Copper(II)ions-immobilized virus-like hollow covalent organic frameworks for highly efficient capture and sensitive analysis of amyloid beta-peptide 1–42 by MALDI-MS
2023-01-30WendeChoZhongJunLinZhulingChenGuorongLiWeiTongYijingWuLnZhngZinLin
Wende M ,Cho Zhong ,Jun Lin,Zhuling Chen,Guorong Li ,Wei Tong ,Yijing Wu,Ln Zhng ,Zin Lin,*
a Ministry of Education Key Laboratory of Analytical Science for Food Safety and Biology,Fujian Provincial Key Laboratory of Analysis and Detection Technology for Food Safety,College of Chemistry,Fuzhou University,Fuzhou 350108,China
b Department of Cardiology,Fujian Provincial Governmental Hospital,Fuzhou 350003,China
Keywords:Virus-like hollow covalent organic frameworks Metal ion immobilization Amyloidβ-peptide 1–42 Capture Matrix-assisted laser desorption/ionization mass spectrometry
ABSTRACT Amyloid beta-peptide 1–42(Aβ1–42)is one of the biomarkers of Alzheimer’s disease,and its selective capture and quantitative detection are important for diagnosis and treatment of Alzheimer’s disease.Herein,copper(II)ions-immobilized virus-like hollow covalent organic frameworks(V-HCOFs@Cu2+)were synthesized by a facile approach.The as-prepared V-HCOFs@Cu2+showed unique morphology,ultrahigh specific surface(2552 m2/g),uniform mesoporous structure(3.2 nm),superior chemical stability and abundant binding sites.Based on these excellent properties,the V-HCOFs@Cu2+could be adopted as an ideal enrichment probe for highly efficient capture of Aβ1–42,exhibiting high adsorption capacity(320 mg/g),and fast adsorption equilibration time(3 min).In addition,an attractive approach of the V-HCOFs@Cu2+-based matrix-assisted laser desorption/ionization mass spectrometry(MALDI-MS)was developed for the rapid screening and quantitative analysis of Aβ1–42 in human serum by using C-peptide as an internal standard,which exhibited low limit of detection(LOD,0.2 fmol/μL),and satisfactory recovery.This work provides an alternative solution for enrichment of biomarkers and also offers the potential applications of COFs in clinical analysis
Alzheimer’s disease(AD)is a classic neurodegenerative disease characterized by memory loss,cognitive decline,and behavioral disturbances.The incidence of AD tends to increase logarithmically with age,which had become an intractable social health problem with an aging population[1,2].Substantial investigations have confirmed that the abnormal aggregation of extracellular amyloid beta-peptides is a major cause of AD,and the main component of aggregates is the Aβ1–42[3].In view of the close relationship between AD and Aβ1–42,Aβ1–42 is widely recognized as an important biomarker for AD research[4].Human serum appears to be more attractive for AD disease diagnosis and prognosis because body fluid assays offer several key advantages,including low invasiveness,minimal cost,and ease of sample collection and processing[5,6].Therefore,the quantitative detection of Aβ1–42 from human serum has an important significance for the early diagnosis and treatment of AD.
To date,several analytical methods have been successfully introduced for Aβ1–42 from human serum involving enzyme-linked immunosorbent assay(ELISA)[7],fluorescence resonance energy transfer(FRET)[8],colorimetric method[9],fluorescence sensor[10],square wave voltammetry(SWV)[11,12]and electrochemical impedance spectroscopy(EIS)[13].Although high selectivity and excellent sensitivity were obtained using ELISA and FRET methods,they require expensive reagents and time consuming(>24 h).The limitation of the colorimetric method and fluorescence sensor is poor sensitivity and reproducibility.The SWV and EIS methods suffered from complex synthesis of biosensor and high nonspecific binding.These drawbacks strongly prevented their applications for rapidly screening of Aβ1–42 in the fluid samples for AD patients.MALDI-MS is a type of soft ionization technique that allows the formation of analyte ions with minimal fragmentation and has the advantages of qualitative accuracy,high sensitivity,fast speed(∼seconds),and high throughput[14–16].Currently,it has been one of the most powerful tools for screening disease markers from biological samples.Since Aβ1–42 exists at ultra-trace levels in human body fluids and coexists with a large number of interfering proteins and other interference substances,direct analysis of the Aβ1–42 remains a great challenge.Therefore,selective enrichment of Aβ1–42 before MS analysis becomes highly necessary for indepth pathological research of AD.
