Development of a prediction model for enteral feeding intolerance in intensive care unit patients: A prospective cohort study
2023-01-06XueMeiLuDengShuaiJiaRuiWangQingYangShanShanJinLanChen
Xue-Mei Lu, Deng-Shuai Jia, Rui Wang, Qing Yang, Shan-Shan Jin, Lan Chen
Xue-Mei Lu, Deng-Shuai Jia, School of Nursing, Shanghai Jiao Tong University, Shanghai 200025, China
Xue-Mei Lu, Lan Chen, Department of Nursing, Shanghai General Hospital, Shanghai Jiao Tong University, Shanghai 200080, China
Rui Wang, Qing Yang, Shan-Shan Jin, Department of Critical Care Medicine, Shanghai General Hospital, Shanghai Jiao Tong University, Shanghai 200080, China
Abstract BACKGROUND Enteral nutrition (EN) is essential for critically ill patients.However, some patients will have enteral feeding intolerance (EFI) in the process of EN.AIM To develop a clinical prediction model to predict the risk of EFI in patients receiving EN in the intensive care unit.METHODS A prospective cohort study was performed.The enrolled patients’ basic information, medical status, nutritional support, and gastrointestinal (GI) symptoms were recorded.The baseline data and influencing factors were compared.Logistic regression analysis was used to establish the model, and the bootstrap resampling method was used to conduct internal validation.RESULTS The sample cohort included 203 patients, and 37.93% of the patients were diagnosed with EFI.After the final regression analysis, age, GI disease, early feeding, mechanical ventilation before EN started, and abnormal serum sodium were identified.In the internal validation, 500 bootstrap resample samples were performed, and the area under the curve was 0.70 (95%CI: 0.63-0.77).CONCLUSION This clinical prediction model can be applied to predict the risk of EFI.
Key Words: Enteral feeding intolerance; Critical care medicine; Clinical prediction model; Nutrition assessment; Nutritional support; Critical care nursing
lNTRODUCTlON
Enteral nutrition (EN) is a preferred and cost-effective approach to nutritional support[1,2].When EN is provided, nutrients in the gastrointestinal (GI) tract activate intestinal endocrine cells and lymphoid tissues, which positively contributes to GI function (e.g., movement, digestion, and immunity)[3,4].However, during the provision of EN, many complications can develop that have an adverse impact on nutritional support[5].Enteral feeding intolerance (EFI) is a common and primary manifestation among many GI complications.
EFI is the inability to deliver adequate energy or nutrients to patients due to GI symptoms in the absence of mechanical obstruction[6].EFI develops in 2%-75% of enteral feeding patients in intensive care units (ICUs)[7].When EFI occurs, prokinetic agents and post-pyloric feeding are recommended[8].If EFI cannot be attenuated by medications or other feeding access, then EN is reduced or suspended[9,10].This may result in an inability to attain nutritional goals or in malnutrition.
Therefore, distinguishing high-risk patients before EFI occurs is very important and has a guiding role in clinical practice.Many studies have explored the mechanics and causes for the development of EFI in clinical practice.A review summarized some of the main reasons: (1) Admission diagnosis of burns, head injuries, sepsis, and multi-trauma; (2) Premorbid conditions of disordered glucose metabolism, age, and sex; (3) Electrolyte disorders; and (4) Use of drugs such as sedatives, analgesics,and catecholamines[9].A recent review of a multicenter and multiyear database indicated that EFI was more likely to occur in burn, cardiovascular/vascular disease, GI disease, and sepsis patients in the ICU[11].However, in recent years, assessment of EFI at the bedside was driven by clinician opinion, which is still subjective to some extent.This may result in misjudgment of EFI occurrence and have an adverse effect on nutrition delivery and clinical recovery.
A clinical prediction model (CPM) is built upon the use of mathematical formulas to estimate the probability that a particular individual will have a disease or an outcome in the future[12,13].CPM can assist clinicians in decision-making and developing therapy programs in complex clinical situations and may help patients have better outcomes.Many studies have identified variables associated with EFI,such as diabetes, abdominal surgery, and head injury.This study aimed to analyze different risk factors for EFI occurrence in the ICU and to construct a CPM that would screen high-risk ICU patients to implement early prevention and intervention methods.
MATERlALS AND METHODS
Study design, setting, and participants
A prospective cohort study was conducted with patients in the ICU at a college hospital, which is a general teaching hospital with 116 ICU beds at the northern and southern campuses.This study was performed in three of the five ICU departments, which included comprehensive ICU, emergency ICU,and neurosurgery ICU.This study was performed between November 2020 and May 2021.
Patients in the ICU were included in the study when EN was started.Patients who received EN for less than 24 h were not included in the model-construction dataset.Eligible patients received the standard nutrition protocol on medical advice (continuous infusionvianutrition pump at rates between 20 mL/h to 150 mL/h).Depending on the patient’s condition, different feeding tubes and formulas were chosen.Exclusion criteria included the following: (1) Age < 18 years; (2) Oral intake; (3) Pregnancy or breastfeeding; (4) Occlusive ileus; and (5) Informed consent not obtained from the patient or their next of kin.
