Boron-promoted reductive deoxygenation coupling reaction of sulfonyl chlorides for the C(sp3)-S bond construction
2023-01-30ShuoChenQingruWenYnqingZhuYnruJiYuPuZhengliLiuYunHeZhngFeng
Shuo Chen,Qingru Wen,Ynqing Zhu,Ynru Ji ,Yu Pu,*,Zhengli Liu,Yun He ,Zhng Feng ,,*
a Chongqing Key Laboratory of Natural Product Synthesis and Drug Research,School of Pharmaceutical Sciences,Chongqing University,Chongqing 401331,China
b Medical Imaging Key Laboratory of Sichuan Province,North Sichuan Medical College,Nanchong 6370 00,China
Keywords:C-S bond formation Reductive deoxygenation coupling Borane Organosulfur compounds Green chemistry
ABSTRACT Herein,we report a borane-promoted reductive deoxygenation coupling reaction to synthesize sulfides.This reaction features excellent functional group compatibility,high efficiency,broad substrate scope,and application in late-stage functionalization of biomolecules.Preliminary mechanistic studies suggest diaryl sulfides are the intermediates of this reaction.Moreover,the real active aryl sulfide anions may be generated in situ with the aid of B2pin2 and react with alkyl tosylates through a concerted SN2 pathway.
Organosulfur compounds,the indispensable chemical substances in our lives,are widely present in many biologically active molecules,materials science,medicine,and agriculture[1–3].Therefore,the carbon-sulfur bonds formation has attracted much attention in organic synthesis[4–8].Undoubtedly,the most classic and important synthetic method for building the C(sp3)-S bond is the substitution reaction of alkyl halides with mercaptans in the presence of strong bases(Scheme 1A)[9,10].However,this strategy suffers from poor compatibility of functional groups and limited substrate scope.Transition metal-catalyzed cross-coupling of aryl halides with alkyl thiols or alkyl halides with aryl thiols has made great progress so far[11],but the undesirableβ-hydride elimination and the metal poisoning problem also restrict its wild applications.Importantly,most mercaptans having unpleasant smells are not commercially available and highly toxic,greatly limiting their applications in organic synthesis.In view of these limitations,much effort has been paid to find new sulfurating agents to replace mercaptans.
In recent years,the use of inorganic sulfur compounds as sulfurating agents has been an alternative strategy for building carbonsulfur bonds(Scheme 1B)[12–15].For example,a copper-catalyzed coupling reaction of aryl iodides and alkyl halides with sulfur powder was reported by Ma[16].In 2018,the Jiang group reported the palladium-catalyzed C-S bond formation using KSAc as a sulfur source[17].Zhou and coworkers also realized a similar transformation by employing KSCN[18].Na2S2O3as a sulfurating agent for the construction of carbon-sulfur bonds has also been achieved by Jiang[19].Another practical protocol is to convert alkyl thiols into electrophiles for the carbon-sulfur bonds formation.For instance,organometallic reagents can react with benzenesulfonothioates to deliver the sulfides through nucleophilic substitution reactions[20].Nevertheless,most organometallic reagents are sensitive to air and moisture,which restricts this method’s application(Scheme 1C).In 2018,a mild and efficient nickel-catalyzed reductive thiolation reaction employing S-phenyl benzenesulfonothioate as the sulfurating electrophiles was reported by Ji and Wang(Scheme 1D)[21].At present,although metal-free and transition metal-catalyzed methods as mentioned above for the C(sp3)-S bond formation have made great developments,it is still very necessary to develop simple,environmentally friendly,safe,and practical methods for the C(sp3)-S bond formation.
