Roles of pH in the NH4+-induced corrosion of AZ31 magnesium alloy in chloride environment
2022-12-30FengGeJiaxuanYinYueLiuWenjunLengXinWangZhongyuCui
Feng Ge,Jiaxuan Yin,Yue Liu,Wenjun Leng,Xin Wang,Zhongyu Cui
School of Materials Science and Engineering,Ocean University of China,Qingdao 266100,China
Abstract Corrosion behavior of AZ31 magnesium alloy in NH4+-bearing chloride solution with controlled pH was investigated by weight loss experiment,hydrogen collection,and electrochemical test combined with surface morphology and topography observation.The results show that NH4+ and pH of the solution can affect the corrosion of AZ31 magnesium alloy.The existence of NH4+ affects the formation and dissolution of corrosion products,which makes the protective effect of the corrosion product layer weaker.The change of pH value not only affects the corrosion mechanism of AZ31,but also alters the concentration of NH4+ ions in the solution,which further influence the corrosion product layer of AZ31,and finall makes the corrosion of AZ31 change significantl.
Keywords: NH4+;pH;AZ31 magnesium alloy;Corrosion.
1.Introduction
In recent years,air pollution is increasing seriously,especially in some industrial places.Several studies show that the oppressive atmosphere of the environmental problem could cause global environmental deterioration,climatic anomaly,and threats to human health [1,2].Besides,researchers found that air pollution also could influenc the corrosion of the metallic materials [3–5].According to previous reports,content of water-soluble ions (like NO3-,SO42-,NH4+,etc.) increases in the atmosphere due to air pollution [6,7].These ions are proved to influenc the metal corrosion in the atmosphere [8–14].The ammonium salt particles formed by the precipitation of NH3become the main component of haze pollution [15,16].For example,Zhang et al.[15] found that NH4+accounted for 17.7% of the total water-soluble ions in Xiamen.Researchers have studied the effects of NH4+on the corrosion of several kinds of materials and demonstrated its deterioration effect [12,17–22].It is worth noting that watersoluble ions become more aggressive on corrosion in the humid air environment.Especially in the marine atmospheric environment,moist air and more Cl-make corrosion easier than anywhere else [23,24].These ions,which dissolve much more into the thin liquid fil on the material surface,could promote the corrosion more distinctly [25–27].
Magnesium alloy,as one of the lightest structural material,is an ideal choice for auto lightweight in the future [13].However,it is difficul to avoid the corrosion of automotive magnesium alloys during service in atmospheric environment.Cui et al.[24] and Li et al.[8,9] studied the atmospheric corrosion of Mg alloys in the coastal and polluted air environments and suggested that the corrosion was promoted in these areas because of the abundant presence of Cl-and SO2,respectively.Acharya and Shetty [10] also pointed out that the corrosion rate of AZ31 increases with the increasing concentration of Cl-and SO4-.The energy barrier for the occurrence of corrosion reaction (Ea) decreased from 54.0 to 16.9kJ/mol as the Cl-concentration is increased from 0.02 to 0.25mol/L.Zeng et al.[28] studied the effect of the ions in saline solution on magnesium alloy corrosion,including HCO3-,SO42-,HPO32-,and H2PO3-and showed that these ions exhibited an impact on the corrosion behavior and mechanism.The fact is that the Mg alloy is easy to be corroded and the corrosion process is also easy to be influence by water-soluble ions and the pH [29–31].
Some researchers investigated the effect of NH4+on the corrosion of magnesium alloy in solution.Battochi et al.[32] found that the corrosion rate of magnesium alloys in dilute Harrison solution (0.05wt.% NaCl+0.35wt.%(NH4)2SO4) was one order higher than that in NaCl(0.1wt.%) solution.Citterio et al.[33] suggested that the NH4+caused the precipitation of Mg(OH)2with dissociation into NH3and H+as local pH increased,which promoted the formation of soluble corrosion products and resulted in the high reactivity of Mg in dilute Harrison solution.Buggio et al.[34] reported that AZ31 magnesium alloy would generate a layer of black corrosion products in the presence of ammonium ions.They declared that the NH4+promoted the formation of magnesium hydride.Cao et al.[35] reported the effect of NH4+on magnesium and demonstrated the accelerated ratio of 30∼100 when the NH4+concentration is increased from 0 to 0.8mol/L as well as the corresponding degradation mechanism.They emphasized the enhanced dissolution of relatively protective MgO by NH4+,which resulted in the acceleration effect on Mg corrosion.
In our previous works [21,22],the corrosion process of magnesium alloy in the NH4+-Cl--NO3-and pure (NH4)2SO4environment was investigated.The results showed that NH4+had a unique effect on the corrosion of magnesium alloy,attributed to the promotion of cathodic reaction and the change of Mg(OH)2.Moreover,the effect of NH4+on the corrosion of magnesium alloy is closely related to the solution pH because of the existence form of NH4+differs with pH.Therefore,in this paper,we designed experiments including the immersion test,hydrogen collection test,polarization curves and surface morphology and topography observation to study the corrosion behavior of magnesium alloy under the various conditions of NH4+and pH values,exploring the effect of pH on the corrosion mechanism of AZ31 in NH4+-containing solution system.