Variables
Outcome measure:According to the results of the literature review and discussion with experts, the primary outcome was patients diagnosed with EFI, including GI symptoms and reduction or suspension of EN.A patient was diagnosed with EFI if one or more listed GI symptoms occurred and resulted in the reduction or suspension of EN within 2 wk of starting EN[7,14].When patients had several symptoms, one symptom was determined to be the main symptom rather than recording several duplicate symptoms.
GI symptoms included the following: (1) Moderate gastric residual volume (defined as GRV, reaching 200 mL)[7,15,16].Ultrasonography was adopted once a day 4 h after completion of EN using the following formula: GRV = 27.0 + 14.6 × gastric antral cross-sectional area - 1.28 × age, where gastric antral cross-sectional area = (anteroposterior diameter × craniocaudal diameter × Ⅱ)/4[17]; (2) Diarrhea,which was defined as having three or more loose or liquid stools within 24 h with a stool weight greater than 200–250 g/day (estimated by assistant nurses)[18]; (3) Vomiting, which was defined as the expulsion of gastric contents from the oropharynx or nasopharynx one or more times a day[19]; (4)Aspiration, which referred to the entry of oropharyngeal food, secretions, or gastroesophageal reflux into the subglottic airway[20]; (5) Regurgitation, which referred to the reflux of gastric contents into the oropharynx without nausea, retching, or straining[21]; (6) Constipation, which was considered a reduction in the frequency of defecation to less than three times a week and difficulty defecating or dry stools[22]; and (7) Abdominal distention, which was considered an uncomfortable feeling of fullness and distension of the abdomen, and abdominal ultrasound showed gas or dilation of the bowel[21].
Predictor selection:We searched databases and consulted with medical experts in GI surgery and critical care medicine (see Supplementary Tables 1 and 2, which demonstrates the literature screening and factor coding results).Eligible studies had a primary endpoint of EFI occurring when diagnosed with GI symptoms.After expert group discussion, the predictor of proton-pump inhibitor use was excluded.The following 14 predictors were selected: age[23-29]; trauma (including blunt trauma,penetrating trauma, and burns)[30-32]; head injury (including postoperative neurosurgery and brain trauma)[33]; sepsis[34]; abdominal surgery[23,31,32]; GI disease (including GI surgery, GI inflammation,etc.)[11,23,28]; blood glucose[35,36]; serum albumin (hypoproteinemia or abnormal content level of albumin)[37]; electrolyte disorders (abnormal content level of K, Na, Cl, Mg, Ca, and P)[38]; mechanical ventilation (had or having mechanical ventilation)[5,23,26]; sedative and analgesic medicine (fentanyl,dexmedetomidine, propofol, and so on)[39]; catecholamine medicine (epinephrine, norepinephrine, and dopamine)[40,41]; early feeding (feeding initiated within 48 h after admission to the ICU)[40]; and tube feeding protocol (feeding formulas, largest feeding speeds, and largest total volume).
Data sources/measurement
A structured form was prospectively used to obtain baseline data for the enrolled patients.When a patient began to receive EN, the nurses responsible for that patient recorded EN and GI symptoms daily.Doctors measured ultrasonographic results daily using a Doppler ultrasound diagnostic apparatus (GE Venue; GE Healthcare, Chicago, IL, United States).The follow-up endpoint was: (1) A diagnosis of EFI; (2) EN for more than 2 wk; (3) Transfer out of the ICU (including to home, to another hospital, and to another department in the hospital); (4) Gastric tube removal; or (5) Death.
Study size
Fourteen predictors were identified based on a literature analysis and expert consultation.The sample size of the case group was calculated to be 10 times greater than the predictors.Considering a 10% dropoff rate, we planned to include at least 155 patients.
Statistical analyses
We searched for predictors of EFI that were repeatedly reported in studies or systematic reviews and could be easily ascertained in different settings by those with various clinical experience.These data were recorded by researchers for many days in the cohort and checked by 2 people.
Data analyses were conducted using IBM SPSS Statistics (version 25.0.Armonk, NY: IBM Corp) and R software (version 4.0.3; R Core Team).Descriptive data, including mean and standard deviation,frequency, percentage, median, and quartile, were used for the univariate analysis.When univariate analysis showed that independent variables were associated with intolerance (P< 0.15), they were included in the multiple logistic regression model.Variables were entered into the logistic regressionanalysis, and we used the stepwise approach to perform the multivariable selection.Finally, we displayed the model using a nomogram because this format is more convenient.
Internal validation was performed using bootstrap validation.We assessed the predictive accuracy of the prognostic instrument with discrimination and calibration.Discrimination was calculated using the area under the curve, ranging from 0.5 (no discrimination) to 1.0 (perfect discrimination).Calibration was assessed using a calibration plot.
