Unmasking lower gastrointestinal bleeding under administration of norepinephrine
2023-01-05DavidJohnWernerNicolaiWenzelNaelAbusalimRalfKiesslichTillBaarAchimTreschJohannesWilhelmRey
David John Werner, Nicolai Wenzel, Nael Abusalim, Ralf Kiesslich, Till Baar, Achim Tresch, Johannes Wilhelm Rey
Abstract BACKGROUND Bleeding in the gastrointestinal tract is common and transarterial embolization enables the clinician to control gastrointestinal bleeding. Contrast extravasation is a prerequisite for successful embolization. Provocative angiography is helpful in the detection of elusive bleeding.AIM We performed a retrospective analysis of angiographic treatment in patients with lower gastrointestinal hemorrhage and initially negative angiographies, as well as the role of norepinephrine (NE) in unmasking bleeding.METHODS We analyzed 41 patients with lower gastrointestinal bleeding after angiography who had undergone treatment over a period of 10 years. All patients had a positive shock index and needed intensive care.RESULTS In three of four patients, angiography disclosed the site of bleeding when NE was used during the procedure for hemodynamic stabilization.CONCLUSION We suggest that angiography performed after the administration of NE in unstable patients with gastrointestinal bleeding and an initially negative angiography has the potential to unmask bleeding sites for successful embolization. However, this statement must be confirmed in prospective studies.
Key Words: Lower gastrointestinal bleeding; Endoscopy; Provocative angiography; Norepinephrine;Radiology; Gastrointestinal bleeding
INTRODUCTION
Bleeding in the gastrointestinal tract may occur at various sites and is frequently encountered in the preclinical and in-hospital emergency setting. Gastrointestinal endoscopy is currently the undisputed method of choice for achieving hemostasis at the bleeding site in the gastrointestinal tract[1]. Bleeding occurs in the upper gastrointestinal tract four to five times more frequently than it does in the lower gastrointestinal tract (LGIB)[2]. The estimated incidence of LGIB is 25per100000 adultsperyear[3]. LGIB is strongly dependent on aging; a 200-fold increase was observed between the third and the ninth decade of life[4]. Diverticular bleeding is the most common cause of LGIB. Endoscopy reveals the bleeding site in a mere 20%-30% of cases. Some patients require surgical or angiographic treatment.Although endoscopic hemostasis is effective and the treatment of choice for LGIB, the optimal technique remains to be determined[5,6]. A bleeding site may not be evident in some patients despite endoscopic evaluation. In fact, the bleeding may cease and thus make it difficult to identify the site.
Provocative angiography with vasodilators, anticoagulants or thrombolytics has been reported to help in the detection of elusive bleeding[7-11]. Bloomfeldet al[8,12] identified bleeding in 37.5% of their patients using intra-arterial urokinase, tolazoline, and heparin during angiography procedures. In a retrospective analysis of 34 patients, provocative mesenteric angiography using systemic anticoagulation with heparin and selective transcatheter injection of a vasodilator disclosed the bleeding site in 31% of cases. Ten patients received embolization[9]. In a recently published case series of pharmacologic provocation combined with endoscopy, the source of bleeding was discovered in 15 of 27 patients with a regimen of antiplatelet/anticoagulant medication[13]. In 2020, Kokoroskoset al[14] described a regimen for stepwise provocation of bleeding with anticoagulants, vasodilators and thrombolytic agents. Twenty-three patients were included in the study; provocation was successful in seven patients,and four of the seven patients were successfully treated by interventional radiological procedures. No complications occurred. The role of vasopressors, such as norepinephrine (NE) in hemorrhagic shock has been reported elsewhere and is well-documented[15-17].
We performed a retrospective analysis of angiographic management of LGIB. In a small number of patients, hemodynamic stabilization with NE disclosed LGIB which had escaped detection until this time. We suspect that, after administering NE, occult bleeding and the concomitant short-term increase in systolic blood pressure become visible on angiography. We retrospectively identified four patients with LGIB who needed NE in the intensive care setting and whose initial angiography had been negative. To the best of our knowledge, provocative challenges with intravenous NE in unstable patients with LGIB have not been reported so far.
Future prospective studies should address the usefulness of inducing blood pressure peaks in patients on NE treatment during angiography in order to disclose occult gastrointestinal bleeding. It may be assumed that this method, when used in eligible patients, may disclose bleeding successfully and help to achieve hemostasis by angiography. However, this assumption should be confirmed in prospective randomized multicenter studies.