2.Experimental
2.1.Material and solution
The material used in this experiment was the commercial standard AZ31 magnesium alloy plate (3.19wt.% Al,0.81wt.% Zn,0.025wt.% Si,0.006wt.% Fe,0.002wt.% Cu,0.001wt.% Ni,Bal.Mg) with a thickness of 3mm (provided by Shanxi United Magnesium Industry Co.,Ltd).According to the different tests,the samples with different dimensions were prepared by wire cutting.The solutions used in the present work were pure NaCl and NH4Cl with different concentrations (0.001M,0.01M,0.1M) and pH(4.67,7.13,8.85).The NaCl solution was employed to exclude the effect of Cl-in the NH4Cl solution and highlight the special effect of NH4+and pH.To adjust and maintain the solution pH,the buffer solutions including 0.22M CH3COONa+0.3M CH3COOH(for pH 4.67),0.95M H3BO3+0.15M Na2B4O7·10H2O (for pH 7.13) and 0.15M H3BO3+0.05M Na2B4O7·10H2O (for pH 8.85) were used.All the solutions were made with analytical grade reagents and high-quality deionized water.The solution temperature was maintained at 25 ± 1 °C by the water bath during the test.
2.2.Immersion test and weight loss
For the immersion test,the samples were cut into pieces with dimensions of 20mm×20mm×3mm.The samples were ground with SiC paper gradually up to 1500 grit and then washed with distilled water,degreased with acetone,and dried with cold air.Before tests,the original weight(w0,mg) of the specimens were recorded by a digital balance with a precision 0.0001g.After immersion with different time intervals,the fina weight of the samples without corrosion products (removed with chromate solution of 200g/L CrO3+10g/L AgNO3+20g/L BaNO3) was obtained(w1,mg).The weight loss (C,mg/cm2) was calculated as follows:

whereS(cm2) is the surface area of the specimens.
Besides,during the immersion test for different concentrations of NaCl and NH4Cl,the pH of the solution was monitored.
2.3.Hydrogen evolution test (HE)
The samples used in hydrogen gas collection and their pretreatment were the same as those in Section 2.2.The evolved hydrogen in different solutions was collected during the immersion.A set of devices assembled by a burette and funnel were used to collect the hydrogen generated by the cathodic reaction.The change of solution volume in the burette displayed the volume of released hydrogen.The ratio of solution volume to sample area was controlled at 40:1 [36].The burette was fille with the initial solution before the test.
2.4.Electrochemical tests
A size of 10mm×10mm×3mm pieces were sealed into epoxy resin with 1 cm2surface exposed for electrochemical tests.CHI 660E electrochemical workstation with a threeelectrode cell was used for measuring potentiodynamic polarization curves.A platinum sheet was used as a counter electrode,and a saturated calomel electrode (SCE) as a reference electrode.For the polarization curves,the potential was scanned from open circuit potential (OCP) to anodic and cathodic directions separately with a rate of 0.333mV/s.For the cathodic curves,the potential was scanned from +50 mVOCPto −500 mVOCP.In the case of anodic curves,the potential was scanned from −50 mVOCPto positive direction until the anodic current density reached 10mA/cm2.
2.5.Surface morphology and topography observation

Fig.1.Weight loss (a) and average corrosion rate (b) of AZ31 alloy during immersion in solutions with different concentrations of NaCl and NH4Cl.
The surface of the samples after immersion test without corrosion products was observed by scanning electron microscope (SEM,Quanta 250) and confocal laser scanning microscope (CLSM,VK-250X).
3.Results and discussion
3.1.Corrosion of Mg in NH4+-Cl- solution environment without pH buffer
3.1.1.Weight loss,hydrogen evolution,and corrosion rate
Fig.1 shows the weight loss and corrosion rate of AZ31 alloy during immersion in solutions with different concentrations of NaCl and NH4Cl.The results display that the weight loss in all solutions increases rapidly during the 24 h immersion.After that,the corrosion rate decreases slowly as the soaking time increases.In Fig.1a,the weight loss curve certifie that the presence of ammonium ions greatly promotes the corrosion of the AZ31 samples despite the NH4+concentration is low.