RESULTS
Participants
The three ICUs had 74 beds, and 684 patients were treated in the three ICUs during the study period.The cohort included 203 EN participants for the final analysis, including 153 patients from the comprehensive ICU, 34 patients from the neurosurgery ICU, and 16 patients from the emergency ICU.Overall,EFI occurred in 37.93% of ICU patients.The baseline characteristics of the enrolled patients are shown in Table 1.
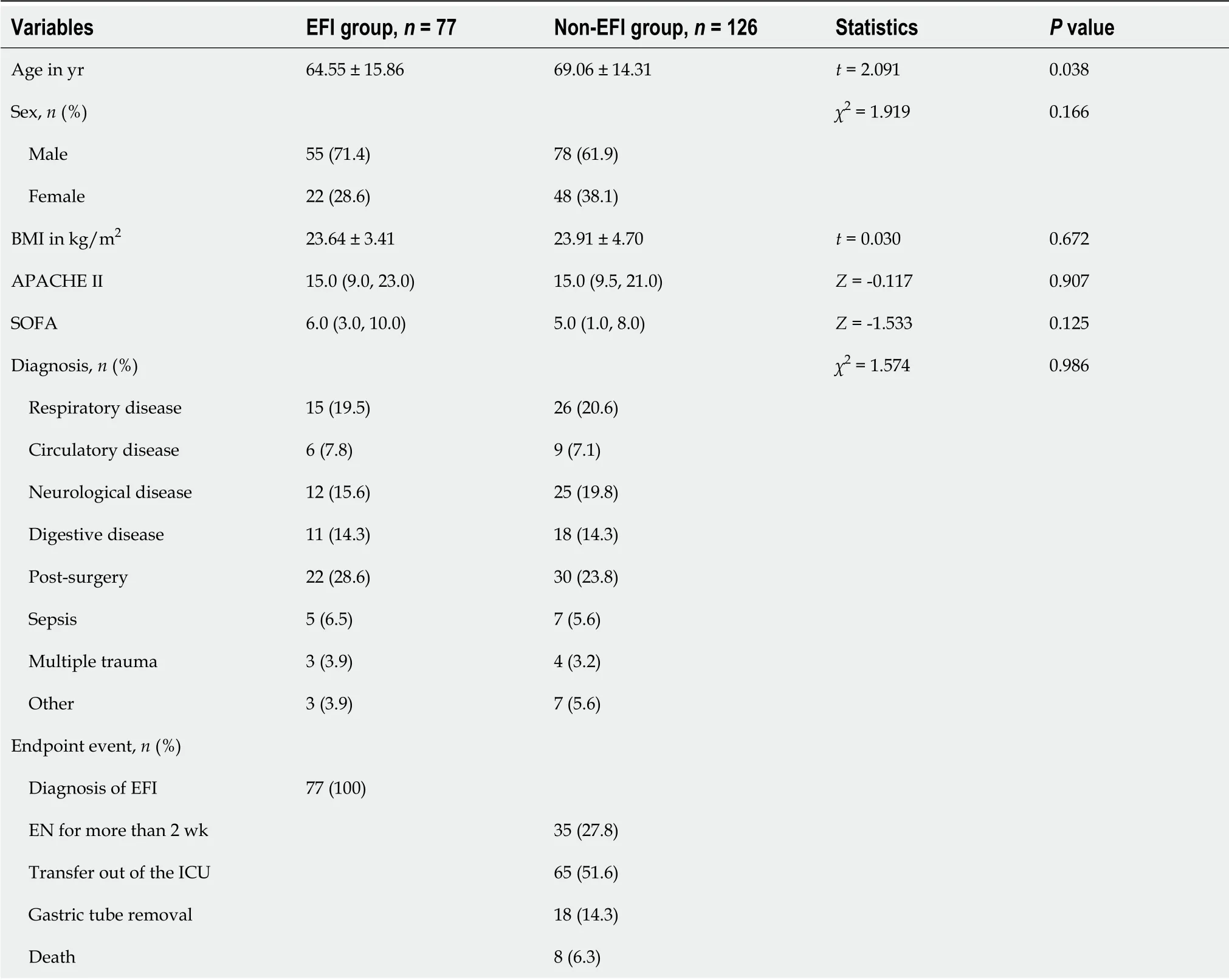
Table 1 Baseline characteristics of the participants included in the analysis
EFI occurrence
A total of 77 patients were included in the case group.EFI occurred more often in the first 7 d after EN started, and more than 90% of EFI cases lasted less than 3 d.Diarrhea, distention, and regurgitation were the most common GI symptoms among patients with EFI.The EFI occurrence in the case group is shown in Table 2.
Selected factors for the model
Univariate analysis of the cohort (Table 3) identified an association between EFI and seven predictors that have been consistently reported in the literature; these include age, GI disease, medical history of mechanical ventilation, mechanical ventilation occupied, sedatives, early feeding, and feeding formula.Four novel potential predictors were also identified, including abnormal serum sodium and serum phosphorus before EN was started and abnormal serum sodium and serum chlorine when EN was started.These variables were entered into a multivariate model.Sepsis was also included in the model because clinical experts strongly recommended it.
