茶多酚对淀粉酯纳米颗粒及其稳定的Pickering乳液性质的影响
2023-01-16钟玉珍张丽红
王 然,钟玉珍,张丽红
茶多酚对淀粉酯纳米颗粒及其稳定的Pickering乳液性质的影响
王 然1,2,钟玉珍1,张丽红1
(1. 长春职业技术学院食品与生物学院,长春 130033;2. 吉林大学生物与农业工程学院,长春 130022)
为探究茶多酚(Tea Polyphenols, TPs)对辛烯基琥珀酸酐(Octenyl Succinic Anhydride, OSA)酯化淀粉纳米颗粒(Starch Nanoparticles,SNPs)及其稳定的Pickering乳液性质的影响,该研究在制备OSA-SNPs的过程中添加TPs,研究TPs对OSA-SNPs的理化性质和乳化性能的影响。结果发现,添加TPs使OSA-SNPs的平均粒径增加、表面Zeta电位绝对值下降、接触角减小(<0.05)。通过傅立叶红外光谱扫描发现,TPs与OSA-SNPs之间存在氢键和疏水相互作用。在TP-OSA-SNPs稳定的乳液中,增加TP-OSA-SNPs的质量浓度(从0.5 g/mL至2.0 g/mL),乳滴平均直径明显减小(<0.05);当TP-OSA-SNPs的质量浓度增加至2 g/mL时,乳液形成了油滴紧密堆积的界面结构,能够抑制油滴迁移。通过加速氧化试验发现,与OSA-SNPs相比,TP-OSA-SNPs稳定的乳液中氢过氧化物值(Peroxide Value, POV)相对较低(<0.05),说明TP-OSA-SNPs具有延缓乳液中油脂氧化的作用。结果表明,这种新型具有抗氧化功能的食品级颗粒乳化剂,对构筑淀粉基Pickering乳液载体具有潜在价值。
淀粉;纳米颗粒;茶多酚;食品级Pickering乳液;氧化稳定性;辛烯基琥珀酸酐酯化淀粉
0 引 言
Pickering乳液是一类由固体颗粒作为乳化剂稳定的乳液[1-2]。相比于传统以分子乳化剂稳定的乳液,Pickering乳液具有颗粒用量少,稳定性好,无污染、无毒,环境友好等优势[3],因此,近年来,研究者们积极开发新型颗粒乳化剂,以期实现Pickering乳液在不同领域的应用。
淀粉是粮食初加工产物,其价格低廉、种类丰富,具有良好的加工适应性[4]。作为食品加工不可或缺的原料或加工助剂,淀粉的多种改性产品已经得到普遍应用[5-6]。在化学结构上,淀粉属于多羟基糖类聚合物,具有较强的亲水性[7-8]。为拓宽淀粉在食品中的应用,研究者在淀粉分子上接枝亲油基团,以提高其乳化性[9-10]。辛烯基琥珀酸酐(Octenyl Succinic Anhydride,OSA)是美国食品药品监督管理局(Food and Drug Administration, FDA)允许使用的酯化试剂[11],中国政府于1997年批准其作为乳化剂和增稠剂在食品加工中使用。许多文献报道了采用OSA改性天然淀粉球制备颗粒乳化剂并将其用于稳定Pickering乳液[12-13]。然而,不同生物源的天然淀粉颗粒的粒径一般为微米级,研究表明,采用微米级的颗粒作为乳化剂,其稳定乳液的乳滴直径会呈指数倍增长,甚至会形成豪米级的乳液[14],这限制了Pickering乳液在食品加工中的应用。因此,采用OSA酯化淀粉颗粒作为乳化剂稳定Pickering乳液,一般要减小淀粉颗粒的粒径。目前,研究者主要采用三种方式降低淀粉颗粒的粒径:研磨、酸或酶水解以及纳米沉淀[15]。由研磨得到的淀粉微粒,其粒度分布相对不均匀,因此,常采用研磨和酸水解相结合的方式来降低淀粉颗粒的粒径。此外,采用乙醇沉淀法制得的淀粉纳米颗粒(Starch Nanoparticles, SNPs),由于没有有毒试剂残留,是较理想的食品级纳米颗粒。OSA酯化淀粉微粒因其具有良好的乳化性,被广泛用于稳定食品级Pickering乳液[16-17]。
脂质氧化是导致食品乳液品质劣变、脂溶性生物活性物质降解甚至功效丧失的主要因素。与纯油脂相比,乳液中油脂更容易发生氧化反应,这主要是因为铁、铜、锰等金属离子助氧化剂分散在水相中,其能够向油水界面传递氧,进而促进乳液中油脂的氧化。颗粒乳化剂通过在油水界面形成物理屏障,阻碍助氧化剂参与油脂氧化反应,因此能延缓乳液中油脂的氧化[18]。目前,已有大量研究报道了利用生物源颗粒制备食品级颗粒乳化剂[19],然而关于具有抗氧化功能的食品级颗粒乳化剂的研究还相对较少。