Covalent organic frameworks(COFs),an attractive new type of crystalline porous organic polymer bonded by strong covalent bonds with different types of organic building units[17–19].Compared to conventional polymers,COFs possess not only offer structural pre-designability,but also many distinct advantages such as large specific surface area,highly tunable pore size,excellent chemical stability and easily tailored functionality[20–23].These fascinating features endow COFs with unique properties and great potential for biological analysis,including nucleic acid analysis,peptidomics analysis,and endogenous substance analysis[24,25].Despite some successes have been made,the variety of functional COFs for biological analysis is still scarce.Therefore,it is necessary to develop more functional COFs for biological analysis.Recently,our groups proposed a facile method for controllable synthesis of flower-like hollow COFs[26],which possessed ultrahigh surface area(>2800 m2/g),and superior chemical stability.Based on the above advantages,the hierarchical flower-like hollow COFs were successfully applied as an attractive adsorption probe and be applied to the highly efficient enrichment of ultratrace endogenous brain natriuretic peptide from human serum.Despite this attractive feature of this type of functional COFs,there have been few studies to date on the use of this type of functional COFs for the separation and enrichment of biological samples.Further development is very necessary to play the advantages and explore new applications of this type of functional COFs.
Herein,a virus-like hollow covalent organic frameworks(VHCOFs)with new functionality was successfully synthesized by using 1,3,5-tri(4-aminophenyl)benzene(TPB)as the node and 2,5-dimethoxyterephthalaldehyde(DMTP)as the linker for the condensation reaction.The resultant V-HCOFs showed unique morphology,high crystallinity,extremely high surface area,abundant active sites,and excellent chemical stability.Taking advantage of the V-HCOFs mentioned above,a fascinating enrichment probe was designed by immobilizing copper(II)ions on V-HCOFs(defined as V-HCOFs@Cu2+)and employed for highly efficient and selective capture of Aβ1–42.In addition,an attractive approach of the V-HCOFs@Cu2+-based MALDI-MS was developed for the rapid screening of Aβ1–42 from human serum.
The preparation procedure of the V-HCOFs and V-HCOFs@Cu2+was shown in Fig.1a.First,the monomers of TPB(14.0 mg)and DMTP(11.6 mg)were selected for the construction of porous frameworks in order to obtain a large number of nitrogen and oxygen elements complexed with copper ions,due to the copper ions have strong interactions with a large number of amino and carboxyl groups of Aβ1–42,which has been reported extensively recent years[27–29].Then the monomers were dissolved in 5 mL ACN containing 8 vol%of 12 mol/L acetic acid.After being placed at room temperature for 72 h,the product(V-HCOFs)was obtained.Finally,the product was vigorously stirred in a solution of copper chloride for 24 h at room temperature,and the V-HCOF@Cu2+could be obtained.
The morphology of V-HCOFs and V-HCOFs@Cu2+was confirmed by transmission electron microscope(TEM)and scanning electron microscopy(SEM).As shown in Figs.1b and c,the COFs displayed virus-like hollower structures consisting of nanorods with vertical spherical centers and a diameter size of about 550 nm.Fourier transform infrared(FT-IR)spectra and solid-state13C NMR spectra analysis demonstrated the existence of the–C=N bonds of VHCOFs(Fig.1d and Fig.S1 in Supporting information),implying the successful condensation reaction between TPB and DMTP.The FTIR and13C NMR spectrum of V-HCOFs@Cu2+(Fig.1d and Fig.S1)was almost identical to that of V-HCOFs in the phenyl areas,indicating the structural preservation of V-HCOFs after the modification of Cu2+.Thermogravimetric(TG)analysis revealed that both V-HCOFs and V-HCOFs@Cu2+were stable up to about 400°C under Ar2atmosphere(Fig.S2 in