Very recently, Radosevich and coworkers found that organophosphines could promote the deoxygenation of sulfonyl chlorides to afford the sulfenyl electrophiles[22].Inspired by Radosevich’s work as well as the role of borane reagents in reductive cross-coupling reactions[23–25],we envisioned that the sulfurating electrophiles would be generatedin situfrom aryl sulfonyl chlorides[26–30]and then underwent the reductive cross-coupling reaction with alkyl halides with the aid of the reducing agent boranes(such as B2pin2)(Scheme 1E).To our continuing interest in green chemical synthesis[31–38],we sought to develop the first example of borane-promoted reductive deoxygenation coupling reaction of aryl sulfonyl chlorides with alkyl halides and alkyl tosylates for the construction of C(sp3)-S bond.
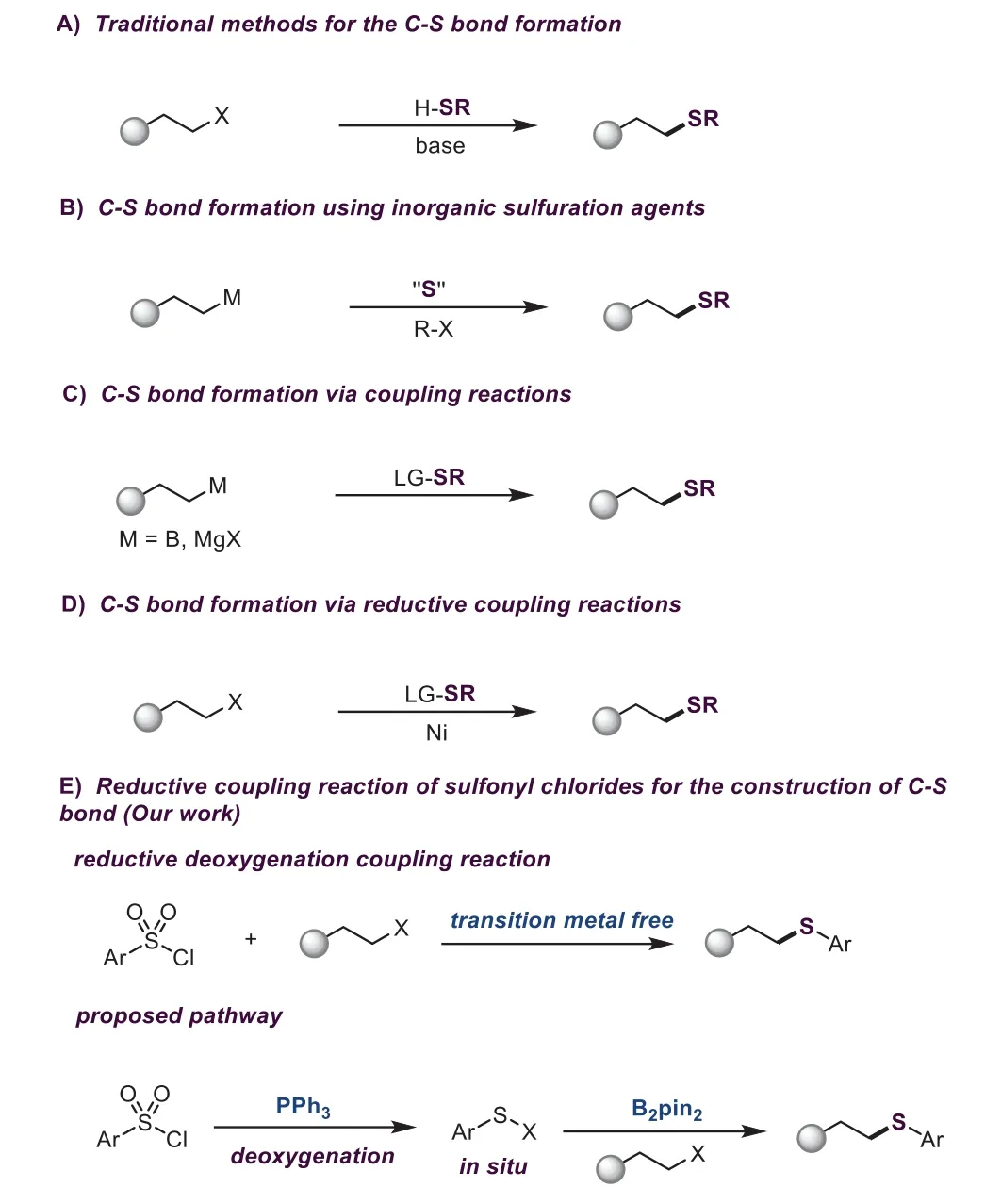
Scheme 1.Methods for the C(sp3)-S bond formation.