In Fig.1b,the average corrosion rate is calculated by the following equation:

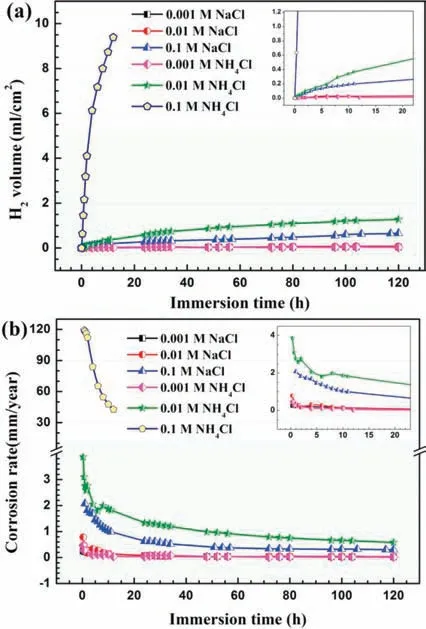
Fig.2.Hydrogen evolution volume (a) and the corresponding corrosion rate(b) of AZ31 magnesium alloy during immersion in solutions with different concentrations of NaCl and NH4Cl.
wherePw(mm/year) is average corrosion rate,C(mg/cm2) is the weight loss,t(h) is the immersion time.Fig.1b demonstrates that the increase of NaCl and NH4Cl concentration increases the corrosion rate of magnesium alloy.However,NH4+can promote the corrosion rate more significantl.Besides,the average corrosion rate of all the samples reduces with extending immersion time.In the 0.1M NH4Cl,the reduction of the corrosion rate is the most remarkable.After 120 h immersion,the corrosion rate of AZ31 samples in 0.001,0.01 and 0.1M NaCl and NH4Cl are 0.18,0.27,0.70,0.35,1.44,and 8.46mm/year.The presence of NH4+increases the corrosion rate of AZ31 magnesium alloy by 1.9,5.3,and 12.1 times when the concentration is 0.001,0.01,and 0.1M,respectively.
Fig.2 shows the dependence of hydrogen evolution volume(a) and the corresponding corrosion rate (b) on the immersion time in the solutions with different concentrations of NaCl and NH4Cl.The hydrogen evolution takes place on Mg surface according to the following reaction equation:
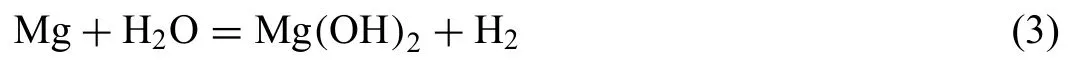
The reaction indicates that corrosion of 1mol Mg (24.31g)will produce 1mol H2(22.4L).Therefore,the average corrosion rate (VH,ml/cm-2·d-1) was calculated by the following equation [37]:


whereVn(mL) is the change of the released hydrogen during immersion,S(mm2) is the surface area,tis the immersion time.PHis the regular corrosion rate inferred by the Faraday’s law.The results show that the samples in all corrosive media continuously evolve hydrogen during the immersion but the hydrogen evolution rate differs from different solutions.The hydrogen evolution is rapid during the initial immersion period,and the increasing degree gradually attenuates with the immersion time in all six solutions,which is consistent with the results of the weight loss.When the concentration of NH4Cl is 0.1M,the detected hydrogen gas volume is much more than the other fi e conditions.After 12h immersion,the hydrogen evolution volume has exceeded the range of hydrogen collection device.In general,the addition of NH4+significantl increases the hydrogen evolution volume.The gas volume is relatively close in 0.001M NaCl,0.01M NaCl,and 0.001M NH4Cl.When the NaCl concentration is 0.1M,the evolved hydrogen volume and corrosion rate of the AZ31 samples are lower than those in 0.01M NH4Cl.During the 120h immersion,the average corrosion rate of AZ31 samples obtained by hydrogen evolution test in 0.001,0.01,and 0.1M NaCl are 0.02,0.03,and 0.30mm/year.The average corrosion rate in 0.001M,0.01M NH4Cl was 0.01 and 0.58mm/year,while it was 42.78mm/year for AZ31 samples in 0.1M NH4Cl during the 12h immersion.
The above results suggest that the presence of NH4+remarkably promotes the corrosion of AZ31 magnesium alloy especially in the solution with high NH4+concentration.Generally,NH4+can be hydrolyzed in the solution as follow reaction equation:resulting in the acidificatio of the solution.Table 1 shows that the initial (0h) pH of different concentrations of NH4Cl solution is slightly acidic.Many studies have proved that acid medium could promote the corrosion of AZ31 alloy[22,32,33].
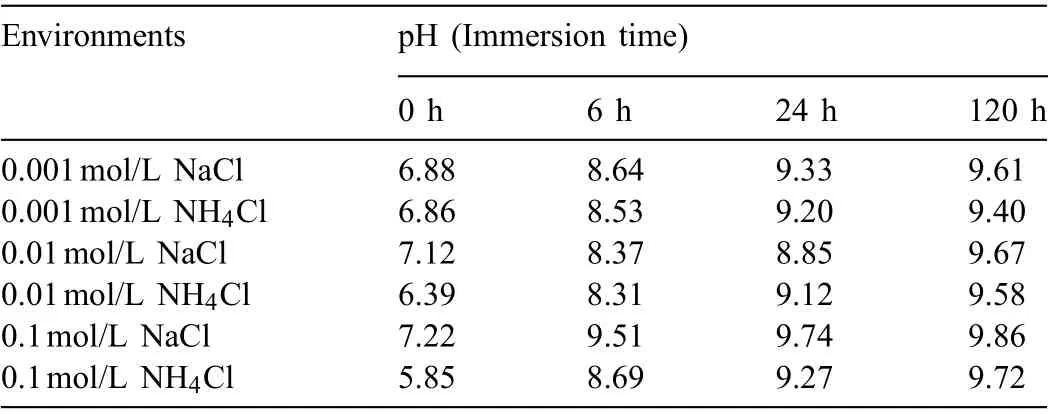
Table 1 Variation of solution pH during the immersion test.