茶多酚(Tea Polyphenols, TPs)是从茶叶中提取的一类多羟基酚类化合物,具有多种生理活性,属于天然强抗氧化剂[20]。已有研究表明,TPs与淀粉等多糖类物质可以通过氢键、范德华力、疏水相互作用等非共价作用方式结合,增进多糖类物质的氧化稳定性[21-23],然而,将TPs用于制备淀粉基颗粒乳化剂以及其对Pickering乳液物理稳定性和氧化稳定性影响的研究还鲜见报道。本研究采用乙醇沉淀法制备OSA-SNPs,分析添加TPs对OSA-SNPs理化性质和乳化性能的影响;采用傅立叶红外光谱(Fourier Transform Infrared Spectroscopy, FT-IR)分析TP-OSA-SNPs的结构性质;采用扫描电子显微镜(Scanning Electron Microscope,SEM)观察颗粒的形貌;采用接触角测量仪检测颗粒的乳化性质;采用激光粒度仪分析由不同颗粒浓度的OSA-SNPs和TP-OSA-SNPs稳定的Pickering乳液的粒径分布和乳滴平均直径;采用共聚焦激光扫描显微镜(Confocal Laser Scanning Microscopy, CLSM)观察Pickering乳液的微观结构;采用加速氧化试验检测乳液中初级氧化产物氢过氧化物值(Peroxide Value, POV)。通过研究TPs与淀粉的相互作用及其对Pickering乳液性质的影响,有助于拓宽淀粉、茶叶等农产品的应用领域,促进茶梗、茶末、滞销茶等茶叶废弃物的再利用,为淀粉基Pickering乳液的开发和TPs等生物活性物质的利用提供新思路。
1 材料与方法
1.1 材料与试剂
辛烯基琥珀酸酐(Octenyl Succinic Anhydride, OSA)酯化淀粉,宜瑞安食品配料有限公司;茶多酚(Tea Polyphenols,TPs),杭州禾田生物技术有限公司;尼罗蓝,美国西格玛奥德里奇贸易有限公司;尼罗红,上海阿拉丁生化科技股份有限公司;油茶籽油,玉山县大成仓食品有限公司;试验所用试剂均为分析纯。
1.2 仪器与设备
Zetasizer Nano ZSE激光纳米粒度仪,英国马尔文仪器有限公司;FA25高剪切分散乳化机,上海弗鲁克科技发展有限公司;Nexus670傅立叶变换红外光谱仪,美国热电集团尼高力仪器公司;JSM-6700冷场发射扫描电子显微镜,日本电子株式会社;UVmini-1240紫外可见分光光度计,岛津仪器(苏州)有限公司;FV3000共聚焦激光扫描显微镜,奥林巴斯(中国)有限公司;Mastersizer3000激光粒度仪,英国马尔文仪器有限公司;SL200KS光学法接触角仪,美国科诺工业有限公司。
1.3 试验方法
1.3.1 茶多酚-OSA酯化淀粉纳米颗粒的制备
参考Peng等[24]和王然[25]方法,将OSA酯化淀粉与去离子水配置成质量浓度为2%的悬浮液,并置于100 ℃恒温水浴中连续搅拌加热40 min;然后加入基于淀粉质量10%的TPs,并置于95 ℃恒温水浴中连续搅拌加热20 min。加热结束后,将混合液冷却至25 ℃,然后以体积比1:5的比例逐滴滴入无水乙醇中进行沉淀;再将混合液于3 500 r/min离心15 min,回收上清液,将沉淀物用无水乙醇洗两遍,然后于−70 ℃进行真空干燥,制得含有TPs的OSA酯化淀粉纳米颗粒(TP-OSA-SNPs)。
采用相同方法制备OSA酯化淀粉纳米颗粒(OSA-SNPs)作为对照组,淀粉悬浮液在100 ℃恒温水浴中连续搅拌加热60 min,不添加TPs,制备过程同上,其中,OSA-SNPs的OSA基团取代度为0.018 3[26]。
1.3.2 茶多酚保留率的测定
TP-OSA-SNPs中TPs保留率的测定参考刘蕾等[27]方法,略有修改。在制备TP-OSA-SNPs过程中,将TP-OSA-SNPs经醇沉和醇洗处理后离心得到的上清液进行混合,并记录上清液体积。根据TPs浓度-吸光度标准曲线,采用紫外分光光度计在274 nm波长下,检测上清液的吸光度。TP-OSA-SNPs中TPs的保留率(,%)按下式计算:

式中M为TPs最初添加量,mg;为上清液中TPs的浓度,mg/mL;为上清液的体积,mL。
1.3.3 接触角的测定
利用接触角测定仪检测OSA-SNPs和TP-OSA-SNPs样品的界面接触角。首先利用压片机将样品压成平整、致密的圆片(直径20 mm,厚度为2 mm),然后使用接触角测量仪测定淀粉样品的水相静态接触角,测量范围为0°~180°。由仪器控制带有毛细针头的微量进样器,将2L超纯水滴在样品圆片表面,待水滴在圆片表面平衡后进行拍摄,通过仪器自带软件分析接触角[28]。每个样品至少在圆片表面取5个点进行检测,测量结果取5个数值的平均值。
1.3.4 傅立叶红外光谱的测定
将OSA-SNPs、TP-OSA-SNPs样品在真空条件下干燥至恒量,然后将其与KBr(光谱纯)以质量比1∶75混合并压制成直径5 mm、厚度1 mm的圆片,使用红外光谱仪在波长4 000~400 cm-1范围内进行光谱扫描,设置光谱分辨率4 cm-1,经32次扫描获得样品红外谱图。
1.3.5 扫描电镜观察
将无水乙醇沉淀后得到的湿基OSA-SNPs、TP-OSA-SNPs样品滴加到硅片上,进行冻干处理。将样品固定在样品台上,经过喷金处理后,置于扫描电子显微镜样品室中,然后进行抽真空,当真空度达到5×10-3时,对样品的表面形态和尺寸进行观察并拍照。
1.3.6 淀粉纳米颗粒粒度分布和Zeta电位的测定
将湿基OSA-SNPs、TP-OSA-SNPs样品与去离子水配置成5∶10 000 (g/mL)的悬浮液,然后超声处理15 min,再将其置于激光纳米粒度仪中进行粒度分布和Zeta电位检测,设置分散剂为水,颗粒折光率为1.530,检测温度20 ℃,检测重复3次,结果取平均值[29]。
1.3.7 水包油Pickering乳液的制备