With these considerations in mind,we set out to investigate the reductive deoxygenation coupling reaction of aryl sulfonyl chlorides.To our delight,as we anticipated,when B2pin2and PPh3were used as additives,this transformation could proceed,and afforded the desired product 1 in 6%yield(Table 1,entry 1).Then,other bases were evaluated,and the best result,32%yield was provided in the presence of NaOH(Table 1,entry 2;for details,please see Supporting information).We thought that the amount of PPh3might be critical to this reaction.Therefore,the loading of PPh3was checked,and 48%yield was obtained when 2.0 equiv.of PPh3was employed(Table 1,entry 3).Furthermore,increasing the loading of PPh3to 3.0 equiv.,the 86%yield of desired product was delivered(Table 1,entry 4).After screening other solvents,DME afforded this desired product in 98%yield(Table 1,entries 5 and 6;for details,please see Supporting information).Subsequently,various borane reagents were tested as well.B2cat2and B2oct2could also promote this reaction smoothly,resulting in the corresponding product in moderate yields(Table 1,entries 8 and 9;for details,please see Supporting information).Control experiments were conducted to reveal the importance of the borane reagent and PPh3(Table 1,entries 10–12).It was found that the efficiency of this reaction was dramatically affected by the amount of B2pin2(Table 1,entry 10).The desired product 1 was not observed in the absence of the borane reagent and PPh3,thus demonstrating the necessity of the borane reagent and PPh3(Table 1,entries 11 and 12).Gratifyingly,the more commonly used inert substrate,cyclopentyl 4-methylbenzenesulfonate,proved to be a suitable substrate,yield-ing the corresponding product 1 in 96%yield(Table 1,entries 7).Remarkably,this transformation can be carried out in undried DME under air conditions without losing its efficiency.
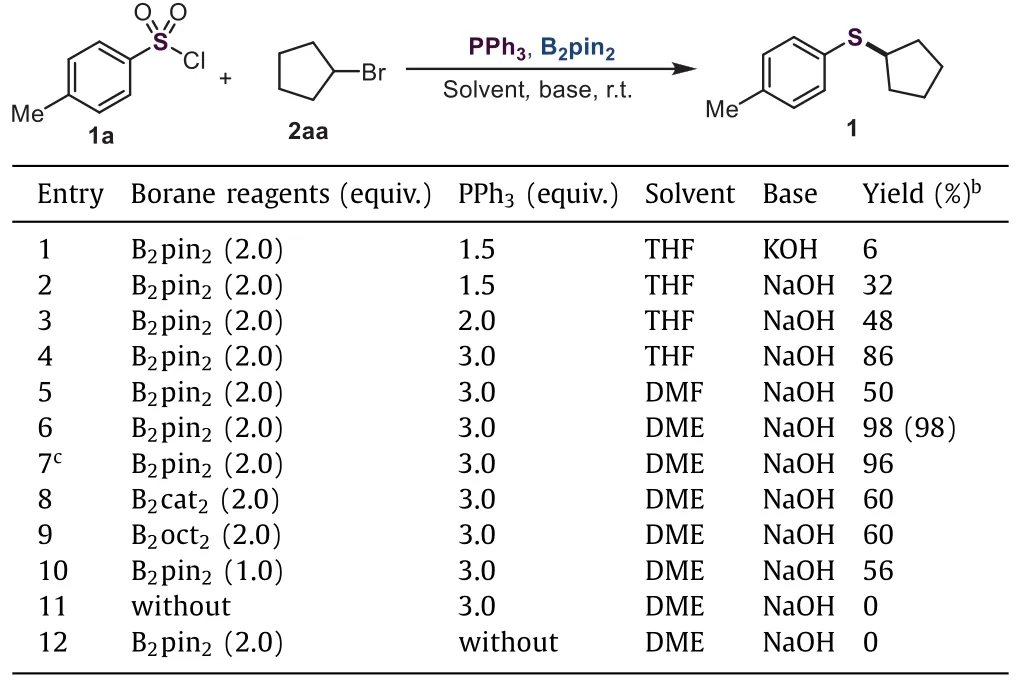
Table 1 Representative results for the optimization of the reaction of aryl sulfonyl chloride 1a with alkyl bromide 2aa.a