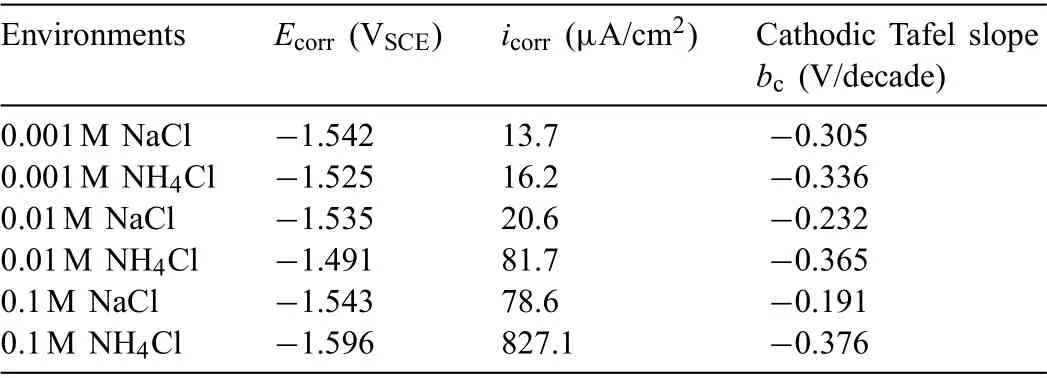
Table 2 Extrapolated parameters from polarization curves of AZ31 specimens in solutions with different concentrations of NaCl and NH4Cl.

By monitoring the pH value of the solution,it is found that the pH value increases with prolonging immersion time.However,at the same concentration,the pH of NH4Cl is always lower than that of NaCl.It means that the change of pH in solution caused by NH4+is a continuous process.That is to say,the reduction of pH is permanent for the promotion of corrosion.It is also important to note that the higher the concentration of NH4+ions,the more significan the change of pH value during the soaking process.It shows that the pH change of the solution caused by the presence of NH4+ions is critical for Mg.

Fig.3.The polarization curves of AZ31 alloys before (a) and after (b) IRcompensation in solutions with different concentrations of NaCl and NH4Cl.
3.1.2.Electrochemical behavior
Fig.3 displays the polarization curves of AZ31 alloy before and after IR-compensation in different solutions.As the polarization curve is tested by separate scanning to the cathodic and anodic directions from the OCP,the corrosion potential is statistically analyzed,and the results are shown in Table 2.Since the cathodic interference is avoided by separate scanning,the corrosion potential is equal to short-term OCP [21].With NaCl concentration increasing from 0.001M to 0.1M,the corrosion potential shows little change but the slope of the anodic and cathode branches is altered.However,in the NH4Cl solution,with increasing NH4+concentration,the cathodic curves are shifted to the right side,indicating the expedition of the cathodic reactions.Even so,the shape of curves does not change,indicating that the reaction mechanism does not change except for the promoted reaction rates.In addition,no limiting current and passivation tendency can be identifie in the polarization curves,suggesting that the corrosion process is controlled by the charge transfer process.The deviation of the anodic polarization curves from the Tafel law in some cases is attributed to the generation of the corrosion products on the magnesium surface [38].
The fitte corrosion current density and Tafel slopes by Tafel extrapolation are listed in Table 2.It should be noted that only the cathodic branch is fitte because the anodic branch does not meet the strict prerequisites for the Tafel fittin [39].By comparing the polarization curves in NH4Cl and NaCl solution with the same concentration,the promotion effect of NH4+on the anodic and cathodic reactions can be found.The acceleration of cathodic reaction kinetics results in the positive shift of the corrosion potential [40],while the facilitation of anodic reactions leads to the negative shift of corrosion potential.In the solution containing 0.01M NH4+,the cathodic reaction process is more heavily promoted,and the corrosion potential rises from −1.535 to −1.494 VSCEand corrosion current density increases from 20.6 to 81.7 μA/cm2.As the NH4+concentration is increased to 0.1M,the anodic process is significantl accelerated,and the corrosion potential declines from −1.545 to −1.599 VSCEaccompanied by increase of corrosion current density from 78.6 to 827.1 μA/cm2.The change of NH4+concentration determines the dynamic trend of anodic and cathodic reactions of AZ31 alloy.