Supporting information).Powder X-ray diffraction(PXRD)analysis was used to determine the crystalline structure of V-HCOFs and V-HCOFs@Cu2+,the most intense diffraction peak of 2.70°could be observed,accompanied by weak diffraction peaks of 4.78°,5.48°,7.32°,9.72°,and 25.2°(Fig.1e),indicating the high crystallinity of V-HCOFs and V-HCOFs@Cu2+,and the structure of the as-synthesized COFs matches with the simulated XRD spectra calculated using the AA stacking model(Figs.S3a and b in Supporting information).In addition,similar PXRD patterns of V-HCOFs and V-HCOFs@Cu2+revealed that the crystal structure of V-HCOFs was well preserved after the treatment with copper chloride.The nitrogen adsorption isotherm further showed the porosity of the materials with Brunauer-Emmett-Teller(BET)surface areas of 2970 m2/g for V-HCOFs and 2552 m2/g for V-HCOFs@Cu2+(Fig.1f).The BET surface areas of V-HCOFs@Cu2+lower than those of V-HCOFs may be due to the fact that the modified Cu2+increased the mass of V-HCOFs.Moreover,the pore size distribution calculated by nonlocal density flooding theory(NLDFT)indicated that V-HCOFs and V-HCOFs@Cu2+had the same mesoporous of 3.2 nm and their pore sizes shown in HRTEM images were in good agreement with the results of NLDFT analysis(Figs.S4 and S5 in Supporting information),indicating that the channels were not blocked during the incorporation of copper ions.The chemical stability of V-HCOFs@Cu2+was further confirmed and the result was shown in Fig.S6(Supporting information).After V-HCOFs@Cu2+powders were immersed into 0.5 mol/L NaOH or 0.5 mol/L HCl for 72 h,the constant position and strength of characteristic peaks in PXRD confirmed that V-HCOFs@Cu2+had good chemical stability.
In order to deeply understand the evolution process of the virus-like hollow structure,the time-dependent studies were performed and the morphologies of structure transformation at the specific times were analyzed using SEM and TEM imaging.As shown in Fig.2a,the isolated precipitates at 1 min revealed the formation of small spherical particles having diameter of approximately 150 nm.After a reaction time of 6 h,these small spherical particles had aggregation to produce spheres with a diameter of about 550 nm(Fig.2b).As the reaction time progressed to 36 h,the spheres with symmetrical core-shell structure were observed(Fig.2c).After 72 h reaction,the spheres with virus-like hollow structure was obtained.During the whole transformation process,the inside-out Ostwald ripening was prominent since the center of the crystal with high surface energy was unstable[30,31],which was easily dissolved and eventually regrown epitaxial on the shell into virus-like hollow structures(Fig.2d).
The distribution of Cu2+on V-HCOFs was investigated.As shown in Figs.3a and b,the energy dispersive spectrometer(EDS)element mapping showed that the element Cu was uniformly distributed throughout the powder with a mass percentage of∼5.89%after V-HCOFs was immersed in copper chloride solution for 24 h(Fig.S7 and Table S1 in Supporting information).To further determine the interaction between Cu2+and V-HCOFs,X-ray photoelectron spectroscopy(XPS)analysis was conducted on V-HCOFs and V-HCOFs@Cu2+.As shown in the XPS full scan-survey spectra(Fig.3c and Fig.S8 in Supporting information),the discovery of Cu(934.6–954.6 eV)signals further confirmed the successful immobilization of Cu2+on V-HCOFs.From the high-resolution XPS spectra of Cu 2p,the characteristic peaks of Cu 2p at 934.6 eV and 954.6 eV were for Cu 2p3/2and Cu 2p1/2,respectively.The satellite peak at 943.5 eV was related to the interactions between Cu2+and functional groups(–N=C,–C–O)(Fig.3d).Furthermore,the N 1s XPS spectrum showed in Fig.3e revealed that the–C=N band shifted from 398.8 eV to 400.0 eV,which should be ascribed to the coordination interaction between N and Cu2+[32].The above results indicated that the copper ion was loaded on the V-HCOFs by chemical rather than physical interactions.
