Protein conformation and electric attraction adsorption mechanisms on anodized magnesium alloy by molecular dynamics simulations
2022-12-30ZhaoQiZhangHongYanWangLiWangXiaoBoChenShaoKangGuanCunGuoLinRongChangZeng
Zhao-Qi Zhang,Hong-Yan Wang,Li Wang,Xiao-Bo Chen,Shao-Kang Guan,Cun-Guo Lin,∗,Rong-Chang Zeng
a Corrosion Laboratory for Light Metals,College of Material Science and Engineering,Shandong University of Science and Technology,Qingdao 266590,China
b School of Materials Science and Engineering,Zhengzhou University,Zhengzhou 450002,China
c State Key Laboratory for Marine Corrosion and Protection,Luoyang Ship Material Research Institute,Qingdao 266101,China
d School of Engineering,RMIT University,Carlton VIC 3053,Australia
Abstract Protein adsorption preferentially occurs and significantl affects the physicochemical reactions once the biodegradable magnesium alloys as bone replacements have been implanted.To date,interactions mechanisms between Mg implants and proteins remain unclear at a molecular level.Thereby,a combination of molecular dynamic (MD) simulations and experimental exploration is used to investigate the adsorption behavior and conformational change of bovine serum albumin (BSA),a representative protein of blood plasma,upon the surface of microarc oxidation (MAO) coated Mg alloy AZ31.The influence of absorbed proteins on the cytocompatibility of MAO coating are evaluated by virtue of cytotoxicity assay.Results indicate that the negatively charged O atoms (BSA) exhibit strong interaction with Mg2+ ions of Mg(OH)2,revealing that BSA molecules are ionically adsorbed on the AZ31 surface.Interestingly,MD simulation reveals that MAO coating demonstrates superior ability to capture BSA molecules during the process of adsorption owing to strong electric attraction between the negatively charged O atoms in BSA molecules with Mg atoms of MgO in MAO coating.Moreover,the α-helix part of absorbed BSA molecules on AZ31 substrate and MAO coating markedly decreases with an increase in β-sheet, β-turn and unordered contents,which is attributed to the reduction in the number of hydrogen bonds in BSA molecules.Furthermore,the adsorbed BSA molecules improve the cytocompatibility of MAO coating since the positively charged -NH3+ group and β-sheet content of absorbed BSA molecules mediate the cell adhesion by interacting with the negatively charged cell membrane.
Keywords: Magnesium alloy;Molecular dynamics simulations;Protein;Conformation;Biomaterial.
1.Introduction
Stainless steel 316 L,Co-Cr-Mo alloys,and titanium(Ti) and its alloys are popular biomaterials in load-bearing orthopedic field [1].But these bio-inert materials need secondary surgeries to remove after recovery [2].Currently,secondary surgeries may be avoided if degradable metals(i.e.magnesium (Mg) alloys) are implanted as bone replacements [3–5].Mg alloys as novel metallic biomaterials are biocompatible,mechanical-compatible and biodegradable[6,7],which may be found applications in orthopedic surgery[8,9].Unfortunately,Mg and its alloys are highly susceptible to corrosion in human body flui and the rapid degradation rate curbs their clinic applications [10,11].Various surface coating techniques have been utilized to improve the corrosion resistance of Mg alloys [12–14],including calcium phosphates (i.e.HA) coating [15],layered double hydroxides(LDHs) or montmorillonite coating [16–18],micro-arc oxidation (MAO) or plasma electrolyte oxidation (PEO) coating[19,20],layer-by-layer assembly (LbL) coating [21,22],silane fil [23],polymeric coating [24] and their composite coatings (i.e.MAO/Poly(L-lactic acid) (PLLA),MAO/silane,MAO/chitosan and MAO/Mg(OH)2[19,20,25,26].In particular,MAO coatings have been widely used,which can be attributed to its outstanding corrosion resistance,high bonding strength and excellent biocompatibility [27,28].