参考Xie等[30]方法,略有修改。将OSA-SNPs和TP-OSA-SNPs样品分别以不同的比例置于超纯水中,然后将油茶籽油以体积比1∶5的比例加入超纯水中,采用高速剪切乳化机在19 000 r/min下剪切乳化3 min,制得淀粉纳米颗粒质量浓度分别为0.5、1.0、1.5、2.0 g/mL的水包油Pickering乳液。将制备完成的Pickering乳液置于4 mL透明样品瓶中,放置15 d,并利用数码相机记录乳液样品贮藏前后的表观。
1.3.8 Pickering乳液粒度分布
利用激光粒度仪分别对不同质量浓度的OSA-SNPs和TP-OSA-SNPs样品制备的Pickering乳液进行粒度分布检测,油相的折射指数和吸收指数分别设置为1.449和0.010,水相的折射指数设置为1.330。每个样品检测3次,结果取平均值。
1.3.9 激光共聚焦荧光显微镜观察
参考Zhu等[31]方法,略有修改。将10L浓度为0.01 mg/mL的尼罗蓝和尼罗红依次加入1 mL乳液样品中,其中采用尼罗蓝对OSA-SNPs和TP-OSA-SNPs进行染色,采用尼罗红对油相进行染色。在避光下对乳液进行染色处理2 min,然后将乳液样品注入玻底平皿;设置尼罗蓝的发射波长为488 nm,激发波长为513 nm,尼罗红的发射波长为633 nm,激发波长为660 nm,然后对乳液进行观察并拍照。
1.3.10 Pickering乳液氧化稳定性的测定
将新制的Pickering乳液样品密闭存放于45 ℃恒温箱中15 d,测定乳液的POV值,测量方法参考Liu等[32],略有修改。在1.5 mL异辛烷和异丙醇(体积比3∶1)混合溶液中,加入不同贮藏时间的Pickering乳液0.3 mL,并以10 000 r/min离心5 min。然后取0.2 mL上清液加入2.8 mL甲醇和正丁醇(体积比2∶1)的混合溶液中,再依次加入3.94 mol/L硫氰酸铵溶液15L与0.072 mol/L亚铁离子溶液15L(0.132 mol/L BaCl2和0.144 mol/L FeSO4混合离心后的上清液),于避光处静置20 min后,于510 nm处测量吸光度值,利用过氧化氢异丙苯标准曲线计算Pickering乳液氢过氧化物的浓度,其中氢过氧化物值(Peroxide Value)以POV表示。
1.3.11 试验数据分析
全部试验重复测定3次,结果表示为平均值±标准差。采用软件Origin 8.5绘图,并用SPSS21.0对试验数据的显著性差异(<0.05)进行单因素方差分析。
2 结果与分析
2.1 茶多酚对OSA-淀粉纳米颗粒性质的影响
2.1.1 红外光谱分析
图1为TPs、OSA-SNPs和TP-OSA-SNPs的FT-IR谱图。从图1a呈现的TPs的红外光谱可以看出,在3 600~3 200 cm-1处形成了较宽的红外光谱特征峰,这是源于羟基(—OH)的伸缩振动,表明分子间或分子内形成了氢键[33];在1 700 cm−1处是羰基C=O的伸缩振动的吸收峰。从图1b可以看出,OSA-SNPs于1 720 cm-1和1 569 cm-1的位置分别出现了C=O酯键基团和-COO-的伸缩振动吸收峰,证明OSA-SNP上存在OSA酯化基团[24, 34-35]。与OSA-SNPs的红外谱图相比,TP-OSA-SNPs的谱带发生了明显的变化,其在3 600~3 200 cm-1处的—OH吸收峰向波数减小的方向发生了移动;与TPs的红外图谱相对照,TP-OSA-SNPs在1 700 cm−1处同样出现了C=O吸收峰,说明TPs与OSA-SNPs的羟基发生了交互作用,形成了氢键。此外,与OSA-SNPs的红外谱带相比,TP-OSA-SNPs在1 720 cm-1和1 569 cm-1两处形成的吸收峰的强度明显下降,说明TPs与OSA-SNPs之间可能存在疏水相互作用。Lv等[36]将TPs与土豆淀粉进行混合湿磨处理,发现茶多酚在1 695 cm−1处出现的C=O吸收峰也同样出现在土豆淀粉的红外谱带中,并发生了轻微的移动,说明TPs和土豆淀粉之间的羟基通过氢键发生了相互作用。Wang等[34]研究了添加TPs对OSA-蜡质玉米淀粉分子结构的影响,发现TPs能够与OSA-蜡质玉米淀粉的亲脂基团产生疏水相互作用,进而改变OSA-淀粉分子结构,这均与本研究结果相符。
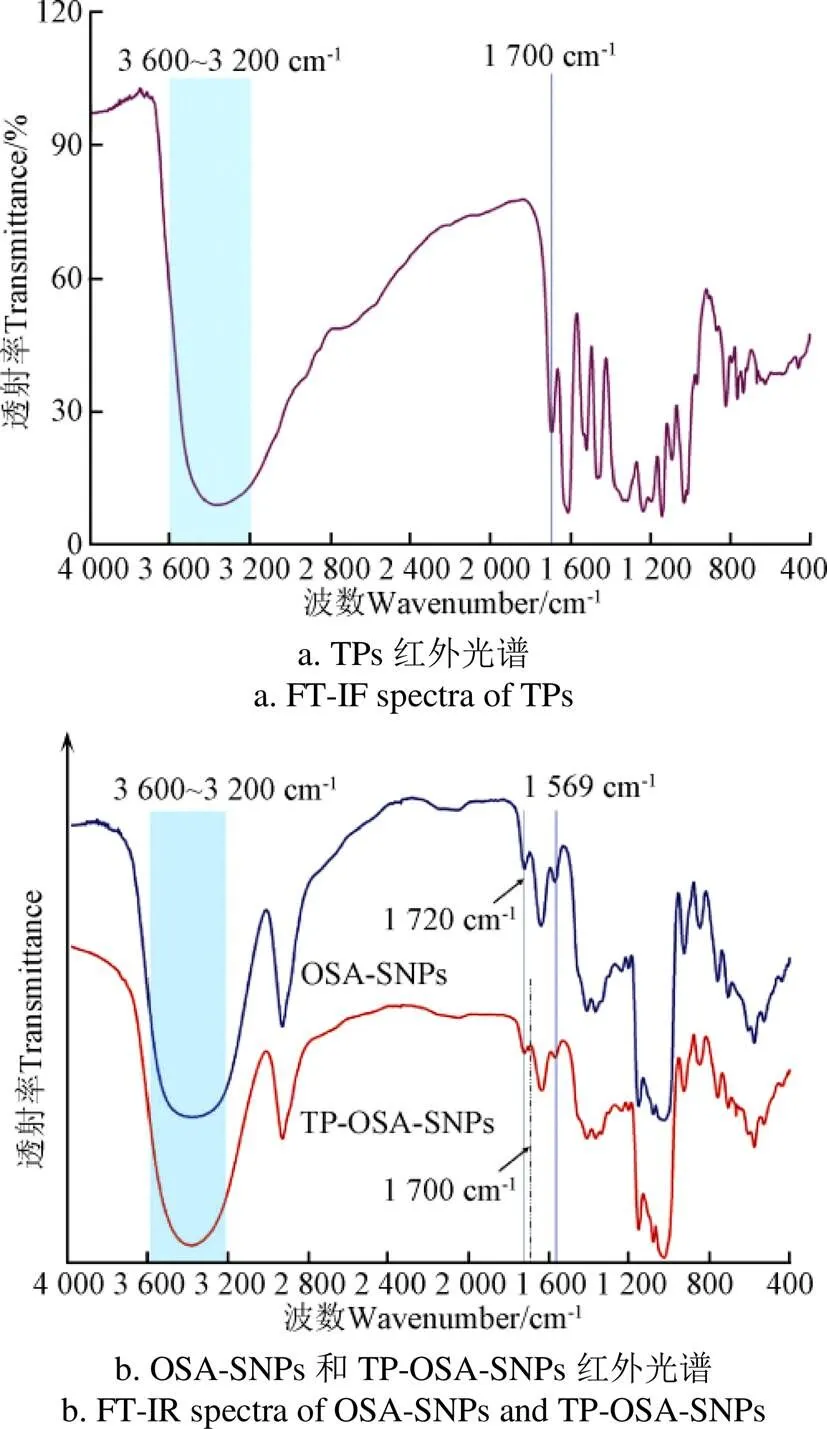
图1 TPs、OSA-SNPs和TP-OSA-SNPs的红外光谱图
2.1.2 茶多酚对OSA-淀粉纳米颗粒微观结构及颗粒性质的影响
图2给出了OSA-SNPs和TP-OSA-SNPs的微观形态。如图2所示,两种纳米颗粒的表面形态十分相似,均为不规则的球形,表面凹凸不平,然而,与OSA-SNPs相比(图2a),TP-OSA-SNPs中存在较多粒径相对较大的颗粒(图2b),说明添加TPs导致OSA-SNPs的粒径增大。Wang等[34]研究了添加不同浓度的TPs对OSA-蜡质玉米淀粉分子结构的影响,发现随着TPs添加量的增加,OSA-淀粉的水力学直径也明显增加。