Model fitting
We applied the stepwise approach to perform multivariable selection, and five variables were included for the final analysis.Age, GI disease, and early feeding decreased the risk of EFI in the ICU.Mechanical ventilation started before EN and abnormal serum sodium when EN was started increased the risk of EFI in the ICU.We fitted the model using the final variables to obtain the final CPM (Table 4).
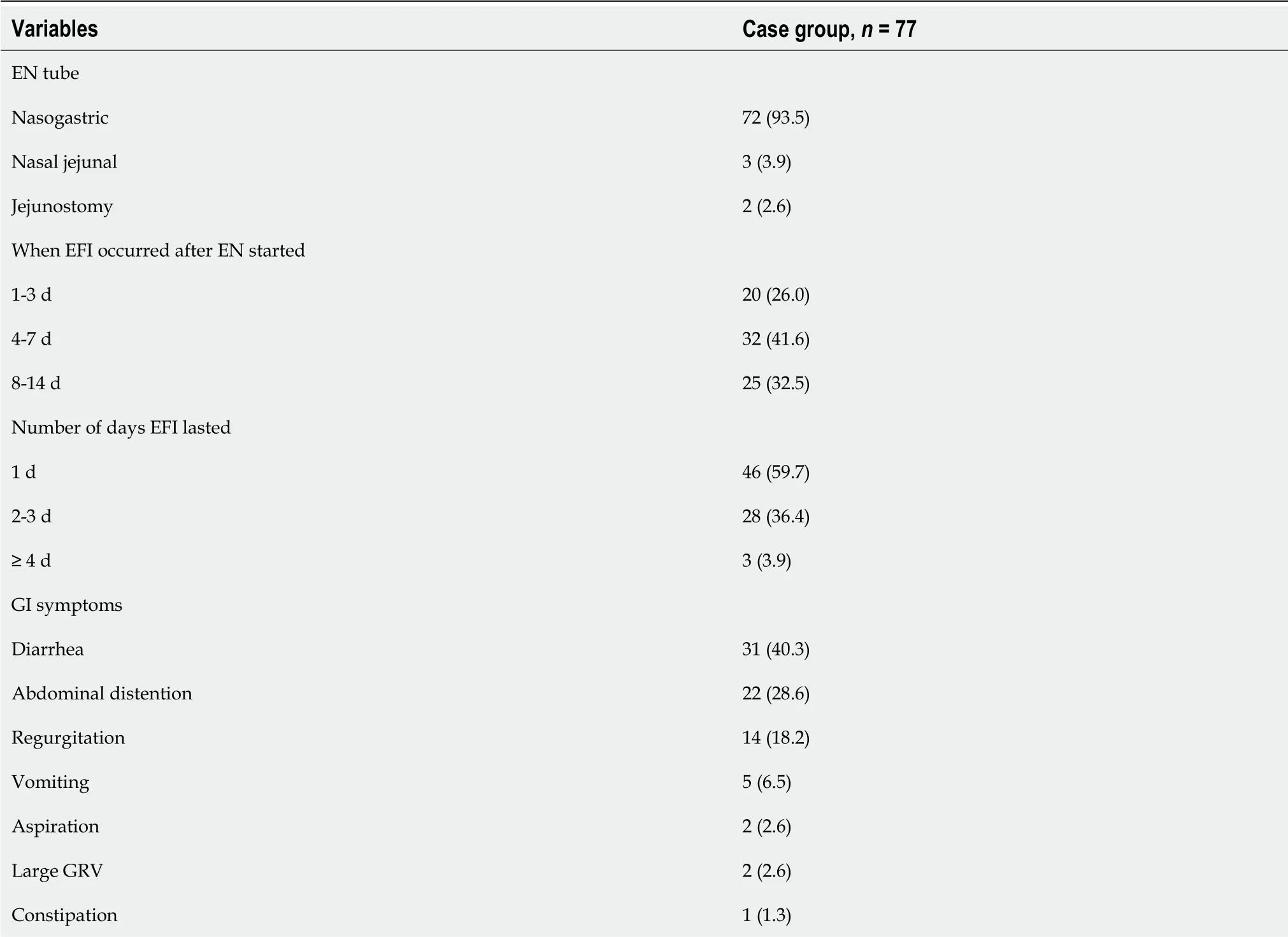
Table 2 Enteral feeding intolerance occurrence in the case group, n (%)
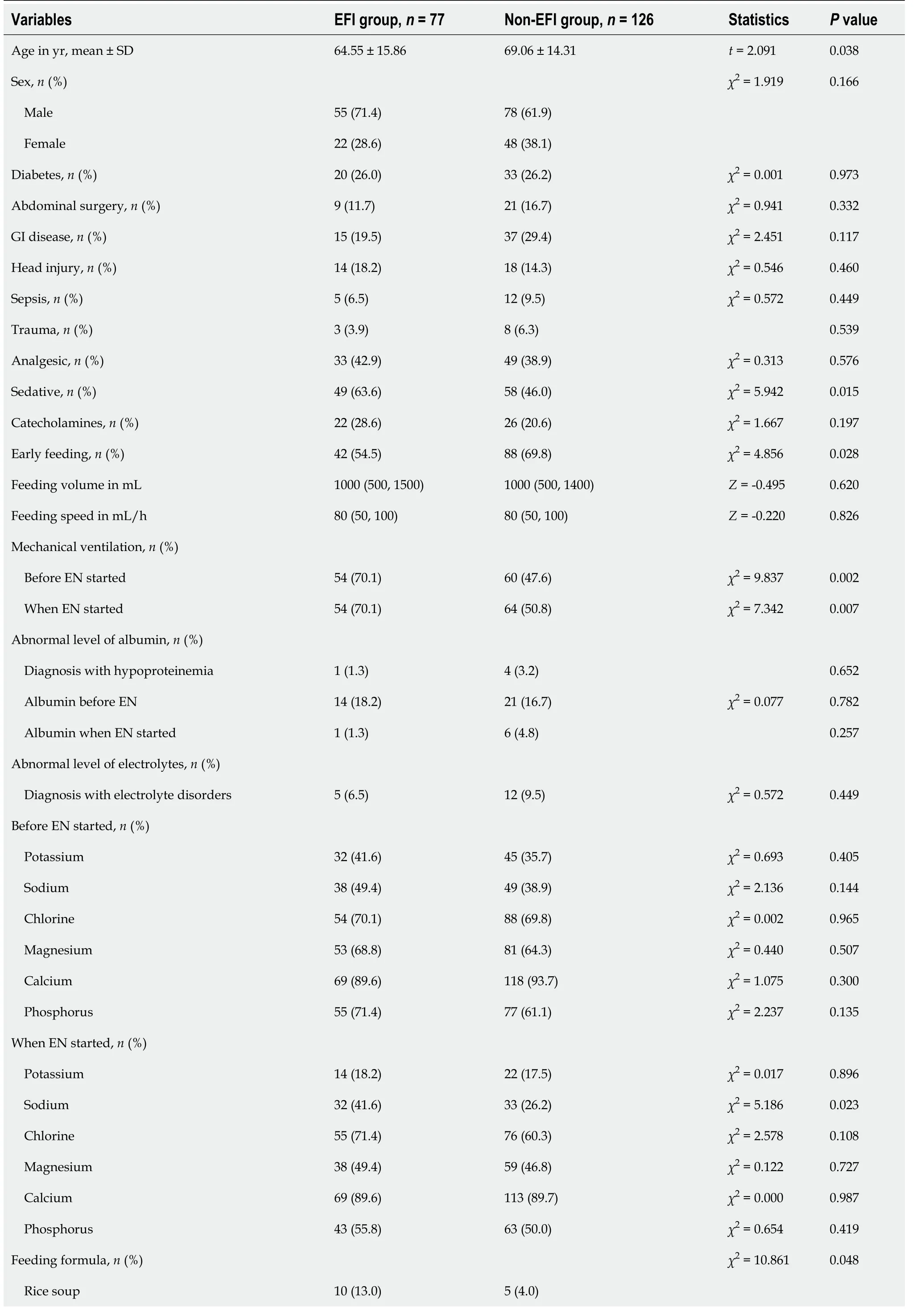
Table 3 Univariate analysis of predictors associated with enteral feeding intolerance

Table 4 Final clinical prediction model of enteral feeding intolerance in the intensive care unit
Predictive nomogram for the probability of EFI
The nomogram illustrated the strength of the association of the predictors with the outcome (Figure 1).The “0” indicated “NO” (i.e.the patient had no history of GI disease, did not receive mechanical ventilation before EN, did not receive early feeding, and/or had no abnormal serum sodium when EN was started), and the “1” indicated “YES” (i.e.the patient had a history of GI disease, received mechanical ventilation before EN, received early feeding, and/or had abnormal serum sodium when EN was started).The variable of “age” was a continuous variable.On the point scale axis, each variable was given a point based on the value.A total score could be easily calculated by adding every single point.By projecting the total points to the lower total point scale, we were able to estimate the probability of EFI.According to statistical standards, if 1 patient’s predictive probability was more than 0.5, then there was a higher possibility that EFI will occur.