After the reaction conditions were established,we tried to examine the scope of this reaction.As shown in Scheme 2,various aryl sulfonyl chlorides underwent this transformation smoothly,delivering the thiolated products in moderate to excellent yields.Notably,this borane-promoted thiolation reaction features excellent functional group tolerance.Functional group such as F,Cl,Br,I,CN,CF3,NO2,OTs,alkenyl,carboxylate,and amine(4–11,23,29,33,34)could be compatible.Notably,iodo and nitro groups that are always sensitive in transition metal-catalyzed reactions,could be well-tolerated in this protocol(7,96%;11,36%).Substrates bearing an electron-withdrawing group(such as CN,CF3)on the aromatic ring performed this reaction well,providing the coupling products in excellent yields(9,96%;10,94%).It was worth mentioning that examples with steric hindrance exhibited good efficiency as well.For instance,aryl sulfonyl chlorides having two groups atortho-position of sulfonyl group were suitable for this transformation,affording the desired products in excellent yields(3,85%;8,95%;18,83%).Moreover,the heteroaromatic sulfonyl chlorides were good coupling partners,delivering the desired products in moderate to good yields(14–18,57%−83%).With respect to another coupling partner,various alkyl tosylates were further evaluated.The primary and secondary alkyl tosylates carried out this reaction well,while tertiary alkyl tosylates were unsuitable for this conversion.This reaction could proceed using alkyl tosylates containing sensitive groups(such as ester and amine)as substrates,and the synthetically useful yields were obtained(33,38%;34,34%).Additionally,to demonstrate the generality of this boranepromoted thiolation reaction,other alkyl electrophiles were also investigated using 4-methylbenzenesulfonyl chloride 1a as the coupling partner.Like alkyl bromides,cyclopentyl iodide showed excellent reactivity,affording the desired product in 98%yield,while cyclopentyl chloride only provided 35%yield.However,tertiary alkyl bromides are not suitable for this transformation.
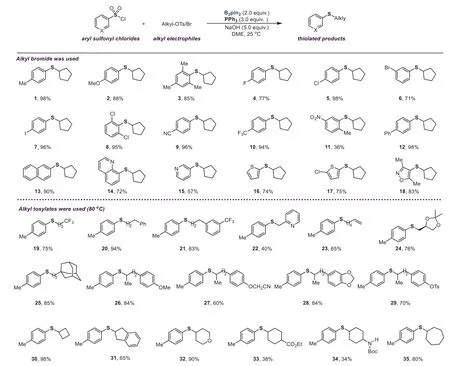
Scheme 2.Scope of the borane-promoted thiolation of(hetero)aryl sulfonyl chlorides.Reaction conditions:aryl sulfonyl chlorides(0.2 mmol,1.0 equiv.),alkyl tosylates(0.3 mmol,1.5 equiv.),B2pin2(0.4 mmol,2.0 equiv.),PPh3(0.6 mmol,3.0 equiv.),NaOH(1.0 mmol,5.0 equiv.),DME(1.0 mL),24 h.