图3呈现了OSA-SNPs和TP-OSA-SNPs的平均粒径、粒度分布、Zeta电位以及接触角。如图3所示,TP-OSA-SNPs平均粒径(222.00±1.40)nm显著大于(<0.05)OSA-SNPs(173.20±0.57)nm;从粒度分布曲线也可以看出,与OSA-SNPs相比,TP-OSA-SNPs粒度分布峰的峰形较窄,峰强度较高,并且向粒径增大的方向发生了平移,说明虽然TP-OSA-SNPs的粒径较大,但粒径分布相对均匀,较小的多分散性指数(Polydispersity Index,PDI)说明了其粒度分布的均匀性(PDI为0.159)。此外,从图3中还可以看出,添加TPs导致OSA-SNPs的接触角由83.20°±1.02°减小至78.60°±0.84°(<0.05),Zeta电位绝对值由|24.20| mV减小至|19.10| mV(<0.05)。OSA-SNPs表面所带负电荷主要来源于OSA分子的烯基长链,添加TPs导致OSA-SNPs表面所带负电荷减少,这会减弱TP-OSA-SNPs之间的静电斥力[37]。同时,OSA分子的烯基长链也是赋予淀粉纳米颗粒疏水性的主要原因,OSA改性使淀粉纳米颗粒具有油水润湿性,研究表明,具有良好油水润湿性的颗粒,其接触角接近90°[38]。本研究发现,添加TPs导致OSA-SNPs接触角有所降低,表明与OSA-SNPs相比,TP-OSA-SNPs的疏水性减弱;这是因为TPs中含有大量的亲脂性成分[39],其与OSA分子的疏水性成分之间可能存在疏水相互作用,阻碍了OSA分子的烯基长链接触油相,因此导致TP-OSA-SNPs的疏水性下降。Peng等[24]和Wang等[34]相继报道了添加TPs导致OSA-蜡质玉米淀粉乳化性减弱,这与本研究结果相符。
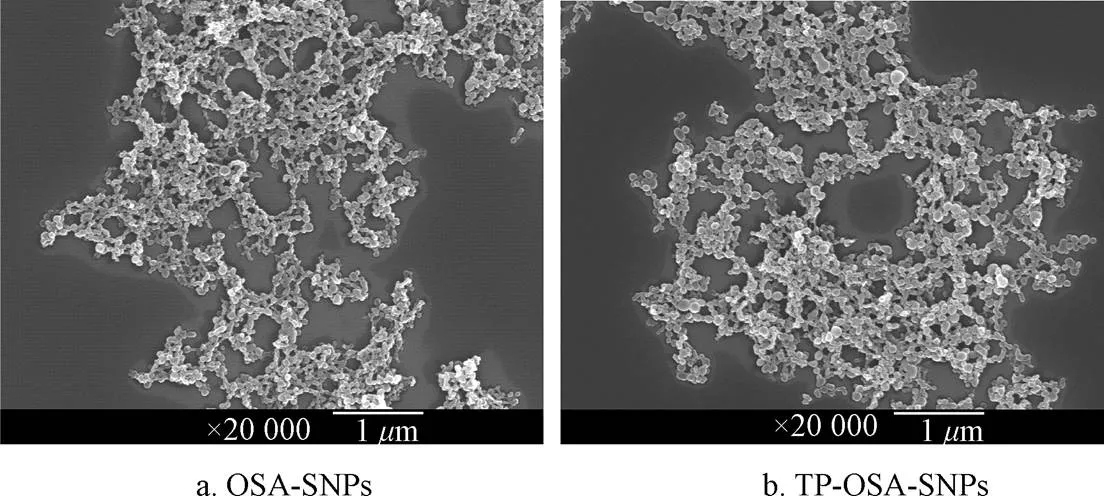
图2 OSA-SNPs和TP-OSA-SNPs的扫描电镜图

注:小写字母上标表示在0.05水平差异显著,PDI为多分散性指数。
2.2 不同颗粒浓度的OSA-SNPs和TP-OSA-SNPs稳定的Pickering乳液
2.2.1 乳液的粒度分布
图4给出了不同质量浓度OSA-SNPs和TP-OSA-SNPs稳定的Pickering乳液在15 d贮藏前后的乳液表观、乳滴平均直径和粒径分布曲线。如图4a所示,当OSA-SNPs的质量浓度从0.5 g/mL增加至2.0 g/mL时,其稳定乳液的乳滴粒径分布曲线峰向粒径增大的方向发生了轻微的平移,并且2.0 g/mL OSA-SNPs稳定的乳液其乳滴粒径分布曲线在主峰旁粒径增加的方向出现了一个小峰,表明乳液样品中形成许多粒径较大的油滴。从OSA-SNPs稳定的乳液的平均粒径[4,3]和(90)也可以看出,乳液的平均粒径随着OSA-SNPs质量浓度的增加而增加。这主要是因为OSA-SNPs的平均粒径较小,根据布朗运动原理,粒径较小的颗粒与较大颗粒相比,其运动速度更快,在稳定乳液时其会先到达油水界面;此外,根据颗粒粒径和乳滴的曲率关系[40],粒径较小的颗粒易于形成直径较小的乳滴,因此,当OSA-SNPs质量浓度增加至2.0 g/mL时,乳液中形成了大量直径小于1m的乳滴,这些直径较小的乳滴通过布朗运动吸附在直径较大的乳滴表面,导致乳滴的平均粒径增加。如图4b所示,由不同质量浓度OSA-SNPs稳定的乳液放置15 d后没有发生明显的油相析出,乳液的[4,3]和(90)有增加的趋势,其中OSA-SNPs质量浓度为2.0 g/mL的乳液样品,在15 d贮藏中其(90)的增加幅度较小。

注:D[4,3]为体积平均直径,D(90)表示小于此粒径的乳滴体积含量占全部乳滴的90%。
如图4c所示,随着TP-OSA-SNPs的质量浓度从0.5 g/mL增加至2.0 g/mL,其稳定乳液的乳滴粒径分布曲线峰从双峰分布变为单峰分布,并且乳滴粒度分布峰向粒径减小的方向发生了平移,乳液的[4,3]和(90)均表现出下降的趋势,这表明随着TP-OSA-SNPs质量浓度的增加,其稳定乳液的乳滴平均直径明显减小,说明TP-OSA-SNPs能够吸附在油水界面上,发挥稳定乳液的作用;当TP-OSA-SNPs的质量浓度较低时,其在乳液油水界面的覆盖程度较低,乳滴易发生聚集导致直径增加;随着TP-OSA-SNPs质量浓度的增加,其能够在油水界面形成稳定的物理屏障,阻碍乳滴聚集,因此乳滴的平均直径降低。如图4d所示,由TP-OSA-SNPs稳定的乳液放置15 d后没有发生明显的油相析出。当乳液中TP-OSA-SNPs的质量浓度为0.5 g/mL和1.0 g/mL时,随着在贮藏时间的延长,乳液的(90)明显增加;当乳液中TP-OSA-SNPs的质量浓度为1.5 g/mL时,在贮藏15 d后,乳液中乳滴粒径分布主峰旁粒径增加的方向出现一个小峰,表明乳滴粒径增加;而乳液中TP-OSA-SNPs的质量浓度为2.0 g/mL时,其乳滴粒径增加幅度较小,说明乳液在15 d贮藏期具有良好的稳定性。
2.2.2 乳液微观结构观察