The physiological solution (i.e.blood plasma) in the human body contains both inorganic ions and organic components.The inorganic ions contain cations (e.g.Ca2+,Mg2+,Na+and K+) and anions (e.g.Cl¯,HCO3¯,H2PO4¯,HPO42−and SO42−)[29,30].The small organic molecules include glucose,amino acids and vitamins;and organic macromolecules e.g.proteins are consisted of twenty amino acids.Once one biological replacement is implanted into the human body,instant protein adsorption occurs at the initial stage of interaction between the interfaces of the implanted material and its physiological microenvironment [31].Indeed,the adsorption of proteins on material surface is complex process,including protein molecular transfer,adsorption,rearrangement,exchange and desorption [32,33].Surface morphology(e.g.roughness) and physical property (e.g.charged state and wettability) can exert critical impacts on protein adsorption[34,35].Deligianni et al.[36] found that proteins are preferentially adsorbed on a smooth Ti substrate,whereas rough surface bound a higher amount of total proteins due to a larger available area than that of the smooth counterpart.However,Rechendorff et al.[37] proposed that bovine serum albumin(BSA),a nearly globular protein,is less influence by surface roughness.He et al.[38] unveiled that hydroxyapatite (HA)coating with negative charges promotes the adsorption of negatively charged BSA molecules on a gold sheet,whereas the positively charged lysozyme molecules are hindered to adsorb on surface.Fabre et al.[39]reported that the hydrophilic functionalized surface limits the adsorption of proteins.However,existing studies of adsorption mechanisms between proteins and implanted biomaterials have mainly focused on stainless steel and Ti alloys for bone repair,but scarcely on Mg alloy or MAO coating.
Protein can alter the physical appearance or topography and chemical property of Mg alloys,and thus affects the degradation behavior of Mg alloys remarkably [40].The representative interactions between protein and material commonly are adsorption and/or chelation.Adsorption of protein upon a surface includes electrostatic attraction,hydrogen bonding and ionic bonding,whereas the chelation is the reaction of protein molecules and metal to form complexes [41].Liu’s et al.[42] postulated that protein reduces the corrosion kinetics of Mg-Ca alloy in PBS solution due to the synergistic effect of OH¯ions and adsorption of negatively charged albumin molecules to inhibit the attack from aggressive Cl¯ions.Similarly,Yamamoto et al.[43]reported that adsorbed protein and formed insoluble salts inhibit the degradation behavior of pure Mg.Our previous work demonstrates that addition of albumin (0.1 g/L) in 0.9 wt.% NaCl solution protects pure Mg from corrosion,which is attributed to the fact that albumin molecules bind to Mg2+ions and adsorb on the surface;however,high concentration (10 g/L) albumin increases the degradation rate of pure Mg due to chelation reaction [3].In contrast,El-Taib Heakal and Bakry [44] found that the presence of albumin (<10 g/L) promotes the degradation rate of AZ80 because of metal chelation,whereas high concentration (10–20 g/L) albumin inhibits the degradation behavior of Mg alloy via an integrated adsorbed layer.This contradiction may stem from the fact that it is challenging to detect the changes occurring on the interface of an implant and its microenvironment on a molecule scale via modern experimental approaches.
Molecular dynamics (MD) simulation provides an insight view into understanding of the protein-metal interactions at atomic level and microsecond to nanosecond time intervals[45,46].Raffaini and Ganazzoli [47] simulated the adsorption of a protein (fibronectin module on the hydrophobic graphite and revealed the strong interaction with the material surface.Zhou et al.[48] applied MD simulation to investigate the interaction between protein (bone morphogenetic protein-7,BMP-7) and hyaluronic acid (HA,001) surface.Similarly,Huang et al.[49] simulated the adsorption process of protein(bone morphogenetic protein-2,BMP-2)on a nanotextured hydroxyapatite (HAP) surface,and demonstrated that the HAP-1:1 (ridge vs.groove = 1:1) surface owns superior ability to capture BMP-2.Utesch et al.[45] simulated the adsorption behavior of small peptide of EAK16 on the titanium dioxide (TiO2) surface and determined the probable conformation on the small dipeptides.Hence,it can be found that MD simulation is very useful for understanding the interfacial interaction of protein at a molecule level [46,50].