3.1.3.Corrosion morphology
The corrosion morphologies after removing corrosion products and the corresponding surface topographies of AZ31 alloy after immersion in NaCl and NH4Cl for different time are shown in Figs.4 and 5,respectively.It is seen that more serious localized corrosion appears with increasing NH4+concentration.Under the same concentration of Cl-,the corrosion is more severe in the NH4Cl than that in the NaCl.Also,the corrosion is aggravated with extending immersion time in all the solutions.It is worth noting that the white Al-Mn phase can be observed on the surface,which is served as the cathodic phase that accelerates the dissolution of anodicα-Mg during the corrosion process.When the NaCl concentration is 0.001M,the sample surface shows slight pitting corrosion after 6h immersion (Fig.4a1).With 0.001M NH4+,corrosion becomes more serious as indicated by the enhanced dissolution area in Fig.4b.In 0.01M NaCl solution,corrosion pits begin to transfer from pitting to filifor corrosion as the immersion time prolongs to 120h (Fig.4c3).The addition of 0.01M NH4+increases the occurrence rate of pitting and changes the corrosion morphology after 24 and 120h immersion (Fig.4d2–d3).Pitting and filifor corrosion (Fig.4e1-f1)appear at the same time after 6h immersion in 0.1M NaCl solution.After 120h immersion,corrosion propagates laterally and gradually and becomes uniform corrosion with isolated cavities (Fig.4e3).With 0.1M NH4+,typical uniform corrosion morphology can be observed,especially when the immersion time is more than 24 h.The serious corrosion caused by the high concentration of NH4+erases the occurrence of localized corrosion (Fig.4f2,f3).
The topography results shown in Fig.5 clearly illustrate the evolution of the corrosion process.With increasing of NH4+content,Mg alloy not only has a higher probability of pitting(Fig.5d1,f1) during the initial immersion stage,but also has a more serious uniform corrosion (Fig.5d2,f2,d3,f3).
3.1.4.Effect of NH4+on corrosion of Mg
The aforementioned results show that NH4+promotes the corrosion of magnesium alloy in the NH4+-Cl-system.It is affected by two ways: one is the hydrolysis of NH4+,which affects the pH of the solution.The other is the influenc of NH4+on the corrosion product layer on the surface of magnesium alloy.
It is generally believed that the main corrosion products on magnesium alloy surface are the outer porous Mg(OH)2and inner protective MgO layer,and the presence of NH4+can accelerate the dissolution rate of the two products.It has been proved that the NH4+-induced dissolution rate of MgO is three times higher than that of Mg(OH)2[35].In this case,the corrosion mechanism of Mg in the NH4+-Cl-system can be summarized.NH4+affects the corrosion of Mg alloy through hydrolysis and the change of dissolution rate of corrosion products.But there is a limit between the two actions.In this study,when the concentration of NH4+is small(less than 0.01M),the solution shows weak acidity,and the presence of H+in the solution promotes the cathodic reaction of magnesium alloy.However,the weak acidity is not enough to encourage the anodic reaction,so the corrosion potential shows a positive shift.Yet,when the NH4+concentration is high (0.1M),the pH change of solution due to hydrolysis has a more significan effect on corrosion,and the corrosion potential has a significan negative shift.The impact of NH4+on corrosion products is positively correlated with its concentration.Under the combined action of NH4+and Cl-in the solution,the local corrosion depth and size of the pits on magnesium alloy surface increase significantl and evolve to uniform corrosion quickly.
3.2.Roles of pH in the NH4+-induced corrosion of AZ31
3.2.1.Weight loss and corrosion rate
Fig.6 shows the weight loss and average corrosion rate of samples in 0.01M and 0.1M pure NaCl and pure NH4Cl solution with different pH.It should be noted that the immersion period is 24h for the solution with pH 8.85 and 7.13.In comparison,it is 6 h for the solution with pH 4.67 because of the relatively high corrosion rate in this environment.6a and c reveal that when the NH4+concentration is 0.01M,pH of the solution has a little influenc on Mg corrosion.Differently,when the NH4+concentration is 0.1M,the weight loss and corrosion rate is increased with the presence of NH4+(6b and d).In addition,the weight loss and corrosion rate increase distinctly with decreasing pH.
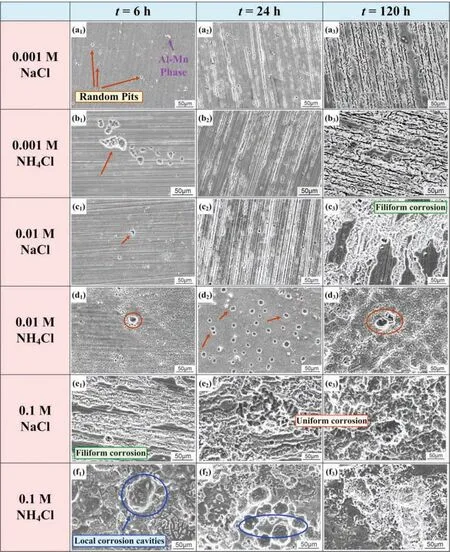
Fig.4.Corrosion morphologies of AZ31 alloy samples after immersion for 6 (a1-f1),24 (a2-f2) and 120 (a3-f3) hours in solutions with different concentrations of NaCl and NH4Cl.