Pickering乳液的微观结构以及不同质量浓度OSA-SNPs和TP-OSA-SNPs在油水界面的吸附情况如图5所示。在图5中,上图呈现为乳液的油滴,其由尼罗红染色,在激发波长488 nm下标记为绿色;下图呈现为乳液中的OSA-SNPs或TP-OSA-SNPs,由尼罗蓝染色,在激发波长633 nm下标记为红色。由不同质量浓度OSA-SNPs和TP-OSA-SNPs稳定的Pickering乳液,其微观结构形态呈现出明显的差异。从图5a可以看出,1.0 g/mL OSA-SNPs稳定的Pickering乳液,其油滴表现出良好的分散性,OSA-SNPs紧密的吸附在油滴表面,形成阻隔层,抑制油滴聚结,然而OSA-SNPs稳定的乳液也表现出油滴尺寸不均匀的现象,乳液中存在大量直径小于1m油滴,同时,也存在许多直径超过20m油滴。如图5b所示,当乳液中OSA-SNPs的质量浓度增加至2.0 g/mL时,乳液中的小油滴增多了,并且一些小油滴倾向于吸附在大油滴的表面,但是OSA-SNPs在油滴之间形成了物理屏障,因此,抑制了油滴的聚结。图5c和d呈现了不同质量浓度的TP-OSA-SNPs所稳定的Pickering乳液的微观形态。如图5c所示,1.0 g/mL TP-OSA-SNPs稳定的乳液中油滴的分散性良好,但是,与1.0 g/mL OSA-SNPs稳定的乳液相比,TP-OSA-SNPs在油水界面上的吸附量明显减少,这是因为与OSA-SNPs相比,TP-OSA-SNPs的接触角较小(图3),疏水性较弱,其在油水界面的吸附能力也有所减弱。此外,TP-OSA-SNPs表面所带负电荷相对较少,颗粒之间的静电斥力较弱,所以颗粒易于发生聚集。从图5d中可以看出2.0 g/mL TP-OSA-SNPs稳定的乳液,形成了油滴堆积的界面结构,TP-OSA-SNPs填充在油水界面之间,抑制油滴的迁移,乳液中的油滴仍然保持圆形,说明乳液具有良好的稳定性。
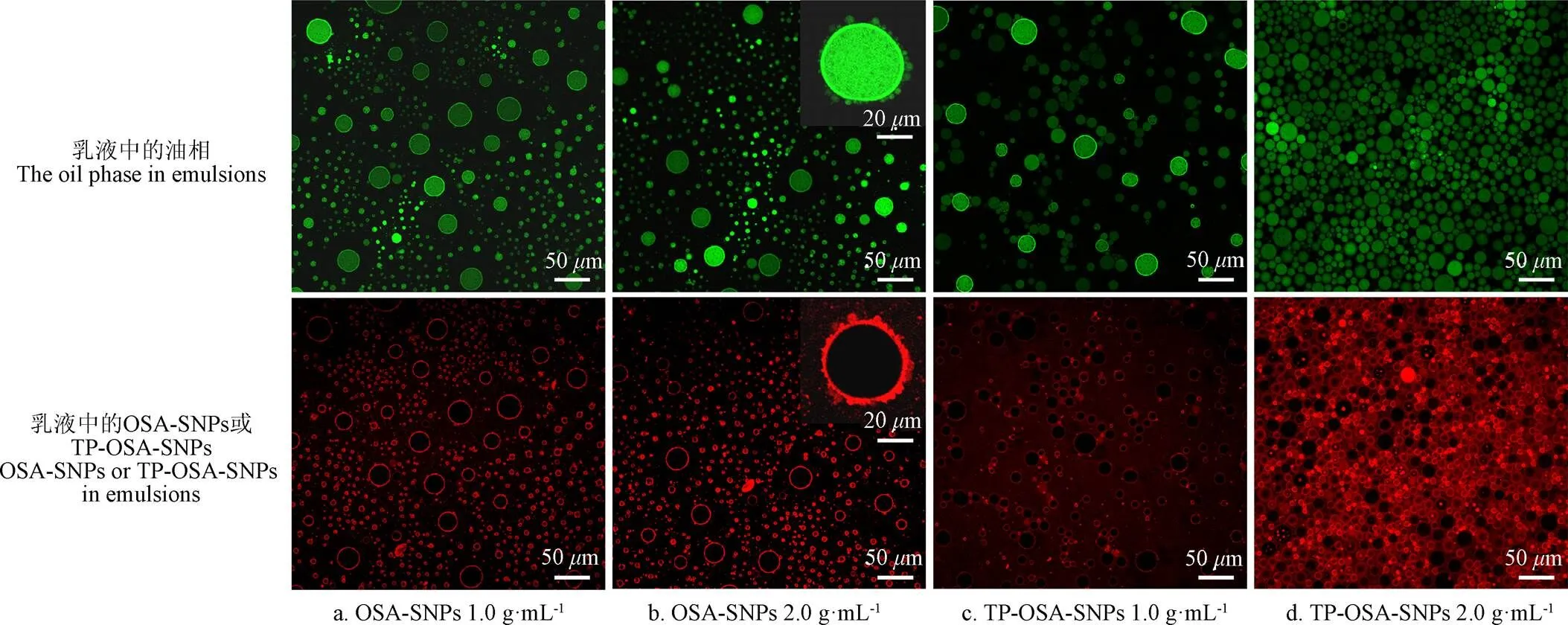
图5 不同浓度的OSA-SNPs和TP-OSA-SNPs稳定的Pickering乳液的共聚焦激光扫描显微镜图
2.3 Pickering乳液的脂质氧化稳定性
图6呈现了在贮藏过程中不同质量浓度OSA-SNPs和TP-OSA-SNPs稳定的Pickering乳液中POV值。如图所示,随着贮藏时间的延长,不同质量浓度的OSA-SNPs和TP-OSA-SNPs稳定的Pickering乳液的POV值均逐渐增加,但是增加的程度不同。从图6a可以看出,当OSA-SNPs质量浓度从0.5 g/mL增加至2.0 g/mL时,其稳定的乳液的POV值明显降低,这可能是由于OSA-SNPs质量浓度增加,其在油水界面形成的吸附层的厚度增加,能起到延缓油脂氧化的作用。如图6b所示,与OSA-SNPs相比,TP-OSA-SNPs稳定的Pickering乳液的POV值明显降低(<0.05),并且随着TP-OSA-SNPs质量浓度的增加,乳液中POV值逐渐下降,这主要与颗粒乳化剂在油水界面形成的阻隔层以及TPs赋予TP-OSA-SNPs的抗氧化活性(TP-OSA-SNPs中TPs保留率为14.49%±2.13%)有关。Miao等[21]利用TPs复合多孔淀粉制备食品级的包装膜,研究发现复合膜具有优异的抗氧化性能。Yuan等[22]在海藻酸钙凝胶中添加TPs制成可食用的生物活性膜,研究发现茶多酚的添加提升了复合膜的抗氧化活性。Zhang等[23]利用TPs与蜡质玉米淀粉复合制成纳米纤维复合膜,研究表明随着TPs浓度的增加,复合膜的抗氧化性增强。上述文献均与本研究结论相符。

图6 不同颗粒浓度的OSA-SNPs和TP-OSA-SNPs稳定的Pickering乳液贮藏15 d的氢过氧化物值
3 结 论
1)茶多酚(Tea Polyphenols,TPs)与辛烯基琥珀酸酐(Octenyl Succinic Anhydride, OSA)酯化淀粉纳米颗粒(Starch Nanoparticles,SNPs)能够通过氢键和疏水相互作用结合,并采用醇沉法制成纳米颗粒,其多分散性指数(Polydispersity Index,PDI)为0.159,说明其粒度分布较均匀。
2)添加TPs能使OSA-SNPs的粒径从(173.20± 0.57)nm增加至(222.00±1.40)nm,表面负电荷数从(−24.20±3.18)mV减少至(−19.10±1.30)mV,接触角从83.20°±1.02°减小至78.60°±0.84°,表明添加TPs导致OSA-SNPs的疏水性下降。
3)由OSA-SNPs稳定的Pickering乳液,随着OSA-SNPs的质量浓度从0.5 g/mL增加至2.0 g/mL,乳液的体积平均直径从24.2m增加至43.2m;由质量浓度为2 g/mL的OSA-SNPs稳定的乳液,形成了大量的小乳滴,一些小乳滴会吸附在较大乳滴的表面,但是乳液没有发生聚结现象。由TP-OSA-SNPs稳定的Pickering乳液,随着TP-OSA-SNPs的质量浓度从0.5 g/mL增加至2.0 g/mL,乳液的体积平均直径从51.1m降低至25.2m;由质量浓度为2 g/mL的TP-OSA-SNPs稳定的乳液,大量的TP-OSA-SNPs填充在油滴紧密堆积的油水界面,并形成有效的物理屏障,维持乳液的稳定。