MATERIALS AND METHODS
All patients who had undergone catheter angiography for gastrointestinal bleeding at our maximumcare hospital between 1 January, 2007 and 31 March, 2018 were included in the study[6]. Predictors of complicated angiographic treatment and the role of specific laboratory parameters and scores in predicting the likelihood of a successful intervention were identified. The study protocol conformed to the 1975 Declaration of Helsinki and was approved by the ethics committee of the Regional Medical Society of Hessen (Landesärztekammer Hessen), approval number FF 95/2017, on 31 August 2017.Written informed consent was obtained from each patient. In three of 41 patients, intravenous administration of NE was followed by contrast media extravasation, although the patients had experienced no extravasation during the initial procedure. In one patient, intravenous administration of NE did not disclose the bleeding. The maximal individual dose of NE was 10 µg. A specific time interval was not applied. For the accompanying anesthetists, the application of NE depended on their observations during monitoring and achieving a mean pressure higher than 60 mmHg. The maximum doseperpatient during an angiography was 40 µg.
From our previous retrospective analysis, we identified a new subgroup of patients with the following common features: LGIB, intensive care, the need for NE, intra-arterial blood pressure measurement, and an intravenous NE bolus of 10-40 μg during angiography (Table 1).
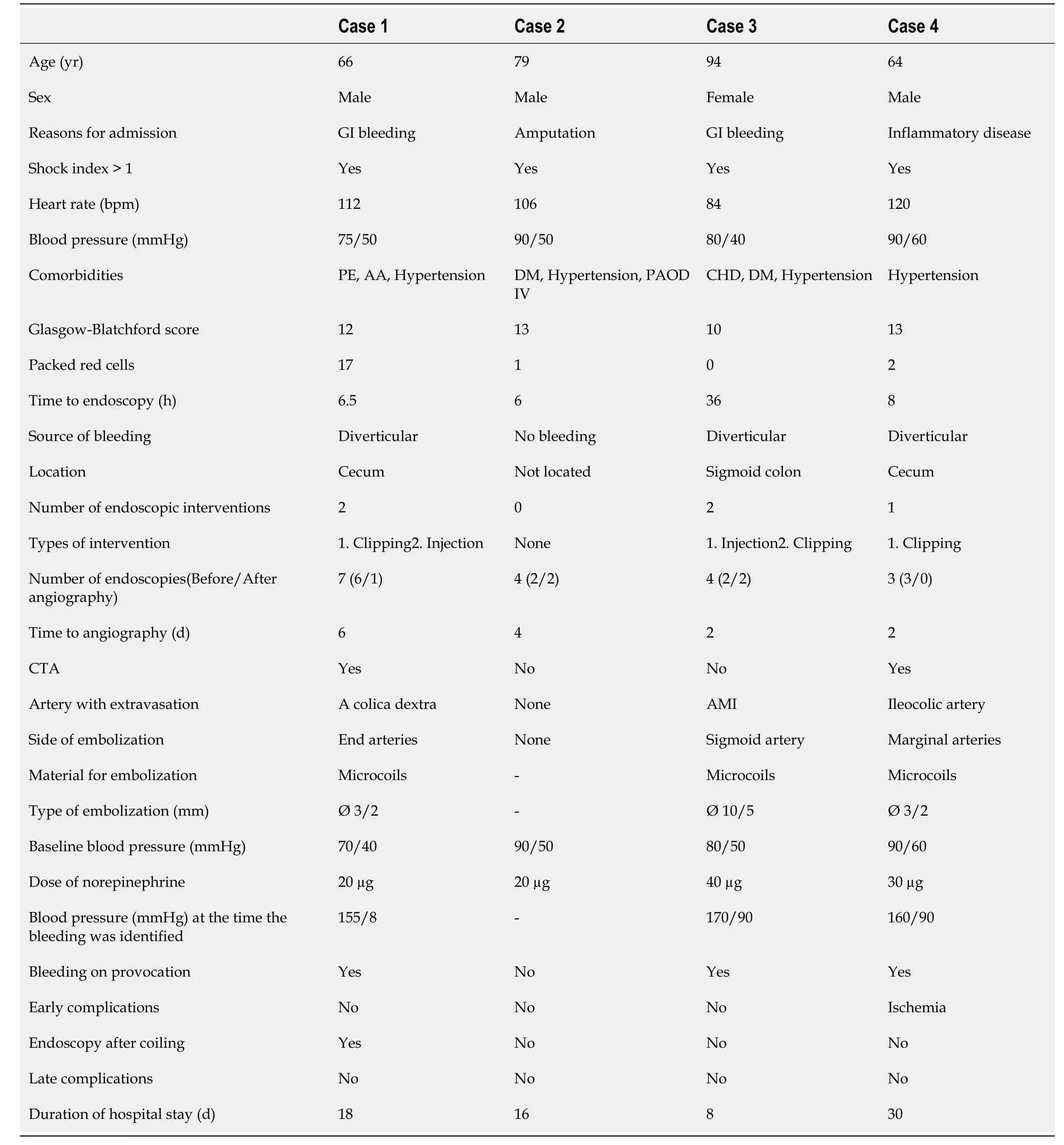
Table 1 Clinical, endoscopic and angiographic characteristics of patients
RESULTS
In a retrospective analysis, we studied 41 consecutive patients with LGIB between 2007 and 2018.Twenty-six of 41 patients had diverticular bleeding. The primary endoscopy failed to achieve hemostasis in any patient (Figure 1). Sixteen of 41 patients underwent pre-interventional computed tomography (CT). The mean systolic blood pressure on the day of angiography was 104 mmHg, and the mean shock index was 0.91 (heart rateperminute/systolic blood pressure). Twenty-five of 41 patients were monitored by an anesthetist. Of these patients, 16 were intubated. Angiography demonstrated the bleeding in 18 of 41 patients, and 20 patients underwent embolization (2 prophylactic embolizations).The angiography was technically successful in 16/18 cases, and clinically successful in 10/18 cases[6].
Patients who needed catecholamines were documented separately. Three patients presented with severe hematochezia and signs of hemorrhagic shock, and a further patient with chronic sigmoid diverticulitis. Only one patient was female. The mean age was 75.8 years. All patients had a positive shock index, and all had at least one notable comorbid condition. The mean Glasgow-Blatchford bleeding score was 10 or higher in all cases (mean score 12). The mean interval