These results once again prove that the effect of NH4+on corrosion of magnesium is related to pH.At the same time,the reason for the limit value caused by NH4+hydrolysis alone cannot be well explained here.Especially when the pH is fi ed to a particular value,the effect of pH by hydrolysis is weak.It shows that the existence of this limit also affects the role of NH4+.
3.2.2.Potentiodynamic polarization curves
Fig.7 shows the polarization curves of the specimens in 0.01M and 0.1M pure NaCl and pure NH4Cl with different pH.Similar to the results shown in Fig.3,the corrosion is controlled by charge transfer process without limiting current and passivation phenomenon.In the solution containing 0.01M Cl-,cathodic reaction is enhanced with addition of NH4+as the buffered pH is controlled at 8.85 (Fig.7a).However,no visible changes can be distinguished between the NH4+-bearing and Na+-bearing solutions when the buffered pH is kept at 7.13 and 4.67 (Fig.7b and c).This phenomenon is consistent with the weight loss results in which only slight increase in corrosion rate caused by NH4+is observed in the pH 8.85 solution when the Cl-concentration is 0.01M.As the Cl-concentration is increased to 0.1M,the reaction rates difference cannot be distinguished.However,the variations of the anodic and cathodic reaction rates differ from solution pH values.When the buffered solution is controlled at 8.85 and 7.13,cathodic reactions are expedited,while the anodic reaction is stimulated at pH 4.67.
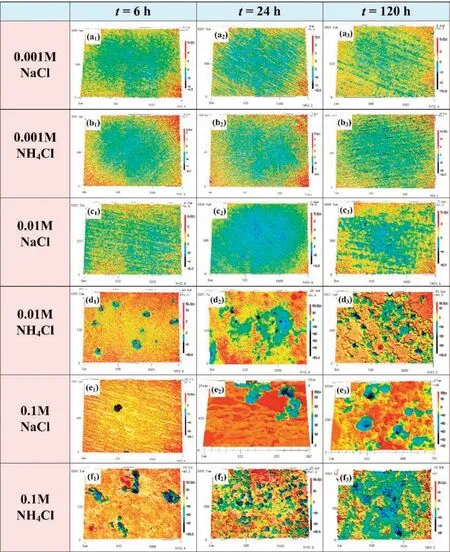
Fig.5.3D profile of AZ31 alloy samples after immersion for 6 (a1-f1),24 (a2-f2) and 120 (a3-f3) hours in solutions with different concentrations of NaCl and NH4Cl.
The Tafel fittin results including the corrosion current density and cathodic Tafel slopes are listed in Table 3.The results show that the presence of NH4+does not affect the electrochemical reaction of magnesium alloy when its concentration is low.At the same pH of NH4Cl and NaCl solution,the shifting trend of polarization curves is the same with the increase of solution concentration and the corrosion current density exhibits little change.So it means that pH controls the corrosion kinetics of Mg alloy in this environment.However,the corrosion rate extrapolated from polarization curves cannot be used to compare the corrosion rate of magnesium because the differences cannot be distinguished when the cathodic branch is fitted As compared with the weight loss data,the acceleration of the anodic reactions especially in the solution with 0.1M NH4+is responsible for the slight increase in weight loss.

Fig.6.Weight loss and average corrosion rate of AZ31 alloy in solution under different pH conditions: (a,b) weight loss in 0.01M NaCl and NH4Cl solution,(c,d) average corrosion rate in 0.1M NaCl and NH4Cl solution.
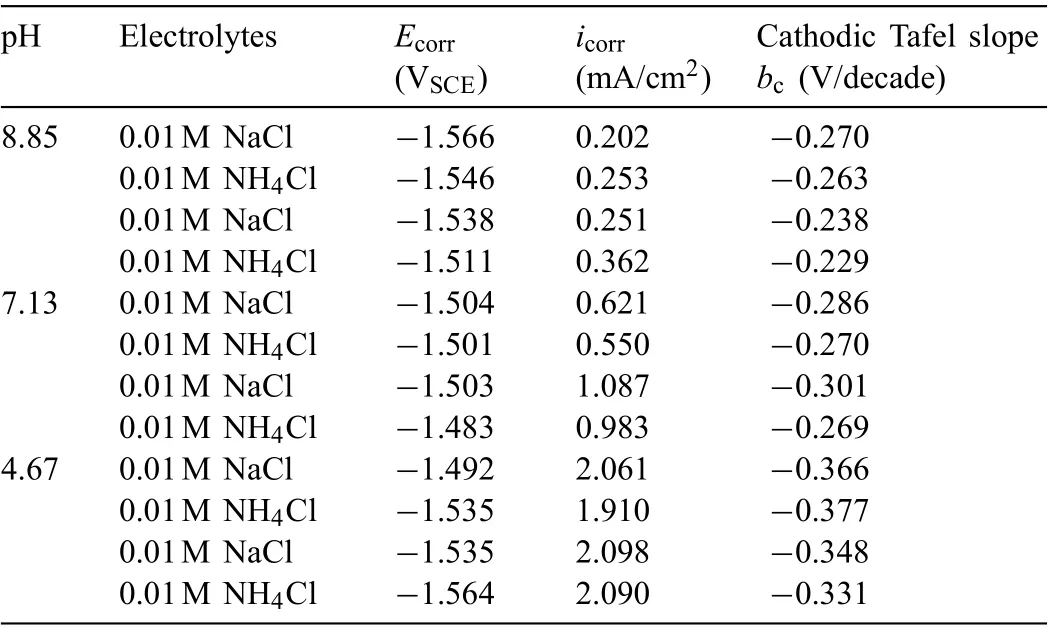
Table 3 Extrapolated parameters from polarization curves of AZ31 specimens in NH4+-bearing and Na+-bearing chloride solution with different pH.