4)随着TP-OSA-SNPs质量浓度的增加,乳液的POV值明显降低,并且质量浓度为2 g/mL的TP-OSA-SNPs稳定的乳液,在贮藏中其氢过氧化物值(Peroxide Value, POV)最小,说明TP-OSA-SNPs在油水界面上,既能形成物理屏障以抑制油滴聚结,又能发挥抗氧化剂效果,提高乳液的氧化稳定性。
通过研究TPs对OSA-SNPs及其稳定的乳液性质的影响,可为开发新型具有抗氧化功能的颗粒乳化剂,为构筑食品级淀粉基Pickering乳液运载体系提供借鉴。
[1] 李松南. 淀粉基Pickering乳液稳定机理及在叶黄素递送中的应用研究[D]. 广州:华南理工大学,2020.
Li Songnan. Starch-based Pickering Emulsion: Stabilizing Mechanism and Application in Lutein Delivery[D]. Guangzhou: South China University of Technology, 2020. (in Chinese with English abstract)
[2] Xia T, Xue C, Wei Z. Physicochemical characteristics, applications and research trends of edible Pickering emulsions[J]. Trends in Food Science & Technology, 2021, 107: 1-15.
[3] Low L E, Siva S P, Ho Y K, et al. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion[J]. Advances in Colloid and Interface Science, 2020, 277: 102117.
[4] Yan X, Diao M, Li C, et al. Formation and properties of starch-palmitic acid complex nanoparticles and their influence on Pickering emulsions[J]. International Journal of Biological Macromolecules, 2022, 204: 685-691.
[5] 赵思琪,张华娟,罗曜,等. 红外辐射制备柠檬酸糯米淀粉酯工艺优化及功能特性[J]. 农业工程学报,2021,37(10): 261-268.
Zhao Siqi, Zhang Huajuan, Luo Yao, et al. Optimization processes and functionality of citric acid esterified glutinous rice starch synthesized via infrared radiation[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2021, 37(10): 261-268. (in Chinese with English abstract)
[6] Song X, Gong H, Zhu W, et al. Pickering emulsion stabilized by composite-modified waxy corn starch particles[J]. International Journal of Biological Macromolecules, 2022, 205: 66-75.
[7] Chen X, Hu Z, Chen D, et al. Preparation and physiochemical properties of enzymatically modified octenyl succinate starch[J]. Journal of Food Science, 2022, 87: 2112-2120.
[8] Li Y, Peng Z, Wu D, et al. Improving hydrophilicity of wheat starch via sodium dodecyl sulfate treatment[J]. Starch-Stärke, 2022, 74(4): 1-6.
[9] Wu Z, Li H, Zhao X, et al. Hydrophobically modified polysaccharides and their self-assembled systems: A review on structures and food applications[J]. Carbohydrate Polymers, 2022, 284: 119182.