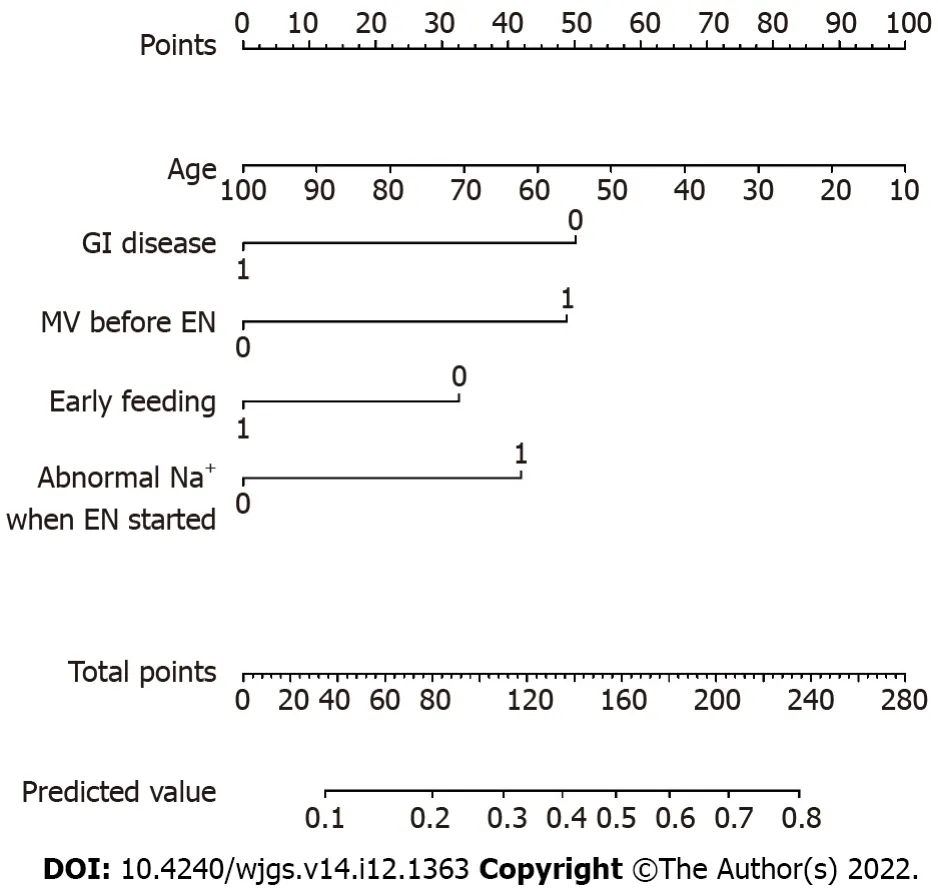
Figure 1 Nomogram for predicting enteral feeding intolerance in intensive care unit patients.0: No; 1: Yes.EN: Enteral nutrition; GI:Gastrointestinal; MV: Mechanical ventilation.
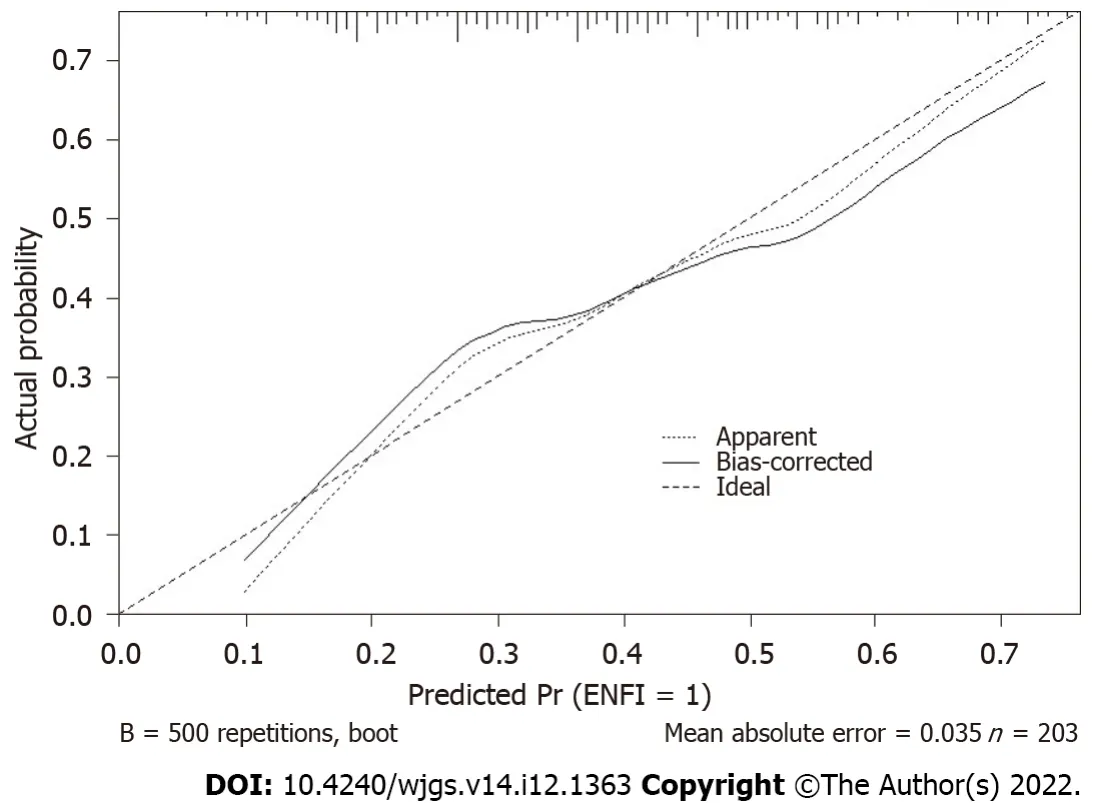
Figure 2 Calibration curve of the prediction model performance during internal validation.