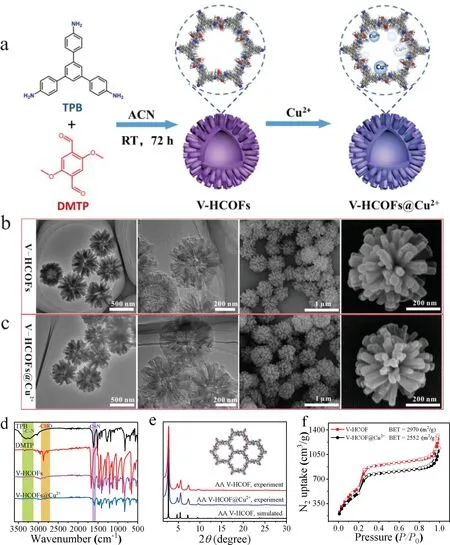
Fig.1.(a)Synthetic procedure for V-HCOFs and V-HCOFs@Cu2+.(b,c)TEM and SEM images of V-HCOFs and V-HCOFs@Cu2+.(d)FTIR spectra of V-HCOFs and V-HCOFs@Cu2+.(e)PXRD patterns of V-HCOFs and V-HCOFs@Cu2+.(f)77 K N2 adsorption-desorption isotherms of V-HCOFs and V-HCOFs@Cu2+.
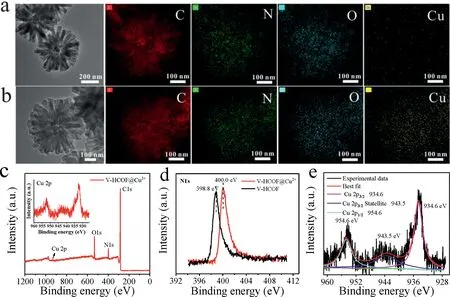
Fig.3.(a,b)EDS mapping of V-HCOFs and V-HCOFs@Cu2+.(c)XPS spectrum of V-HCOFs@Cu2+.(d)N 1s XPS spectra of V-HCOFs and V-HCOFs@Cu2+.(e)Cu 2p XPS spectrum of V-HCOFs@Cu2+.
To investigate the feasibility of V-HCOFs@Cu2+as an adsorbent for Aβ1–42 enrichment,the adsorption capability of VHCOFs@Cu2+for Aβ1–42 was investigated by using the standard Aβ1–42.As presented in Fig.4a,the adsorption capacity of Aβ1–42 absorbed in the V-HCOFs@Cu2+was measured to be 320 mg/g,which was much higher than that of V-HCOFs.The results indicated the V-HCOFs@Cu2+possessed superior adsorption ability for Aβ1–42,which attributed to the ultra-high surface area of VHCOFs@Cu2+and the high affinity of Cu2+toward Aβ1–42.Besides,Fig.4b exhibited the adsorption kinetics of the Aβ1–42 on the V-HCOFs@Cu2+,and the result showed that 90%of the maximum adsorption amounts were reached when the enrichment time was up to 1 min,and the adsorption equilibrium could be reached within 3 min.This was mainly attributed to the unique morphology of V-HCOFs@Cu2+that facilitated the rapid diffusion of Aβ1–42,which was very beneficial for the rapid separation and enrichment of Aβ1–42 from complex biological samples.To further confirm the advantage of virus-like hollow structure of COFs for Aβ1–42 enrichment,the spherical COFs with the same building units were also synthesized according to the previous strategy[25]and further adopted for comparison(Figs.S9a and b in Supporting information).Although the spherical COFs modified Cu2+(SCOF@Cu2+)also possessed good crystallinity(Fig.S9c in Supporting information)and high specific surface area(1240 m2/g,Fig.S9d in Supporting information),the adsorption capacity and kinetics of the SCOF@Cu2+toward Aβ1–42 were 161 mg/g with an equilibration time of∼10 min,which was inferior to that obtained with the V-HCOFs@Cu2+(Figs.S9e and f in Supporting information).The good result was mostly ascribed to the fact that V-HCOFs@Cu2+had higher surface area(2552vs.1240 m2/g)and the unique viruslike hollow structure.The large surface area exhibited much better adsorption capability,and the unique virus-like hollow structure made the diffusion path of the target in the pore channel of virus-like hollow COFs shorter than that of spherical COFs[33],and the mutual obstruction of the target diffusion in different nanorods was smaller compared to the spherical COFs[34],as a result,it helped to achieve faster adsorption equilibrium.