from the time of hospitalization and the endoscopic investigation was 14.1 h. All patients received an emergency colonoscopy.Three of four colonoscopies revealed the stigmata of hemorrhage with no evidence of manageable bleeding. Only one case of diverticular bleeding was classified as active bleeding that could not be managed by endoscopy, and was directly treated by radiological procedures. Two of four patients underwent pre-interventional CT. In patient 1 we performed a triphasic CT scan and found no contrast medium extravasation on the CT, but did observe signs of previous hemorrhage in the colon. Patient 4 also underwent a triphasic CT scan and revealed contrast medium extravasation in the lower gastrointestinal tract as evidence of active bleeding.
The mean period of time until angiography was 3.5 d. The primary angiography failed to reveal active contrast media extravasation in any patient. In three of four cases (75%), the bleeding was seen after the application of noradrenaline for cardiovascular stabilization (Figures 2 and 3). In all cases,microcoils (Tornado®Embolization Coil, Cook Medical) were used for embolization. Superselective embolization failed in one patient; multiple feeding vessels were embolized using a front-door and back-door technique, which resulted in confirmed hemostasis. It should be noted that extended dearterialization caused mesenteric ischemia and necessitated a right-sided hemicolectomy the following day.The mean duration of the hospital stay for patients in the NE population was 18 d.
DISCUSSION
The fact that NE is able to unmask LGIB in unstable patients has not been reported so far in the published literature. The pharmacological basis is alpha1-induced global vasoconstriction with an increase in peripheral vascular resistance and a resulting increase in systolic and mean arterial blood pressure, provoking a morphologically visible contrast media extravasation.
Our case series must be viewed in the context of other scientific investigations on bleeding site provocation in patients with GIB, using established endoscopic or angiographic procedures[6].Permissive hypotension under sedation is a common phenomenon in patients with GIB and hemorrhagic shock. These conditions are usually treated effectively by intravenous volume substitution and intravenous NE. In our analysis, this treatment disclosed the source of bleeding in patient 1.
The bleeding site is rarely detected by endoscopy, especially in patients with LGIB[18]. Previously,bleeding provocation procedures were usually performed in conjunction with an intervention in the coagulation system[8,9,11,13]. Although safe and uncomplicated provocation has been reported in all publications, we believe that the use of an anticoagulant as provocation in patients with GIB is a critical measure and is associated with a potential risk of bleeding complications.
