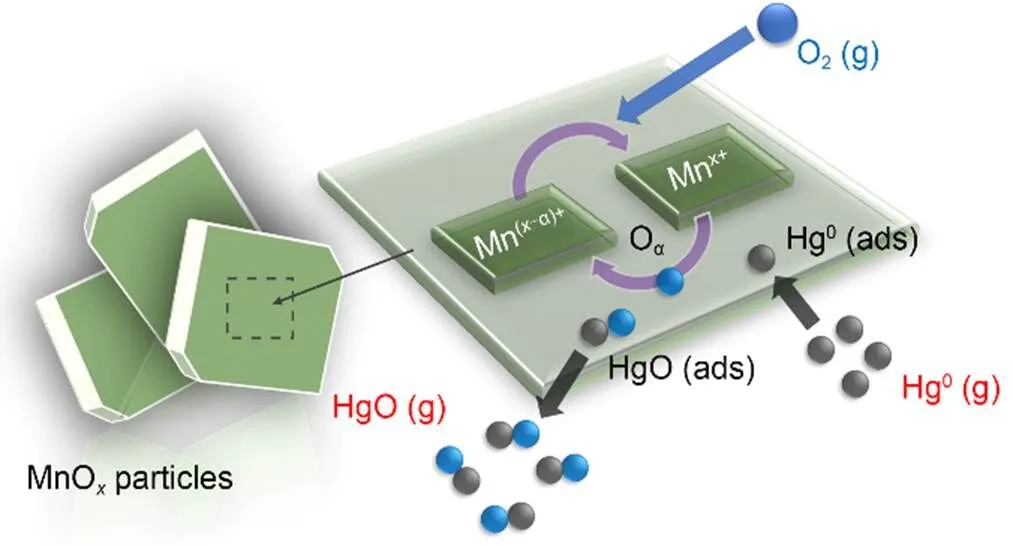
Fig. 10 Hg0 removal mechanism over Mn-SG
In summary, acidity and oxidizability both play a role in the Hg0capture capability of an adsorbent. A satisfactory acidity can ensure sufficient physisorption of Hg0and the sites with a superior oxidative ability will then contribute to the efficient oxidation of the physisorbed Hg0to HgO, and enable Hg0to be effectively removed from the flue gas.
4 Conclusions
In this study, we synthesized MnOadsorbents using the SG, PR, and HT methods and tested their Hg0removal performance. Some conclusions are drawn below:
(1) Adsorbent prepared using the SG method exhibits the best performance; ca. 90% Hg0removal performance is reached at 150 ℃, almost doubling compared with that of Mn-PR.
(2) The presence of plentiful acid sites facilitates the physisorption of Hg0, providing ample Hg0for oxidation to HgO by the redox sites and subsequently enabling Mn-SG with a relatively high Hg0removal efficiency.
(3) Chemisorption dominates in all the Hg0abatement cycles, in which the initial adsorption stage constitutes the rate-determining step. The Mars-Maessen mechanism can explain the Hg0capture pathways over the adsorbents with Mn ions in a high valence state and Oαdirectly participating in the oxidation of Hg0.
Acknowledgments
This work is supported by the Fundamental Research Funds of China Jiliang University and the Zhejiang Provincial Natural Science Foundation of China (No. LQ22E060003).
Author contributions
Yongjin HU and Zhichang JIANG designed the research. Dong YE processed the corresponding data. Dong YE wrote the first draft of the manuscript. Xin LIU helped to organize the manuscript. Haining WANG and Dong YE revised and edited the final version.
Conflict of interest
Dong YE, Yongjin HU, Zhichang JIANG, Xin LIU, and Haining WANG declare that they have no conflict of interest.