Performance of the nomogram
We did the bootstrap validation, and model performance showed an area under the curve of 0.70.The calibration curve of the model’s performance is demonstrated in Figure 2.
DlSCUSSlON
We developed a novel practical prognostic instrument for predicting the risk of EFI in the ICU that may support clinicians when making treatment recommendations for patients receiving EN.Development of the model followed established recommendations.We identified three protective predictors, namely age, GI disease, and early feeding.Moreover, two risk factors were determined, namely mechanical ventilation before EN started and abnormal serum sodium.The internally validated area under the curve was 0.70 for the model to predict EFI outcomes.
We developed the CPM using an assembled population from three different ICU departments at one center.We made every effort to enroll patients with different diseases.Therefore, our model could apply to most situations in the ICU.To control for potential bias, the data of every patient were divided into three parts.The basic information was recorded by a researcher, the daily EN data were recordedby clinical nurses, and the ultrasonographic data were recorded by ICU doctors trained in performing ultrasonography.The researcher was unable to obtain the other data before the follow-up ended.In addition, we utilized the quantitative method of content analysis to guarantee the scientific rationality of our study.
1TPF-D: Enteral Nutritional Emulsion “RuiDai,” suitable for diabetics.2TPF-T: Enteral Nutritional Emulsion “RuiNeng”, rich in fat and calorie.EN: Enteral nutrition; EFI: Enteral feeding intolerance; GI: Gastrointestinal.
Alternative predictors were found from the literature and clinical experts.When we performed univariate analysis, we included predictors withPvalues smaller than 0.15 with the aim that no possible significant factors were omitted.We determined the potential effective predictors based on thePvalue and by considering those predictors recommended by experts or that were highly suspected.These predictors were well-defined, easily measured, and routinely available.In internal validation, we used the bootstrap validation to assess discrimination and calibration and repeated the validation 500 times for accuracy.
In our study, we found that older patients were less likely to develop EFI.This result is similar to the results of existing studies[28,29] but contrary to conventional wisdom.After a literature review, expert consultation, and clinical observation, we identified some reasons that explain this counterintuitive result.Older patients are given less EN because of their energy requirements and physical condition.In our study, patients aged 60 years or older received on average less EN per day than younger patients[900 (500, 1200)vs1000 (500, 1500), respectively].Critically ill elderly patients have many chronic diseases, such as diabetes, chronic gastroenteritis, and hepatic dysfunction.To promote GI motility and regulate water balance, nutrition teams often use water or rice soup as the initial nutrition for the elderly.Water or rice soup is used for a period of time to facilitate a later transition to an EN emulsion,which may reduce stimulation of the GI tract in elderly patients[42].The direct relationship between age and EFI requires further experimental analysis to completely understand the relationship.
Similarly, we found that ICU patients with GI disease (e.g., pancreatitis, post-gastrectomy, or upper GI hemorrhage) were less likely to experience EFI.In our study, patients with GI disease were likely given less feed to avoid worsening their health issues [patients with GI disease: 575 (275, 975)vspatients without GI disease: 1000 (725, 1500),P< 0.000].In clinical practice, the intention of a small volume of EN is not to meet energy requirements but to maintain the structural and functional integrity of the GI tract[43].Therefore, GI symptoms may be slight and difficult to observe in this circumstance.In addition,medical interventions (e.g., metoclopramide, probiotics, acupuncture, and enema) are administered to patients diagnosed with GI disease[44-46].This advance treatment may lead to a decreased occurrence of EFI.
A previous attempt to develop a prediction model yielded promising results but had limited applicability because its target population was patients with gastrectomy for gastric cancer rather than ICU patients[47].Some preventive measures have been implemented to reduce EFI occurrence in ICU patients, such as fat-modified enteral formula and bolus enteral feeding methods[48-50].However, there is a gap in the knowledge of distinguishing patients at high risk of EFI.Medical workers can apply our model when it is recommended that a patient receives EN.By analyzing the conditions between theperiod of being admitted to the ICU and receiving EN, patients at high risk are determined and are given a set of preventive measures, which is an effective measure for reducing the occurrence of EFI.Notably, experienced clinical workers already have some knowledge of which patients will be high risk for EFI and have put protective measures into clinical practice.Based on the current nutritional management practices in our center, the predictive model should be used knowing that high-risk patients may have already received preventive measures.