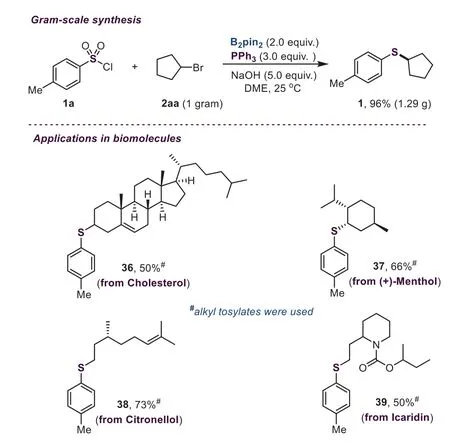
Scheme 3.Gram-scale synthesis and late-stage functionalization of biomolecules.
A gram-scale synthesis was carried out to demonstrate the excellent applicability of this borane-promoted thiolation reaction.As shown in Scheme 3,this transformation proceeded well on a 1-gram scale,and 96%yield was afforded.Furthermore,we also investigated the late-stage thiolation of biomolecules(Scheme 3).The cholesterol derivative underwent this reaction smoothly,providing the desired product in an acceptable yield(36,50%).The substrate with steric hindrance derived from menthol could react well,and a moderate yield was afforded(37,66%).The citronellol derivative bearing an alkenyl group successfully carried out this coupling reaction,delivering the corresponding product in good yield(38,73%).Notably,when the icaridin derivative containing an ester group was used as the substrate,a reasonable yield was provided(39,50%).These results indicate that this boranepromoted thiolation reaction could provide an efficient method to access valuable molecules in medicinal chemistry.
To understand the mechanism of this borane-promoted crosscoupling reaction,some critical experiments were conducted.In the presence of a radical scavenger TEMPO(50 mol%)or a radical inhibitor BHT(100 mol%),this reaction proceeded well without loss of efficiency(Scheme 4A).Moreover,a radical clock experiment was performed as well,and the coupling product 41 could be obtained in excellent yield instead of the ring-opening product(Scheme 4B).The outcomes suggest that free radicals may not be involved in the reaction system.Furthermore,the EPR experiment also supported no free radical in this reaction(for details,see Supporting information).
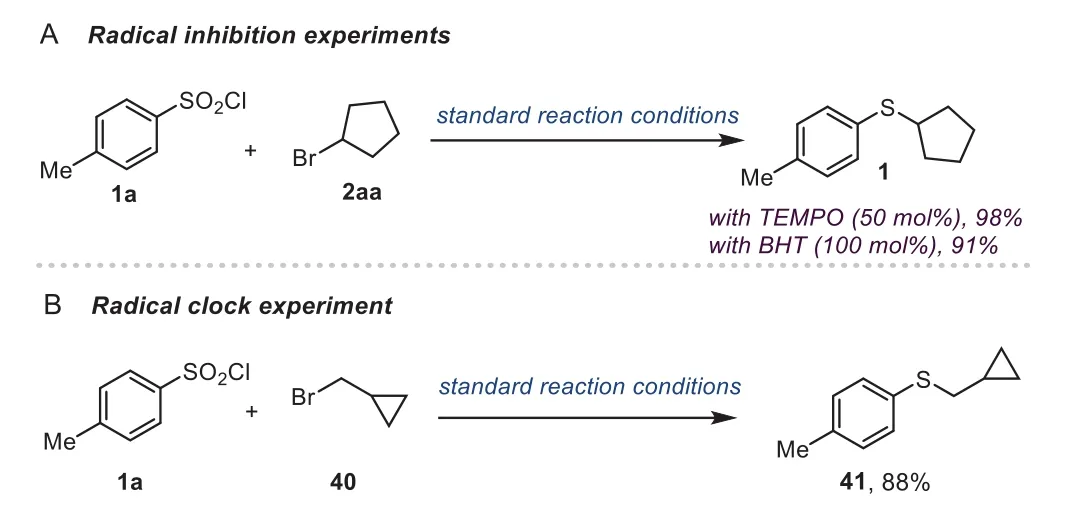
Scheme 4.Mechanistic studies.