3.2.3.Corrosion morphology and topography observation
The SEM and CLSM morphologies of the samples after immersion in the solution with different pH are shown in Figs.8 and 9,respectively.In solution with pH 7.13,the surface morphology of the samples is almost the same.But in the solution with pH 8.85 and 4.67,the corrosive degree is different between the samples in NH4Cl and NaCl.It should be noted that the NH4+causes corrosion aggravation in these two solutions.The 0.01M NaCl and NH4Cl solution only slightly corrode the surface of magnesium alloy at pH 8.85 (Figs.8a1,b1,and 9a1,b1).But there is no pitting,which could influenc the protection and stability of the corrosion product layer under this condition.With increasing concentration,pitting occurs in NaCl solution and pitting turns into uniform corrosion in the NH4Cl solution.In pH 7.13 environment,the probability of shallow dish pitting is high,and uniform corrosion tends to cover the metal surface in a large area.When the pH value is 4.67,AZ31 samples in 0.01M NaCl and NH4Cl contains corrosion pits that connects into shallow dish.When the concentration is increased to 0.1M,dense pitting morphology with larger pit diameter can be found (Fig.8c3,d3).Under the same pH value,the addition of NH4+does not change the corrosion morphology.Still,it promotes the occurrence of uniform corrosion morphology which may be transformed from the early pitting corrosion.It also can be found that in the solution with low pH value and high concentration,the localized corrosion is easier to appear and is more obvious.

Fig.7.The polarization curves of AZ31 alloys before (a,c,d) and after (b,e,f) IR-compensation in different concentrations of NaCl and NH4Cl solution under different pH conditions.
The corrosion morphology still shows that pH controls the corrosion surface morphology of Mg alloy.Under the condition of pH control,NH4+did not show the same effect as a result in Section 3.1.In the alkaline and neutral environment,the presence of NH4+only shows an impact on the increase of pitting corrosion.However,under the condition of pH value being 4.67,7.13,and 8.85,the pitting density and size become smaller than those under the same NaCl condition,attributed to the accelerated corrosion process that promotes the evolution from pitting corrosion to uniform corrosion.
3.2.4.Effect of pH on the corrosion of Mg induced by NH4+
According to Pan et al.[21],the effect of the initial pH value of the solution due to NH4+hydrolysis is negligible.Through the above results,it can be concluded that pH can not only control the corrosion of Mg alloy,but also control the action of NH4+.First of all,it should be noted that pH can control the proportion of NH4+in solution.The percentage of NH4+in solution can be obtained by the following formula[41]:
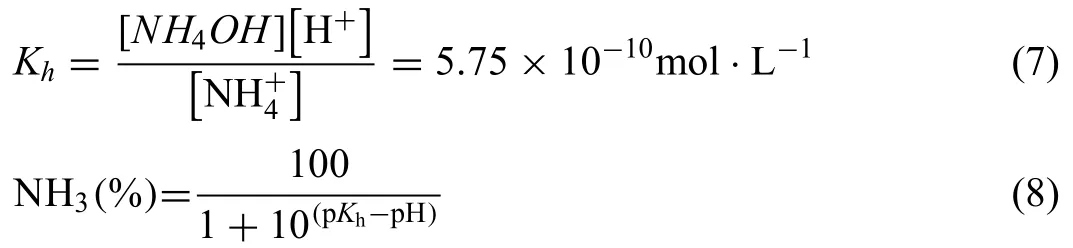
whereKhis the hydrolysis constant of NH4+.According to the different pH values (8.85,7.13,and 4.67),the proportion of NH4+is 71.1%,99.2%,and 99.9% in the alkaline,neutral,and acidic solutions,respectively.The lower the pH value is,the higher the percentage of NH4+.Even so,both NH4+and NH4OH can affect the corrosion of Mg [22].
In this study,the effect of pH induced by NH4+is mainly attributed to the fact that pH controls the hydrolysis of NH4+,affecting the proportion of NH4+,and thus influencin the state of corrosion product layer on Mg surface.It is worth mentioning that the different corrosion products layers generating in different solution could change the corrosion process and mechanism of Mg alloys [42].In the solutions with different pH,the model of the NH4+-induced corrosion of AZ31 magnesium alloy is shown in Fig.10.