[10] Mirzaaghaei M, Nasirpour A, Keramat J, et al. Chemical modification of waxy maize starch by esterification with saturated fatty acid chlorides: Synthesis, physicochemical and emulsifying properties[J]. Food Chemistry, 2022, 393: 133293.
[11] Gharaghani M, Mousavi M, Khodaiyan F, et al. Octenyl succinylation of kefiran: Preparation, characterization and functional properties[J]. International Journal of Biological Macromolecules, 2021, 166: 1197-1209.
[12] 余振宇. OSA改性芋头淀粉基Pickering乳液运载体系稳定机制及其特性研究[D]. 合肥:合肥工业大学,2020.
Yu Zhenyu. Stabilization Mechanism and Characteristics of Pickering Emulsion Delivery System Based on OSA Modified Taro Starch[D]. Hefei: Hefei University of Technology, 2020. (in Chinese with English abstract)
[13] 刘微. OSA淀粉的结构特征与乳化性质的相关性分析[D]. 无锡:江南大学,2019.
Liu Wei. Correlation Analysis Between Structural Characteristics and Emulsification Capacity of OSA Starch[D]. Wuxi: Jiangnan University, 2019. (in Chinese with English abstract)
[14] Binks B P, Lumsdon S O. Pickering emulsions stabilized by monodisperse latex particles: Effects of particle size[J]. Langmuir, 2001, 17: 4540-4547.
[15] Lu H, Tian Y. Nanostarch: Preparation, modification, and application in Pickering emulsions[J]. Journal of agricultural and food chemistry, 2021, 69(25): 6929-6942.
[16] Li W, Yu Y, Peng J, et al. Effects of the degree of substitution of OSA on the properties of starch microparticle-stabilized emulsions[J]. Carbohydrate Polymers, 2021, 255: 117546.
[17] Zhu F. Starch based Pickering emulsions: Fabrication, properties, and applications[J]. Trends in Food Science & Technology, 2019, 85: 129-137.
[18] Keramat M, Kheynoor N, Golmakani M-T. Oxidative stability of Pickering emulsions[J]. Food Chemistry: X, 2022, 14: 100279.
[19] Li W, Jiao B, Li S, et al. Recent advances on Pickering emulsions stabilized by diverse edible particles: Stability mechanism and applications[J]. Frontiers in Nutrition, 2022, 9: 864943.
[20] Yin Z, Zheng T, Ho C-T, et al. Improving the stability and bioavailability of tea polyphenols by encapsulations: A review[J]. Food Science and Human Wellness, 2022, 11: 537-556.
[21] Miao Z, Zhang Y, Lu P. Novel active starch films incorporating tea polyphenols-loaded porous starch as food packaging materials[J]. International Journal of Biological Macromolecules, 2021, 192: 1123-1133.
[22] Yuan B, Cao Y, Tang Q, et al. Enhanced performance and functionality of active edible films by incorporating tea polyphenols into thin calcium alginate hydrogels[J]. Food Hydrocolloids, 2019, 97: 105197.
[23] Zhang D, Chen L, Cai J, et al. Starch/tea polyphenols nanofibrous films for food packaging application: From facile construction to enhance mechanical, antioxidant and hydrophobic properties[J]. Food Chemistry, 2021, 360: 129922.
[24] Peng S, Xue L, Leng X, et al. Slow digestion property of octenyl succinic anhydride modified waxy maize starch in the presence of tea polyphenols[J]. Journal of Agricultural and Food Chemistry, 2015, 63(10): 2820-2829.
[25] 王然. 辛烯基琥珀酸纳米淀粉酯颗粒的制备及其食品级Pickering乳液的特性[J]. 食品科学,2019,40(20):94-99.
Wang Ran. Preparation and characterization of a food-grade pickering emulsion stabilized by octenyl succinic anhydride-modified starch nanoparticles[J]. Food Science, 2019, 40(20): 94-99. (in Chinese with English abstract)
[26] Wu X, Liu P, Ren L, et al. Optimization of corn starch succinylation using response surface methodology[J]. Starch, 2014, 66: 508-514.
[27] 刘蕾,袁芳,高彦祥.-乳白蛋白提高-胡萝卜素乳液稳定性[J]. 农业工程学报,2016,32(增刊2):423-429.
Liu Lei, Yuan Fang, Gao Yanxiang.-lactalbumin enhancing emulsion stability of-carotene[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2016, 32(Supp.2): 423-429. (in Chinese with English abstract)
[28] Shao P, Zhang H, Niu B, et al. Physical stabilities of taro starch nanoparticles stabilized Pickering emulsions and the potential application of encapsulated tea polyphenols[J]. International Journal of Biological Macromolecules, 2018, 118: 2032-2039.
[29] 李书红,周军君,陈桂芸,等. 玉米醇溶蛋白-壳聚糖纳米营养递送粒子的制备及性质[J]. 农业工程学报,2021,37(16):279-286.
Li Shuhong, Zhou Junjun, Chen Guiyun, et al. Preparation and properties of zein-chitosan nano-nutrient delivery particles[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2021, 37(16): 279-286. (in Chinese with English abstract)
[30] Xie Y, Liu H, Li Y, et al. Characterization of Pickering emulsions stabilized by OSA-modified sweet potato residue cellulose: Effect of degree of substitute and concentration[J]. Food Hydrocolloids, 2020, 108: 105915.
[31] Zhu W, Zheng F, Song X, et al. Influence of formulation parameters on lipid oxidative stability of Pickering emulsion stabilized by hydrophobically modified starch particles[J]. Carbohydrate Polymers, 2020, 246: 116649.
[32] Liu Y, Zhang C, Cui B. Carotenoid-enriched oil preparation and stability analysis during storage: Influence of oils’ chain length and fatty acid saturation[J]. LWT-Food Science and Technology, 2021, 151: 112163.
[33] Li X, Sun C, Luo L, et al. Determination of tea polyphenols content by infrared spectroscopy coupled with iPLS and random frog techniques[J]. Computers and Electronics in Agriculture, 2015, 112: 28-35.
[34] Wang X, Leng X, Zhang G. The loosening effect of tea polyphenol on the structure of octenyl succinic anhydride modified waxy maize starch[J]. Food Hydrocolloids, 2020, 99: 105367.
[35] Bai Y, Kaufman R C, Wilson J D, et al. Position of modifying groups on starch chains of octenylsuccinic anhydride-modified waxy maize starch[J]. Food Chemistry, 2014, 153: 193-199.
[36] Lv Y, Zhang L, Li M, et al. Physicochemical properties and digestibility of potato starch treated by ball milling with tea polyphenols[J]. International Journal of Biological Macromolecules, 2019, 129: 207-213.