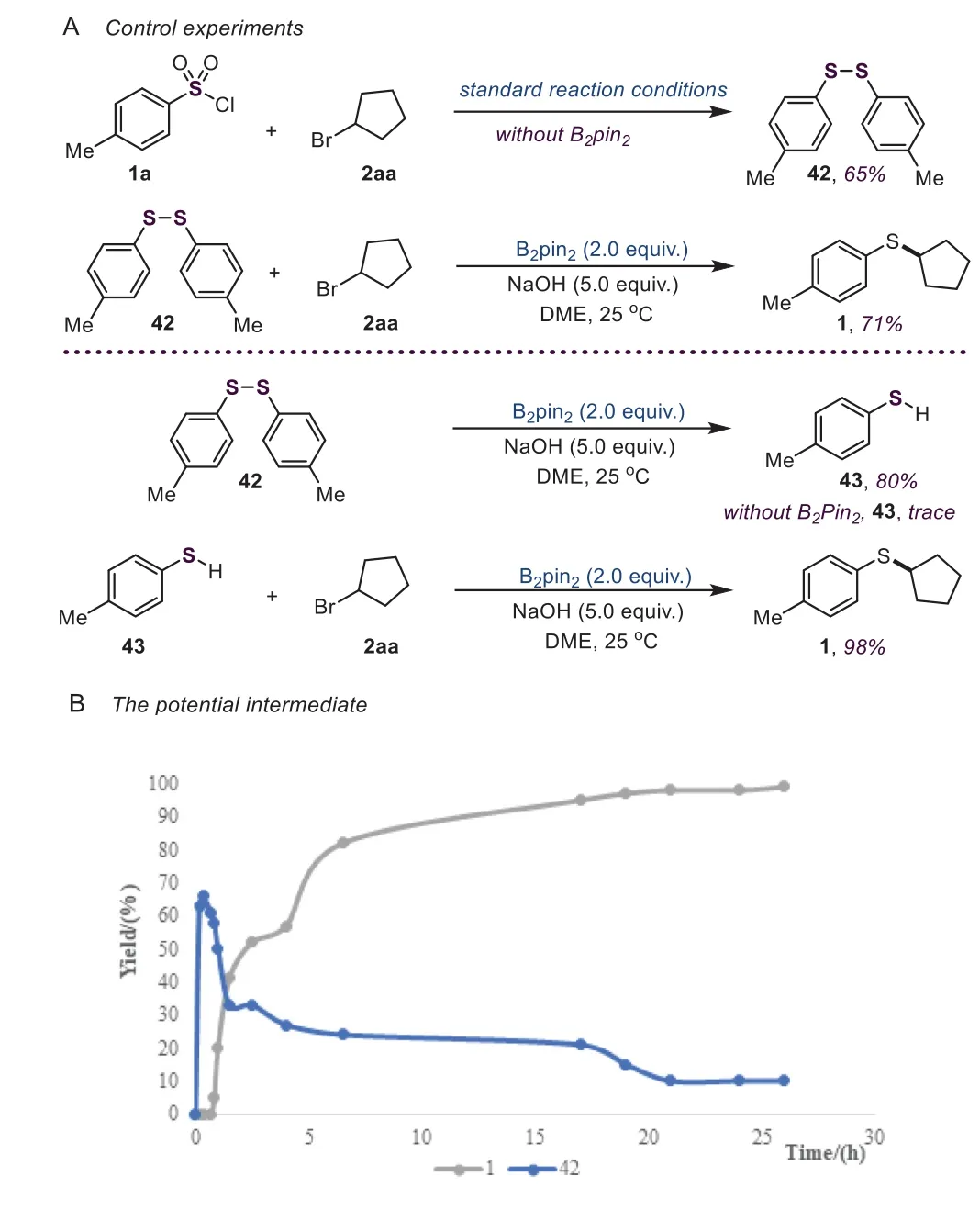
Scheme 5.Investigation of the borane effect(A)and the potential intermediate(B).