In fact,protein can experience a change in conformation structures upon adsorption,and the impact of biomaterials on protein conformation has been extensively studied [51,52].The composition of proteins secondary structures has been studied by a variety of spectroscopic methods such as circular dichroism (CD),X-ray crystallography,nuclear magnetic resonance (NMR),and Fourier transform infrared (FT-IR) [53].CD is a common method for studying proteins secondary structures,but this means has inherent inconsistency in determination of absolute secondary structures,and it is only suitable for optically clear solution.X-ray crystallography can provide specifi atomic level information about protein structures;however,it is not feasible to obtain sufficientl highquality crystals for such analysis.NMR is limited to a low molecular weight protein at least in the current state.FT-IR possesses the advantage of being a technology that enables conformation analysis of protein in crystals,solids and aqueous solution.Normally,infrared spectra of proteins are mainly composed of amide bands vibrations [54].The amide I region(˜1700 – 1600 cm−1) is mainly due to a C = O stretching vibration [55].The amide II region (1600 – 1500 cm−1) can be attributed to the in-plane N–H bend and C–N stretch of amide band.Owing to the difference in secondary structure,the orientation of amide bonds is different in protein skeleton.The conformational states of proteins,causing different vibrational frequencies,are observed via component peaks which contribute to characteristic amide I band.The overall shape and maxima of the groups are determined by the secondary structure of the analyzed protein [53].Roach et al.[54] used FT-IR to study the conformation change of protein induced by alkane(CH3)surface.It is revealed that protein lost a large part of itsα-helix,whereas the component ofβ-sheet or random structure increased simultaneously.Hu and Yang [56]indicated that the different bioactive Ti surfaces (alkali-heat and alkali-acid treated) induce the different conformation change of protein by means of FT-IR.Changes in proteins structures induced by adsorption process,and the interaction with bio-implants may lead to a decreased or increased biological activity.However,little information is available in literature about structural changes of proteins adsorbed on Mg-based materials.
In this paper,for the firs time to the best of our knowledge,we have demonstrated the adsorption behavior of BSA molecules on Mg alloy AZ31 or MAO coating by combining experimental methods and MD simulations.The paper aims to take insight into the interaction mechanisms between protein and Mg-based biomaterials at an atomic and molecular level,and to understand the influenc of absorbed protein on the biocompatibility of MAO coating.
2.Experimental
2.1.Materials
The as-extruded Mg alloys AZ31 (Al 2.5–3.0,Zn 0.7–1.3,Mn>0.20 and the balance Mg) were supplied by the Shandong Yin Guang Yu Yuan Light Metal Precision Molding Co.,Ltd.,China.Bovine serum albumin (BSA) was purchased from Sinopharm Chemical Reagent Co.,Ltd.,China.The AZ31 substrates were cut into squares with a dimension of 20 mm × 20 mm × 5 mm,and ground with sandpaper up to 1200 #.And then the polished AZ31 Mg substrate was cleaned with deionized water and alcohol;subsequently,it was dried in warm air.
2.2.Coating preparation
The self-made micro arc oxidation(MAO)device was used to anodize the AZ31 Mg alloy,and the power supply unit was controlled by a single chip micyoco.The AZ31 substrate was used as anode,a stainless-steel plate was used as the cathode and a cooling system.The electrolyte includes 10 g/L of Na2SiO3,8 g/L of NaOH and 5 g/L of KF.MAO coatings were performed at a constant voltage of 400 V at 350 Hz for 3 min using an AC power supply with a duty cycle of 30%.Subsequently,prepared MAO coating was cleaned with deionized water,and dry out [26].And the prepared MAO coating was tested as soon as possible,because newly generated MAO coating would inevitably adsorb airborne organics during storage,leading to a change in the sample properties.
2.3.Surface analysis
Surface morphologies and chemical compositions of MAO coating were characterized via a field-emissio scanning electronic microscope(FE-SEM,Hitachi S-4800,Japan)equipped an energy dispersive X-ray spectrometer (EDS,Oxford Isis,UK).The crystal structures of samples were analyzed via Xray diffractometer (XRD,Rigaku D/MAX 2500 PC,Japan)using a Cu-Kαradiation.The confocal laser scanning microscopy (CLSM,OLS 4000,Japan) was applied to study the surface roughness of the coating.