In order to obtain the optimal conditions of V-HCOFs@Cu2+for Aβ1–42 enrichment,related parameters affecting the enrichment efficiency of V-HCOFs@Cu2+involving the adsorbent amount,the concentration of trifluoroacetic acid(TFA)or ammonia in the eluent,the volume ratio of ACN/water of eluent,eluent volume,and elution time were investigated in detail.In this experiment,1.0 mL standard solution(Aβ1–42,5 fmol/μL)were selected to investigate the performance of V-HCOFs@Cu2+under different experiment conditions and the MS signal intensity of Aβ1–42 were used to evaluate the enrichment performance.As shown in Fig.S10(Supporting information),the optimized performance of VHCOFs@Cu2+on Aβ1–42 enrichment parameters were determined:V-HCOFs@Cu2+of 0.25 mg,TFA content of 0.1%in eluent,eluent of 20μL 80%ACN solution,and desorption time of 10 min,respectively.Besides,Fig.S11(Supporting information)showed that 77%of elution efficiency of the V-HCOFs@Cu2+towards Aβ1–42 was obtained under the above optimal elution conditions.In addition,the reusability of V-HCOFs@Cu2+for Aβ1–42 was examined under the optimum conditions,and the results demonstrated that the extraction efficiency was nearly 30%loss after three extraction cycles(Fig.S12 in Supporting information).The decrease of enrichment performance was mainly due to the loss of Cu2+ions caused by the relative strong acid eluent.However,the enrichment performance could be restored if V-HCOFs@Cu2+were reincubated in copper chloride solution before the next cycle.It should be noted that the V-HCOFs@Cu2+morphology remained unchanged after reuse(Fig.S13 in Supporting information),indicating the good stability of the V-HCOFs@Cu2+.
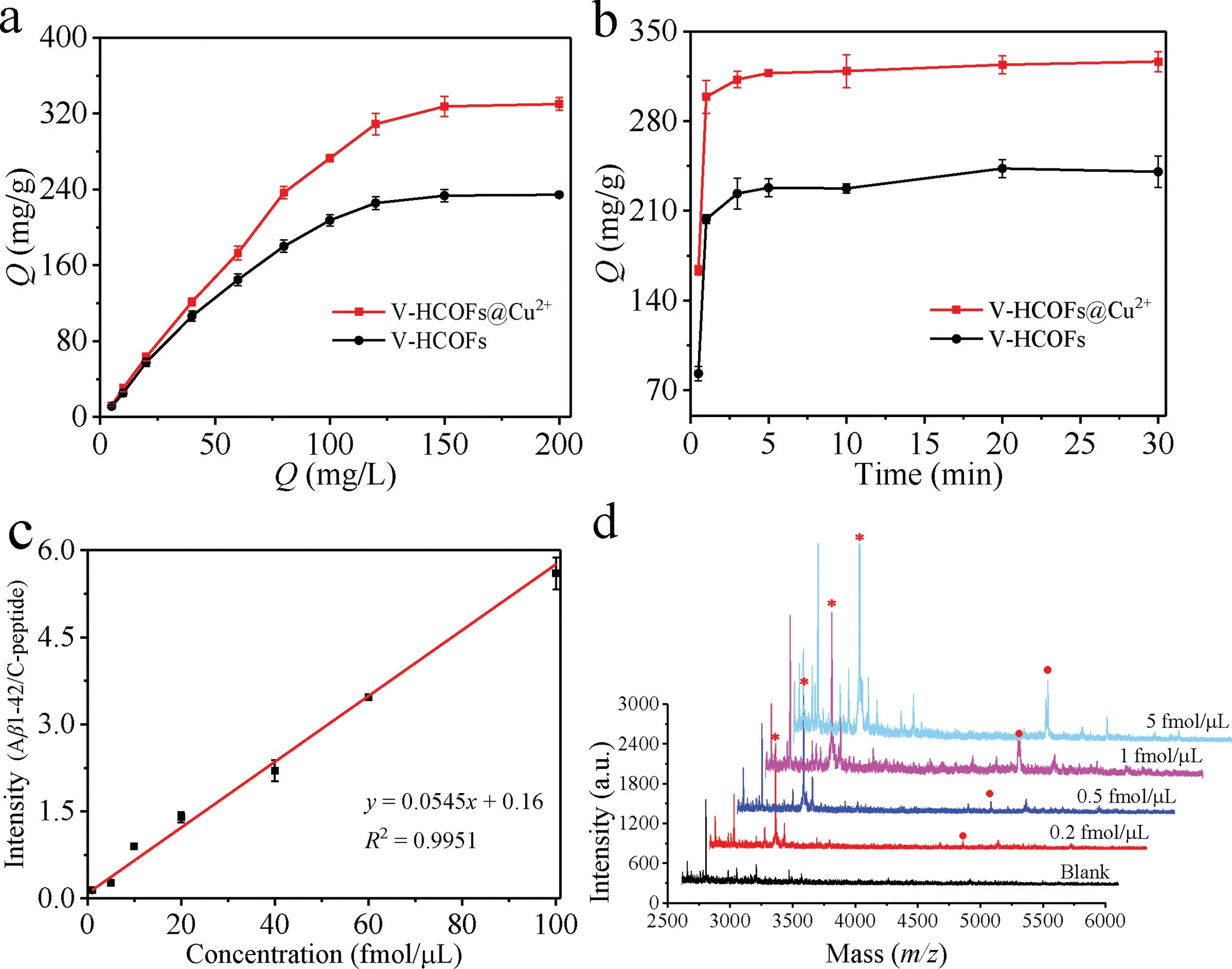
Fig.4.(a)Adsorption isotherms of the Aβ1–42 on the V-HCOFs and V-HCOFs@Cu2+.(b)Adsorption kinetics of the Aβ1–42 on the V-HCOFs and V-HCOFs@Cu2+.(c)The calibration plots of Aβ1–42 established by MALDI-MS(C-peptide as an internal standard for quantitative analysis).(d)MALDI-MS of Aβ1–42([M+H]+,m/z=4515.5)after enrichment by V-HCOFs@Cu2+with the concentration of 5 fmol/μL,1 fmol/μL,0.5 fmol/μL and 0.2 fmol/μL(in 20-fold diluted human serum).Mass spectrometric peaks of Aβ1–42 are marked with red circles and the internal standard C-peptide are marked with red stars.