[37] Nilsson L, Bergenståhl B. Adsorption of hydrophobically modified anionic starch at oppositely charged oil/water interfaces[J]. Journal of Colloid and Interface Science, 2007, 308: 508-513.
[38] Hunter T N, Pugh R J, Franks G V, et al. The role of particles in stabilising foams and emulsions[J]. Advances in Colloid and Interface Science, 2008, 137: 57-81.
[39] He Q, Lv Y, Yao K. Effects of tea polyphenols on the activities of α-amylase, pepsin, trypsin and lipase[J]. Food Chemistry, 2006, 101: 1178-1182.
[40] Kim I, Worthen A, Johnston K, et al. Size-dependent properties of silica nanoparticles for Pickering stabilization of emulsions and foams[J]. Journal of Nanoparticle Research, 2016, 18: 1-12.
Effects of tea polyphenols on the properties of starch ester nanoparticles and their stabilized Pickering emulsions
Wang Ran1,2, Zhong Yuzhen1, Zhang Lihong1
(1.,,130033,;2.,,130022,)
Pickering emulsions can be expected to serve as a promising method against coalescence during storage in the food industry. Food-grade oil-in-water Pickering emulsions are stabilized by the solid particles on the oil-water interface, where a dense film can be formed against the aggregation of droplets. In this study, the food-grade oil-in-water Pickering emulsion was prepared using Octenyl Succinic Anhydride (OSA) modified waxy maize Starch Nanoparticles (SNPs) in the presence of Tea Polyphenols (TPs). An investigation was also made to clarify the effects of TPs on physicochemical properties and emulsifying capacity of OSA-starch nanoparticles (OSA-SNPs). The potential of TP-OSA-SNPs was examined after the combination of OSA-starches with TPs. The ethanol precipitation was then selected as a kind of particle emulsifier. An analysis was also conducted for the properties of Pickering emulsions that stabilized by OSA-SNPs and TP-OSA-SNPs at different concentrations. The mean particle size of OSA-SNPs and TP-OSA-SNPs was measured by the laser diffraction particle size analyzer and Scanning Electron Microscope (SEM). The results showed that the mean particle size of TP-OSA-SNPs (222.00±1.40) nm was larger than that of OSA-SNPs (173.20±0.57) nm. The absolute value of Zeta potential for the TP-OSA-SNPs (|-19.10±1.30| mV) was less than that of OSA-SNPs (|-24.20±3.18| mV). The contact angle of TP-OSA-SNPs (78.60°±0.84°) was also smaller than that of OSA-SNPs (83.20°±1.02°). It infers that there was a weaker hydrophobicity of TP-OSA-SNPs, compared with the OSA-SNPs. Furthermore, the structural properties of OSA-SNPs and TP-OSA-SNPs were analyzed by Fourier Transform Infrared spectroscopy (FT-IR). Specifically, the characteristic absorbance peak at 3 600-3 200 cm-1of TP-OSA-SNPs was shifted towards the lower wavenumber, compared with the OSA-SNPs. Moreover, the FT-IR spectra of TP-OSA-SNPs appeared a peak at the same wavenumber, indicating that the hydrogen bonds were formed in the interactions of TPs with OSA-SNPs, compared with the absorbance peak at 1 700 cm-1of TPs. There were the decreased intensities of peaks for the TP-OSA-SNPs at 1 720 and 1 569 cm-1, indicating the hydrophobic association between TPs and OSA-SNPs. There was an outstanding change in the Pickering emulsions that stabilized by OSA-SNPs and TP-OSA-SNPs at different concentrations. The mean droplet size of the OSA-SNPs emulsions increased slightly, as the concentration of OSA-SNPs increased from 0.5 g/mL to 2.0 g/mL. By contrast, the mean droplet size of the TP-OSA-SNPs emulsions decreased with the increasing TP-OSA-SNPs additions from 0.5 g/mL to 2.0 g/mL. The droplet size distributions of the TP-OSA-SNPs emulsions at different concentrations were changed from the bimodal to the unimodal distribution. The trend was attributed to the various compositions of interfacial structure with the different particle emulsifiers. The morphologies of Pickering emulsions were visualized by Laser Confocal Fluorescence Microscopy (CLSM). Specifically, a large quantity of OSA-SNPs was absorbed closely on the surface of oil droplets, inhibiting the droplets from gathering. There was a weaker absorbing capacity of TP-OSA-SNPs, compared with the OSA-SNPs. The reason was the smaller quantities of absorbed TP-OSA-SNPs on the oil-water interface.Additionally, an interfacial droplet-compacted structure was formed in the TP-OSA-SNPs emulsion with a concentration of 2 g/mL, which was in favor of inhibiting oil droplets migration. A rapid oxidation test was carried out to determine the oxidative stabilities of OSA-SNPs and TP-OSA-SNPs emulsions. The Peroxide Value (POV) was also selected to evaluate the formation of primary oxidative products in the emulsions. There was an increase in the POV of the OSA-SNPs and TP-OSA-SNPs emulsions during storage. However, the POV descended with the increasing concentration of OSA-SNPs or TP-OSA-SNPs in the emulsion. Furthermore, the POV values in the TP-OSA-SNPs emulsions were smaller after 15 days of storage, compared with the OSA-SNPs emulsions. It infers that the oxidation stabilities of the TP-OSA-SNPs emulsions were better than that of the OSA-SNPs emulsions. Consequently, the interactions between TPs and starch-based nanoparticles emulsifier can greatly contribute to developing the better carrier for the bioactive substances. The finding can also provide a potential application to fabricate the delivery system of food-grade starch-based Pickering emulsion.
starch; nanoparticles; tea polyphenols; food-grade Pickering emulsion; oxidative stability; octenyl succinic anhydride modified starch
10.11975/j.issn.1002-6819.2022.17.033
TS235.1
A
1002-6819(2022)-17-0303-08
王然,钟玉珍,张丽红. 茶多酚对淀粉酯纳米颗粒及其稳定的Pickering乳液性质的影响[J]. 农业工程学报,2022,38(17):303-310.doi:10.11975/j.issn.1002-6819.2022.17.033 http://www.tcsae.org
Wang Ran, Zhong Yuzhen, Zhang Lihong. Effects of tea polyphenols on the properties of starch ester nanoparticles and their stabilized Pickering emulsions[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2022, 38(17): 303-310. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2022.17.033 http://www.tcsae.org
2022-04-21
2022-08-04
长春职业技术学院应用技术研究与开发项目(YY-2019B25)
王然,博士生,讲师,研究方向为农产品加工及贮藏工程、食品级淀粉基乳液递送体系。Email:ranwang13@mails.glu.edu.cn