In our initial studies,we found that the borane reagent was necessary to this reaction.Therefore,control experiments were carried out to illustrate the role of B2pin2.Without the borane reagent,the diaryl sulfide 42 could be isolated in 65%yield(Scheme 5A);meanwhile,no thiolated product 1 was obtained.Furthermore,when diaryl sulfide 42 was used as the substrate,the coupling product 1 could be afforded in 71%yield.These results reveal that diaryl sulfide 42 might be an intermediate of this reaction.In addition,we also monitored this reaction and found that in the initial stage of this reaction,intermediate 42 was rapidly formed and then converted into the desired product 1(Scheme 5B).Importantly,in the presence of B2pin2,diaryl sulfide 42 could convert into 4-methylbenzenethiol 43,which readily reacted with 2aa to produce the thiolated product 1 in excellent yield.Nevertheless,only a trace amount of 4-methylbenzenethiol 43 was observed in this reaction.Therefore,these results suggest that the aryl sulfide anion might be the real active species generatedin situwith the aid of B2pin2.
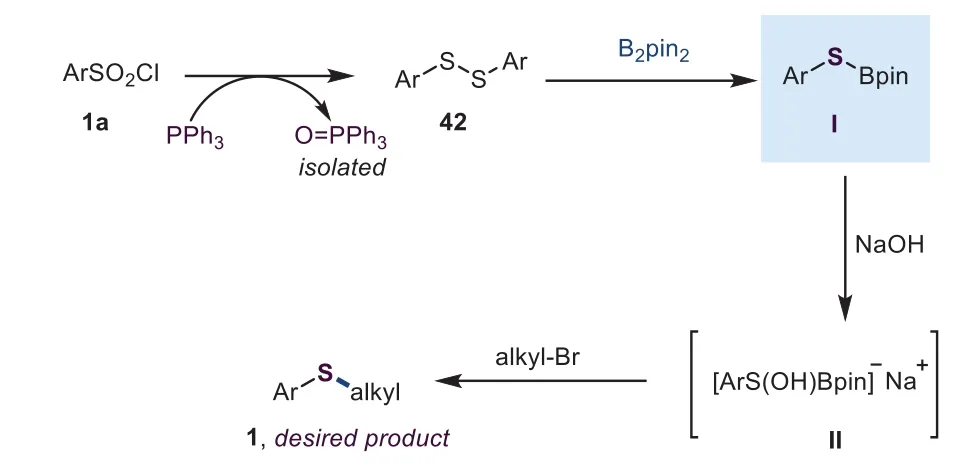
Scheme 6.Proposed mechanism.
According to these preliminary results,the mechanism of this borane-promoted thiolation reaction was proposed,as shown in Scheme 6.Firstly,the aryl sulfonyl chloride was reduced by PPh3to form a diaryl sulfide,which could be observed in this reaction[39,40].Subsequently,the resulted diaryl sulfide might react with B2pin2to offer an intermediate I[41],which then was triggered by NaOH to deliver a boronate complex II.Finally,theinsitu-generated aryl sulfide anion reacted with the alkyl bromide through a SN2 pathway to produce the desired sulfide.
In conclusion,we have developed an efficient borane-promoted reductive deoxygenation coupling reaction to construct the C(sp3)-S bond.This transformation without the use of unpleasant mercaptans exhibits excellent functional group tolerance and a wide range of substrates,thus providing an excellent strategy to access the valuable sulfide compounds.Moreover,this reaction has also shown good applications in the late-stage thiolation of biomolecules and offers a good platform for drug discovery and development.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgments
We thank for the financial support from Natural Science Foundation of Sichuan(No.2021YJ0413),Sichuan Key Laboratory of Medical Imaging(North Sichuan Medical College,No.SKLMI201901),Strategic Cooperation of Science and Technology between Nanchong City and North Sichuan Medical College(Nos.19SXHZ0441,19SXHZ0227),Chongqing Postdoctoral Science Foundation(No.cstc2020jcyj-bshX0052),and China Postdoctoral Science Foundation(No.2020M673121).We also thank Analytical and Testing Center of Chongqing University for assistance with NMR spectrum analysis.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.04.022.
杂志排行
Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