Chalkidis A, Jampaiah D, Amin MH, et al., 2019a. CeO2-decorated α-MnO2 nanotubes: a highly efficient and regenerable sorbent for elemental mercury removal from natural gas. Langmuir, 35(25):8246-8256. https://doi.org/10.1021/acs.langmuir.9b00835
Chalkidis A, Jampaiah D, Hartley PG, et al., 2019b. Regenerable α-MnO2 nanotubes for elemental mercury removal from natural gas. Fuel Processing Technology, 193:317-327. https://doi.org/10.1016/j.fuproc.2019.05.034
Chen JH, Cao FF, Chen SZ, et al., 2014. Adsorption kinetics of NO on ordered mesoporous carbon (OMC) and cerium-containing OMC (Ce-OMC). Applied Surface Science, 317:26-34. https://doi.org/10.1016/j.apsusc.2014.08.067
Chen JH, Cao FF, Qu RY, et al., 2015. Bimetallic cerium–copper nanoparticles embedded in ordered mesoporous carbons as effective catalysts for the selective catalytic reduction of NO with NH3. Journal of Colloid and Interface Science, 456:66-75. https://doi.org/10.1016/j.jcis.2015.06.001
Fulazzaky MA, Khamidun MH, Omar R, 2013. Understanding of mass transfer resistance for the adsorption of solute onto porous material from the modified mass transfer factor models. Chemical Engineering Journal, 228:1023-1029
Gao X, Jiang Y, Fu YC, et al., 2010. Preparation and characterization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3. Catalysis Communications, 11(5):465-469. https://doi.org/10.1016/j.catcom.2009.11.024
Jia JB, Zhang PY, Chen L, 2016. The effect of morphology of α-MnO2 on catalytic decomposition of gaseous ozone. Catalysis Science & Technology, 6(15):5841-5847. https://doi.org/10.1039/C6CY00301J
Liu CX, Chen L, Chang HZ, et al., 2013. Characterization of CeO2-WO3 catalysts prepared by different methods for selective catalytic reduction of NOx with NH3. Catalysis Communications, 40:145-148. https://doi.org/10.1016/j.catcom.2013.06.017
Liu DJ, Zhang Z, Luo F, et al., 2020a. Elemental mercury capture from simulated flue gas by graphite-phase carbon nitride. Energy & Fuels, 34(6):6851-6861. https://doi.org/10.1021/acs.energyfuels.0c00457
Liu DJ, Li CE, Wu J, et al., 2020b. Novel carbon-based sorbents for elemental mercury removal from gas streams: a review. Chemical Engineering Journal, 391:123514. https://doi.org/10.1016/j.cej.2019.123514
Liu H, Chang L, Liu WJ, et al., 2020. Advances in mercury removal from coal-fired flue gas by mineral adsorbents. Chemical Engineering Journal, 379:122263. https://doi.org/10.1016/j.cej.2019.122263
Liu SL, Ji J, Yu Y, et al., 2018. Facile synthesis of amorphous mesoporous manganese oxides for efficient catalytic decomposition of ozone. Catalysis Science & Technology, 8(16):4264-4273. https://doi.org/10.1039/C8CY01111G
MEP (Ministry of Environmental Protection of the People’s Republic of China), 2011. Emission Standard of Air Pollutants for Thermal Power Plants, GB13223-2011. National Standards of the People’s Republic of China (in Chinese).
Qu RY, Gao X, Cen KF, et al., 2013. Relationship between structure and performance of a novel cerium-niobium binary oxide catalyst for selective catalytic reduction of NO with NH3. Applied Catalysis B: Environmental, 142-143:290-297. https://doi.org/10.1016/j.apcatb.2013.05.035
Ramesh K, Chen LW, Chen FX, et al., 2008. Re-investigating the CO oxidation mechanism over unsupported MnO, Mn2O3 and MnO2 catalysts. Catalysis Today, 131(1-4):477-482. https://doi.org/10.1016/j.cattod.2007.10.061
Shi YJ, Deng S, Wang HM, et al., 2016. Fe and Co modified vanadium-titanium steel slag as sorbents for elemental mercury adsorption. RSC Advances, 6(19):15999-16009. https://doi.org/10.1039/c5ra26712a
Wang HN, Ma W, Yan JB, et al., 2020. Smart modification of HZSM-5 with manganese species for the removal of mercury. ACS Omega, 5(30):19277-19284. https://doi.org/10.1021/acsomega.0c02877
Wang Z, Liu J, Yang YJ, et al., 2020. Insights into the catalytic behavior of LaMnO3 perovskite for Hg0 oxidation by HCl. Journal of Hazardous Materials, 383:121156. https://doi.org/10.1016/j.jhazmat.2019.121156