The quantitative analysis of Aβ1–42 was performed by MALDIMS using internal standard method,and the C-peptide was selected as internal standard based on its similarity in quality and physicochemical properties to Aβ1–42.The validation of method was subsequently studied under the optimum conditions as described above.The dynamic linear range and limits of detection(LODs)of the method were studied.A range of Aβ1–42 solutions with different concentrations were added to 20-fold diluted human serum and then captured with V-HCOFs@Cu2+.Subsequently,200 fmol/μL of C-peptide was added to the eluent as an internal standard before MALDI-MS analysis.The calibration graph was plotted by the ratio of the peak intensity(Aβ1–42/C-peptide)versusthe concentration of Aβ1–42(Fig.4c).The developed method exhibited good linearity in the concentration ranges of 1.0–100 fmol/μL with the correlation coefficients higher than 0.9951.Besides,the LODs for Aβ1–42 were calculated to be 0.2 fmol/μL(Fig.4d),which was much lower than those obtained without treatment(Fig.S14 in Supporting information).Compared with the previously reported analysis methods,as shown in Table S2(Supporting information),the results exhibited that the developed method was fast,simple,highly sensitive and efficient to Aβ1–42.
In order to examine the practicability of the developed method for real sample analysis,20-fold diluted human serum samples spiked with different amounts of Aβ1–42 were determined by using V-HCOFs@Cu2+.Fig.S15(Supporting information)described the mass spectra of the samples with various concentrations from 1.0 fmol/μL to 40 fmol/μL after enrichment with V-HCOFs@Cu2+;the peaks of Aβ1–42 could be clearly identified.In addition,as shown in Table S3(Supporting information),satisfactory recoveries of the three spiked serum samples were achieved.
In summary,the Cu2+-immobilized virus-like hollow covalent organic frameworks(V-HCOFs@Cu2+)were preparedviaa simple strategy.These as-prepared V-HCOFs@Cu2+probes showed great potential in the analysis of the Aβ1–42 in human serum,because of the unique morphological structure,large surface area,uniform mesoporous pores,high copper ions loading,and excellent chemical stability.Furthermore,the combination with MALDI-MS exhibited high sensitivity,good linearity range,and satisfying high recovery.This study not only provides a potential selective capture platform for in-depth mapping of Aβ1–42,but also offers promising guidance for development of novel enrichment probes combined with MALDI-MS for the detection of other biomarkers in biological fluids.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This project was supported by a grant from the National Natural Science Foundation of China(Nos.21974021,22036001,and 91843301).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.01.047.
杂志排行
Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