There are potential limitations to our study.Because of time and manpower, we developed the model using a small sample size in a single center.The effect of sepsis, trauma, electrolytes could not be properly addressed because of the small sample size.The differences between these factors between the two groups may be overlooked.Due to the actual situation, the effect of various formula feeds could not be ascertained because of use of several feeding formulas.In addition, the representativeness and predictive performance of our model may have limitations.However, this limit may be slight because the final model includes only five variables.Moreover, the delivery strategy of intermittent or continuous feeding and the temperature of the nutrient solution contribute to EFI occurrence[51].Our study did not consider these effects because all included patients received room temperature continuous feeding in our medical center.During the study, there may have been some confounding factors that we did not consider, including etiology, medications, and fluids.For future research, these factors should be considered, and we suggest external validation in different centers over additional time periods.In the future, we hope to be able to analyze the effect of individual factors on EFI on the basis of expanding the sample size.In addition, applying our prediction model to additional interventional studies as a tool to optimize clinical management is a long-term goal.
CONCLUSlON
We have developed and internally validated a CPM for predicting the risk of EFI in patients receiving EN in the ICU.The developed nomogram is easy to use and might help clinicians make individualized predictions of each patient’s probability of experiencing EFI.Early identification of patients at high risk of EFI can greatly help doctors and nurses better manage clinical care.Clinical nurses can implement different nursing measures according to each patient’s risk.These measures will ultimately help ICU patients achieve better nutritional support and a quicker recovery.
ARTlCLE HlGHLlGHTS
Research background
Enteral nutrition (EN) is essential for critically ill patients, but some patients develop enteral feeding intolerance (EFI).Intolerance can hinder a patient’s energy intake and recovery.Therefore, predicting EFI is of vital importance in clinical practice.
Research motivation
Determining which patients are at high risk of developing EFI based on their current physical condition and medical treatment will allow physicians and nurses to individualize medical care and begin EFI preventative measures for the high-risk patients.
Research objectives
To develop a clinical prediction model (CPM) to predict the risk of EFI in patients receiving EN in the intensive care unit (ICU).We currently know that many factors can influence the development of EFI.
Research methods
A prospective cohort study was performed, and we prospectively recorded enrolled patients’ data.Prospective cohort studies can more realistically document patient data and clinical responses, reducing human intervention.We used ultrasound measurement of the antrum cross-sectional area to measure gastric residual volume, which can effectively reduce the occurrence of complications and increase the efficiency of feeding.
Research results
We developed and internally validated a CPM for predicting the risk of EFI in patients receiving EN in the ICU.After univariate and multivariate analyses, five factors were used for the CPM, including age,gastrointestinal disease, early feeding, mechanical ventilation before EN started, and abnormal serum sodium when EN started.
Research conclusions
This model can help clinical workers to identify patients at high risk for EFI earlier, which will allow these patients to receive preventative measures in advance.
Research perspectives
In the future, an increased sample size and analyzing more variables will develop a more accurate clinical predictive model.Prospective cohort studies and randomized control studies are the best methods for the future research.
ACKNOWLEDGEMENTS
We thank the patients whose anonymized data were used for this research.We acknowledge all collaborators in this study and all clinical workers in the intensive care units.We thank the nurses responsible for helping us collect data every day.We thank Professor Lu and Professor Fang for their guidance on the research project.We thank Mrs.Kang, the statistician, for her help in the data analysis stage.
FOOTNOTES
Author contributions:Lu XM contributed to conceptualization, methodology, formal analysis, investigation, data curation, writing the original draft, and project administration; Jia DS contributed to conceptualization, methodology,investigation, and writing the original draft; Wang R and Yang Q contributed to methodology, investigation, and resources; Jin SS contributed to investigation and resources; Chen L contributed to conceptualization, methodology,resources, review and editing of the manuscript, supervision, and project administration; All authors read and approved the final manuscript.
lnstitutional review board statement:The study protocol was approved (numbered 2020KY230) by the appropriate ethics committee (Medical Ethics Committee of Shanghai General Hospital) on December 23, 2020.
lnformed consent statement:Before we enrolled patients, informed consent was obtained from the patient or next of kin.Since we needed to complete daily ultrasonography of patients, we needed to obtain their informed consent.
Conflict-of-interest statement:The authors declare having no conflicts of interest.
Data sharing statement:If there is a need to get the dataset, please contact Xue-Mei Lu (lu_xm1118@163.com).The information of the patients in the dataset is anonymized.
STROBE statement:The authors have read the STROBE Statement–checklist of items, and the manuscript was prepared and revised according to the STROBE Statement–checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Lan Chen 0000-0002-4309-0240.
S-Editor:Zhang H
L-Editor:A
P-Editor:Zhang H
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Timing of individualized surgical intervention in Crohn’s disease
- Acute appendicitis in the short term following radical total gastrectomy misdiagnosed as duodenal stump leakage: A case report
- Malignant transformation of perianal tailgut cyst: A case report
- Rectal tubular adenoma with submucosal pseudoinvasion misdiagnosed as adenocarcinoma: A case report
- Secondary sclerosing cholangitis in a young COVlD-19 patient resulting in death: A case report
- Meta-analysis of transanal vs laparoscopic total mesorectal excision of low rectal cancer: lmportance of appropriate patient selection