Xu HM, Xie JK, Ma YP, et al., 2015. The cooperation of Fe-Sn in a MnOx complex sorbent used for capturing elemental mercury. Fuel, 140:803-809. https://doi.org/10.1016/j.fuel.2014.10.004
Yang LT, Wu J, Li B, et al., 2021. Defective molybdenum disulfide nanosheet for elemental mercury capture in simulated flue gas. Journal of the Energy Institute, 94:120-128. https://doi.org/10.1016/j.joei.2020.11.007
Yang R, Mei CL, Wu XS, et al., 2019. Mn-Cu binary metal oxides with molecular-scale homogeneity for Hg0 removal from coal-fired flue gas. Industrial & Engineering Chemistry Research, 58(41):19292-19301. https://doi.org/10.1021/acs.iecr.9b04005
Yang SJ, Guo YF, Yan NQ, et al., 2011a. Capture of gaseous elemental mercury from flue gas using a magnetic and sulfur poisoning resistant sorbent Mn/γ-Fe2O3 at lower temperatures. Journal of Hazardous Materials, 186(1):508-515. https://doi.org/10.1016/j.jhazmat.2010.11.034
Yang SJ, Guo YF, Yan NQ, et al., 2011b. Elemental mercury cap ture from flue gas by magnetic Mn-Fe spinel: effect of che mical heterogeneity. Industrial & Engineering Chemistry Research, 50(16):9650-9656. https://doi.org/10.1021/ie2009873
Yang SJ, Guo YF, Yan NQ, et al., 2011c. Nanosized cation-deficient Fe-Ti spinel: a novel magnetic sorbent for elemental mercury capture from flue gas. ACS Applied Materials & Interfaces, 3(2):209-217. https://doi.org/10.1021/am100835c
Yang Y, Zhang SZ, Wang SW, et al., 2015. Ball milling synthesized MnOx as highly active catalyst for gaseous POPs removal: significance of mechanochemically induced oxygen vacancies. Environmental Science & Technology, 49(7):4473-4480.
Ye D, Wang XX, Wang RX, et al., 2021. Recent advances in MnO2-based adsorbents for mercury removal from coal-fired flue gas. Journal of Environmental Chemical Engineering, 9(5):105993. https://doi.org/10.1016/j.jece.2021.105993
Ye D, Wang RX, Wang XX, et al., 2022a. Effects of synthesis methods on the physicochemical properties and Hg0 capture capability of MnO2-CeO2 mixed oxides. Applied Surface Science, 578:151998. https://doi.org/10.1016/j.apsusc.2021.151998
Ye D, Wang RX, Wang XX, et al., 2022b. Improvement in the Hg0 removal performance of CeO2 by modifying with CuO. Applied Surface Science, 579:152200. https://doi.org/10.1016/j.apsusc.2021.152200
Ye D, Wang XX, Wang RX, et al., 2022c. Relationship between Hg0 capture performance and physicochemical properties of CeO2-CrOx mixed oxides. Journal of Environmental Chemical Engineering, 10(5):108252. https://doi.org/10.1016/j.jece.2022.108252
Ye D, Wang XX, Wang RX, et al., 2022d. Review of elemental mercury (Hg0) removal by CuO-based materials. Journal of Zhejiang University-SCIENCE A (Applied Physics & Engineering), 23(7):505-526. https://doi.org/10.1631/jzus.A2100627
Zhao HT, Ezeh CI, Yin SF, et al., 2020. MoO3-adjusted δ-MnO2 nanosheet for catalytic oxidation of Hg0 to Hg2+. Applied Catalysis B: Environmental, 263:117829. https://doi.org/10.1016/j.apcatb.2019.117829
July 14, 2022; Revision accepted Aug. 18, 2022; Crosschecked Dec. 6, 2022
https://doi.org/10.1631/jzus.A2200388
Haining WANG, whnfyy@163.com
Haining WANG, https://orcid.org/0000-0003-4653-0819
© Zhejiang University Press 2023
杂志排行
Journal of Zhejiang University-Science A(Applied Physics & Engineering)的其它文章
- Multiscale multiphysics modeling in geotechnical engineering
- CFD-DEM modelling of suffusion in multi-layer soils with different fines contents and impermeable zones
- Effect of hydraulic fracture deformation hysteresis on CO2 huff-n-puff performance in shale gas reservoirs
- Finite volume method-based numerical simulation method for hydraulic fracture initiation in rock around a perforation
- Numerical investigations of the failure mechanism evolution of rock-like disc specimens containing unfilled or filled flaws
- Modelling and applications of dissolution of rocks in geoengineering