When the pH is 8.85,the presence of OH-in the solution promotes the formation of Mg(OH)2.Although Mg(OH)2is porous with partially protection [34],it still plays a vital role in the corrosion process of magnesium alloy.Therefore,the corrosion loss of Mg alloy is the smallest and the corrosion degree is the lightest.Under this condition,the proportion of NH4+in the solution is the lowest.However,the enrichment of OH-can still attract the NH4+,but the same electricity will exclude Cl-.Therefore,NH4+can still improve the dissolution of Mg(OH)2,which increases weight loss and corrosion rate.

Fig.8.Corrosion morphologies of AZ31 alloys after immersion for 6 (a3-c3) or 24 (a1-d1,a2-d2) hours in 0.01M and 0.1M NaCl and NH4Cl solution with solution pH of 8.85 (a1-d1),7.13 (a2-d2),and 4.67 (a3-d3).(The immersion test lasts 6 h in the acidic environment because of the high corrosion rate.).
When the pH is 7.13,NH4+has the least effect on the corrosion of Mg alloy.Under this condition,because the pH value of the solution is neutral,the anodic and cathodic reactions are also in equilibrium during the dissolution of magnesium alloy.Therefore,the corrosion potential of the polarization curve is not shifted with changing solution concentration.In this case,some NH4+is hydrolyzed to produce H+.This part of H+promotes the cathodic reaction,and the anodic reaction is also facilitated to keep the balance,resulting in the formation of thicker corrosion product layer.When the concentration of NH4+is low,the effect is not significant As the concentration of NH4+increases,the effect of NH4+on the dissolution of Mg(OH)2becomes more significant At this time,the amount of H+produced by NH4+hydrolysis also increases,which can make the local environment more acidic and the cathodic reaction is promoted,resulting in the positive shift of the corrosion potential.
When the pH is 4.67,the formation and dissolution rate of Mg(OH)2and MgO on the alloy surface are enhanced by excessive H+,resulting in the acceleration of the anodic reactions and the negative shift of the corrosion potential.At this time,99%of NH4+is in the form of ions,so the H+produced by hydrolysis is very little.Therefore,the increase of NH4+on the dissolution of corrosion product fil is the reason for the negative shift of corrosion potential at the same concentration of NH4Cl and NaCl.In this case,the pitting corrosion is accelerated.In addition,the falling off of the corrosion product layer is enhanced in this environment,which further accelerates the corrosion rate.
Therefore,it can be concluded that the pH of the solution plays an important role in controlling the corrosion of Mg in NH4+-Cl-system.On the one hand,pH controls the kinetics of the corrosion reaction of Mg alloy.On the other hand,pH can control the hydrolysis of NH4+,which makes the content of NH4+significantl related to the pH of the solution.When the concentration of NH4+is low,the pH plays a significan role,and the presence of NH4+will affect the corrosion of Mg.When the concentration of NH4+is high,the effect of NH4+on the corrosion product fil of the Mg surface becomes significant and the promotion effect of NH4+on the corrosion of Mg alloy becomes more apparent.

Fig.9.3D profile of AZ31 alloys after immersion for 6 or 24 h in 0.01M,0.1M NaCl and NH4Cl solution under different pH conditions.

Fig.10.Schematic diagram of the NH4+-induced corrosion of AZ31 alloy in the solutions with different pH.
4.Conclusion
(1) NH4+can affect the corrosion of Mg alloy in two aspects.One is that NH4+can affect the formation and dissolution of corrosion products on the magnesium alloy surface,and the other is that NH4+can promote the cathodic hydrogen evolution reaction.However,NH4+does not change the electrochemical corrosion reactions of magnesium alloy in the chloride solution.
(2) The pH of the solution can affect the corrosion process of NH4+on Mg.There is no synergistic effect between NH4+and solution pH on the corrosion of Mg alloy,and the mechanism between them is independent.
(3) In the presence of NH4+,the pitting corrosion is accelerated especially during the initial immersion stage,which may be attributed to the partial destruction of NH4+on the corrosion product layer.
Declaration of Competing Interest
None.
Acknowledgement
The authors wish to acknowledgement the financia support of the National Natural Science Foundation of China (Nos.51601182),the Fundamental Research Funds for the Central Universities (No.201762008) and Shandong Provincial Key R &D plan (No.2019GHY112050).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Grain refinemen of Mg-alloys by native MgO particles: An overview
- Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis
- In silico studies of magnesium-based implants: A review of the current stage and challenges
- Investigating local corrosion processes of magnesium alloys with scanning probe electrochemical techniques: A review
- A tightly bonded reduced graphene oxide coating on magnesium alloy with photothermal effect for tumor therapy
- Nucleation of recrystallization in magnesium alloy grains of varied orientation and the impacts on texture evolution