2.4.Protein adsorption tests
The phosphate-buffered saline (PBS,pH 7.4) without and with BSA were prepared,and the 1 g/L BSA-containing PBS solution was designated as BSA-PBS.The AZ31 substrate and its MAO coating were immersed in 6-well plate containing 10 mL of BSA-PBS solution at 37 °C for different times(10,30 and 60 min).Subsequently,the samples were rinsed with PBS solution,and dried in N2.Then,the adsorbed BSA on the samples was labeled via fluorescei isothiocyanate(FITC) [57].And ultraviolet (UV) analysis lamp and fluo rescence microscope (FM,Leica DM2500,Germany) were employed to observe the adsorbed BSA.The chemical composition of the immersed sample was characterized via X-ray photoelectron spectroscopy(XPS,EscaLab 250Xi,USA)with Al Kαradiation (1486.6 eV) as an excitation source [58].Fourier transform infrared spectrophotometer (FT-IR,Nicolet 380,USA)was recorded at 25°C with a resolution of 4 cm−1(32 scans).Besides,the secondary structures (i.e.α-helices,β-sheet,β-turn and unordered) of the adsorbed BSA on the surface were identifie by treating amide I band.Component peaks were fitte with Gaussian curves.Peak position was estimated through Fourier deconvolution [59],and the specifi parameters were set to an initial Lorentzian line-shape function with a full bandwidth at half height of 52 cm−1and a resolution enhancement factor of 3.3.
2.5.Molecular dynamics (MD) simulations of the adsorption of protein
The crystal structure of BSA(PDBID:3v03)[60]was used in present work,and which was obtained from the RCSB(Research Collaboratory for Structural Bioinformatics)protein data bank.The firs 111 amino acids in chain A of BSA contains were selected as the model protein to explore the adsorption behavior.The protein has a net charge of −4e.All MD simulations were performed with LAMMPS[61]in this work.Mg(OH)2used as the substrate of protein adsorption was the main corrosion product on Mg alloy.The lattice constants of Mg(OH)2and MgO area=b= 3.141 °A,c= 4.677 °A,α=β= 90°,γ= 120° [62] anda=b=c= 4.211 °A,α=β=γ=90° [63],respectively.CLAYFF3 force fiel which had been used successfully in many systems of clay minerals,oxide and hydroxide [64,65],was used to describe the interaction of MgO and Mg(OH)2.An -OH bond stretch and an additional Mg-O-H angle bending term were described the surface hydroxyl behavior of Mg(OH)2[66].The supercell of MgO (001) consisted of 3528 atoms with a dimension of 64.0 °A × 64.0 °A × 8.4 °A.The 8969 water molecules were fille into the box and the whole box size of systems was 64.0 °A × 64.0 °A × 73.9 °A.The supercell of Mg(OH)2(001)with dimensions of 64.0 °A×69.0 °A×9.5 °A contained 4800 atoms.The box was fille with 10,174 water molecules and the whole box size of the systems was 64.0 °A × 69.0 °A × 81.7 °A.There were 0.1 M Mg2+ions fi ed within a distance of 8 °A from the top layer atoms of Mg(OH)2(001) so as to be consistent with aggressive environment.The initial minimum distance between BSA molecules and the surfaces for MgO (001) system was 5.0 °A to ensure the occurrence of spontaneous adsorption,while the distance between BSA molecules and the surface magnesium ions for Mg(OH)2(001) was 5.0 °A.Sodium or chloride ions were dissolved into the water to keep the system neutral.The parameters of CHARMM22 force fiel were used for ions,TIP3P water molecules and BSA molecules.The parameters of Lennard–Jones (L-J) potential followed the Lorentz-Berthelot mixing rule for the substrates,protein molecules,ions and water molecules cross interactions between nonbonding atoms [67].The periodic boundary condition was applied in all three directions.SHAKE algorithm was used to constrain bands connected to H atoms and maintain the rigidity of the water molecules.The time step was selected as 1 fs,and the coordinates were saved every 2 ps.All of the MD simulations were performed using the Nose-Hoover thermostat in the NVT ensemble [68].The long-range electrostatic interactions were computed by means of the particle-particleparticle-mesh (PPPM) method.The solutes were kept fi ed firstl to randomize the position of water molecules.Then,the temperature of water box was increased gradually from 0 K to 300 K in 300 ps to balance water box.Solute constraint was removed,and the temperature of the system was increased gradually from 0 to 300 K for 500 ps to make the system temperature reach stability.Finally,a typical 20 ns production simulation was performed for each molecular assembly at 300 K.The trajectories were visualized by the Visual Molecular Dynamics (VMD) program [69].