All patients were in a state of hemorrhagic shock and had to be treated in an intensive care unit.Blood pressure was measured invasively. The shock index was elevated in 3/4 patients. A previously undetectable extravasation appeared in three of four cases after the administration of a cumulative dose of NE. Two of three patients had no complications after embolization. Nevertheless, the use of NE requires thorough knowledge of its pharmacological properties, especially in unstable patients. NE has a number of potential side effects, such as dizziness, headache, angina, ischemia, necrosis, and vasospasm[17]. Vasospasms occurred in one of our cases (Figure 3).
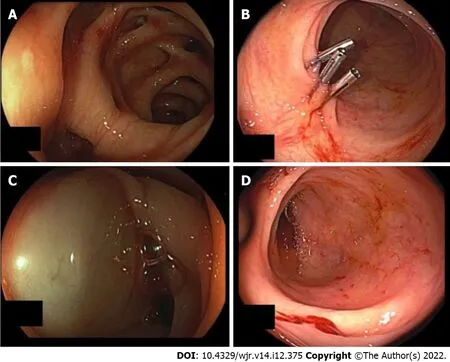
Figure 1 Urgent colonoscopy in case 3. A: Revealed diverticular bleeding in the sigmoid colon; B: The endoscopic procedure was performed with three hemoclips; no further signs of bleeding; C: Injection therapy was used; D: Diverticular bleeding of the right colon was detected in case 4.
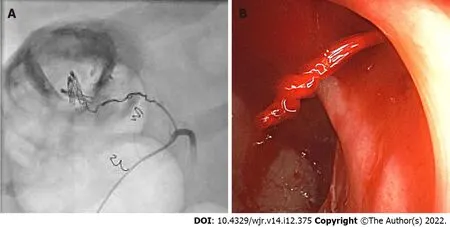
Figure 2 Final colonoscopy in case 1, which revealed a spurt of bleeding at the right colon during norepinephrine treatment of hemorrhagic shock. A: The bleeding site was clip-marked before the patient was transferred for angiographic treatment; B: Superselective view of the right colic artery after norepinephrine application and coiling (the cumulative dose of norepinephrine was 20 µg).
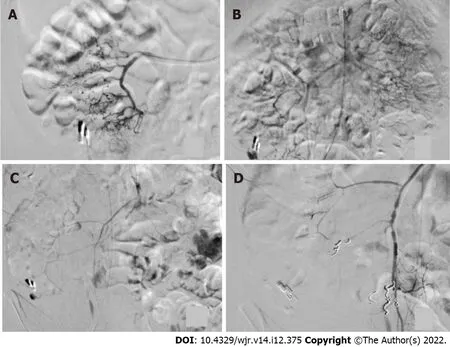
Figure 3 Angiographic procedure in case 4. A: The ileocolic artery after application of 10 µg of norepinephrine is shown; no sign of extravasation; B:Selective view of the superior mesenteric artery, showing bleeding next to the hemoclip after repeated administration of a cumulative dose of 20 µg of norepinephrine; C: Arterial vasospasm was observed during an ongoing extravasation in the cecum; D: Following extensive embolization, no further bleeding was seen after a cumulative dose of 30 µg of norepinephrine.
In view of the high re-bleeding rates reported under NE, its use as a means of provocation calls for further investigation[19]. Other measures, such as the administration of at least three erythrocyte concentrates, may also improve the detection of bleeding[20]. However, transfusions with concentrates are known to cause side effects, and contrast extravasation after transfusion takes longer than it does after the administration of NE. Lianget al[21] used an intra-arterial epinephrine bolus with a small dose of vasopressin to induce vasospasm in the intra-arterial treatment of acute LGIB. The technical success rate in 21 procedures (16 patients) was 100%.
Bowel ischemia is a complication of embolization. In retrospective analyses, 10% of patients who underwent treatment for LGIB required surgical intervention 24 h after the procedure. Long-term survival rates were 70.6% (1 year), 56.6% (3 years) and 50.8% (5 years)[22]. This complication has also been reported in other studies[23-25]. We believe that the case of intestinal ischemia and subsequent hemicolectomy in the present study was not caused by provocation but by unsuccessful superselective embolization. However, this notion cannot be proved conclusively. Bowel ischemia may also have been induced by the administration of NE, which was a clinical necessity.
In view of this ischemic complication, patients with LGIB who have received interventional radiological treatment with provocation must be monitored closely in terms of laboratory parameters as well as follow-up endoscopies, although some authors do not advocate routine colonoscopy after angiography[23]. The need for a standard follow-up colonoscopy and its ideal timing must be investigated further.
We are aware of the crucial importance of patients being monitored by qualified intensive care specialists, including the administration of fluids, as well as the need to monitor other organ functions such as the kidneys, bowel and extremities by specialists and experienced physicians. In the future it may be meaningful to perform an angiography at the time when NE is administered, in order to identify an accidentally provoked contrast media extravasation. In the present investigation, NE was administered for the purpose of cardiovascular stabilization and the extravasation of contrast medium was a positive additional effect.
Limitations: This was a retrospective investigation and was accompanied by the known difficulties incumbent upon a study of this nature. If further investigations show that the suggested approach might be successful, the outcomes will have to be validated prospectively under controlled conditions.Furthermore, the data were not obtained for the purpose of the study and data collection may have been subject to structural inaccuracies. NE is a highly potent substance and should only be used by experienced clinicians. In one of our cases, the complication of bowel ischemia may have been an effect of NE. Further prospective studies are needed to address this problem. The limitations of the previously published study[6] also apply. Many crucial points such as the fluctuating nature of GIB, the time point of endoscopy, the number of endoscopies to be performed, the role of CT diagnosis, and the optimal time point of angiography have not yet been clearly specified, and must be clarified in future investigations[6].
CONCLUSION
In summary, bleeding was discovered by the administration of NE in three of four patients with LGIB.NE was needed in all cases as a means of cardiovascular support.
Future prospective studies should address the usefulness of inducing blood pressure peaks in patients on NE treatment during angiography in order to disclose occult gastrointestinal bleeding. It may be assumed that this method, when used in eligible patients, may disclose bleeding successfully and help to achieve hemostasis by angiography. However, this assumption should be confirmed in prospective randomized multicenter studies.
ARTICLE HIGHLIGHTS
Research background
Gastrointestinal endoscopy is the undisputed method of choice for achieving hemostasis in the gastrointestinal tract. Provocative angiography has been reported to help in the detection of elusive bleeding.
Research motivation
We discovered contrast extravasation following the application of norepinephrine (NE) in angiographic procedures. Our purpose was to investigate the detection of masked bleeding during NE therapy.
Research objectives
The aim was to describe the procedure for the detection of elusive bleeding under the administration of NE and intensive care therapy.
Research methods
We performed a retrospective analysis of 41 patients with lower gastrointestinal tract bleeding treated by radiological procedures. Four patients received NE during angiography.
Research results
A previously undetected bleed was found in three patients. The bleeding was embolized without complications in two of four patients. In one patient we observed no bleeding after the administration of NE. One patient experienced bowel ischemia and had to be treated surgically.
Research conclusions
Bleeding was discovered in three of four cases. No complications were observed in two of three cases of embolization. Our results suggest that the use of NE may have the potential to improve the angiographic therapy of lower gastrointestinal bleeding in critically ill patients.
Research perspectives
Future studies should be focused on a prospective validation of the procedures described here but with a larger number of patients.
FOOTNOTES
Author contributions:Rey JW and Werner DJ designed the topic and wrote the paper; Wenzel N collected the data and edited the text; Baar T and Tresch A performed data analysis; Kiesslich R performed endoscopy and Abusalim N performed interventional angiography.
Institutional review board statement:The study was reviewed and approved by the Ethik-Komission bei der Landesärztekammer Hessen Institutional Review Board (Approval No. FF95/2017).
Informed consent statement:Patients were not required to give informed consent to the study as the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement:All authors declare that they have no conflicts of interest.
Data sharing statement:If there is a need, you can contact the corresponding author to share data at any time.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non
commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Germany
ORCID number:David John Werner 0000-0003-1828-2969; Nicolai Wenzel 0000-0002-8820-2461; Nael Abusalim 0000-0003-3594-6050; Ralf Kiesslich 0000-0003-0199-6412; Till Baar 0000-0002-6744-1463; Achim Tresch 0000-0002-4146-6371;
Johannes Wilhelm Rey 0000-0001-9905-4444.
S-Editor:Liu GL
L-Editor:Webster JR
P-Editor:Liu GL