2.6.Cytocompatibility tests
Mouse pre-osteoblasts MC3T3-E1 (iCell Bioscience Inc,Shanghai) were cultured in alpha-Minimum Essential Medium (α-MEM,Gibco) supplemented with 1% penicillinstreptomycin solution and 10% fetal bovine serum (FBS) in a humid atmosphere with 5% CO2at 37 °C.Indirect contact assay was used to evaluate the cytotoxicity.Prior to the cell assay,all samples were placed inα-MEM (12 mL)containing 10% FBS and 5% CO2at 37 °C for 3 d The extract was diluted to 20% usingα-MEM containing 10%FBS.The cultured MC3T3-E1 cells were seeded in a 96-well plate (4 × 103cells/well) until the cells adhere to the wall.Subsequently,the MC3T3-E1 cells in diluted extracts were incubated for 1 and 3 d The extracts in each well were replaced by 100 μL of 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide (MTT,Solarbio,Beijing) solutions,and the cells were cultured for 4 h at 37 °C.Per well was added to 100 μL of dimethyl sulfoxide(DMSO)and shaken for 10 min.Then,the absorbance was detected by a microplate reader (SPARK 10 M) at a fi ed wavelength of 570 nm.
MC3T3-E1 cells were cultured in a 6-well plate (4 × 104cells/well) for 24 h in a humid atmosphere with 5% CO2at 37 °C.The initial cell media in 6-well were replaced by 1 mL diluted extract media (20 V% extracts and 80 V%α-MEM).Then,the cells were incubated for 72 h and stained by Dead/LIVE D® assay (BestBio).Briefl ,the mixed staining reagent (PI and Calcein-AM) was diluted to 1000 times and added to each well.Then,the reagent and cells were cultured for 15 min in the dark.After that,the live/dead cells were observed by a confocal laser scanning microscope.The dead and living cells were stained by PI and Calcein-AM into red and green colors,respectively.
2.7.Statistical analysis
Data of the cell viability were reported as the mean ±standard deviation (SD).Statistical analysis was conducted by independent samplest-test.Differences were considered significan at∗∗P <0.01 or∗P <0.05.All the tests were carried out three times.
3.Results
3.1.Surface analysis
Fig.1 shows SEM images of MAO coating.A typical porous structure of MAO coatings is evident (Fig.1a–c).And the corresponding element compositions of MAO coating are mainly O,Mg and Si as well as trace of Al (Fig.1e),demonstrating a successful preparation of MAO(MgO,MgSiO3etc.)coating on AZ31 Mg substrate.Micro-cracks are found,too.The detailed EDS data (Spectrums #1–3) is listed in Table 1.Moreover,cross-sectional microphotograph displays that the thickness of the MAO coating is 6.10 ± 0.22 μm (Fig.1d).3D morphology of the AZ31 substrate and its MAO coating is depicted in Fig.S1.The porous and rough morphology of MAO coating is observed,and the surface roughness(Ra) is 0.490 ± 0.098 μm,confirmin that the MAO coating has higher surface roughness than the AZ31 substrate(Ra = 0.053 ± 0.005 μm).

Table 1 EDS compositions for MAO coating in at.(wt.)%.
XRD patterns (Fig.1f) reveal the characteristic peaks of MAO coating are MgSiO3and MgO.Of note,some Mg peaks can be observed on the MAO coating,which is related to Xray penetrated thin MAO coating into the AZ31 substance.
3.2.Adsorption of protein and its change in conformation
For the AZ31 substrate (Fig.2a),the adsorbed area of BSA gradually increases with the extending immersion time.Interestingly,it can be found that the BSA is mainly adsorbed in the corrosion region of the Mg substrate,revealing that the adsorption of BSA on the AZ31 substrate is related to the formation of corrosion products (i.e.Mg(OH)2).The adsorbed BSA for MAO coatings (Fig.2b) progressively increases and uniformly distributes on the surface during the immersion.The adsorbed area on MAO coating is larger than that of AZ31 Mg alloy,suggesting that the porous MAO coating promotes the adsorption of BSA.The results are consistent with the fluorescenc microscope images (Fig.S2).SEM images further show that the absorbed BSA gradually increases as increasing immersion time,and the pores in MAO coatings are gradually fille with BSA (Fig.2c).
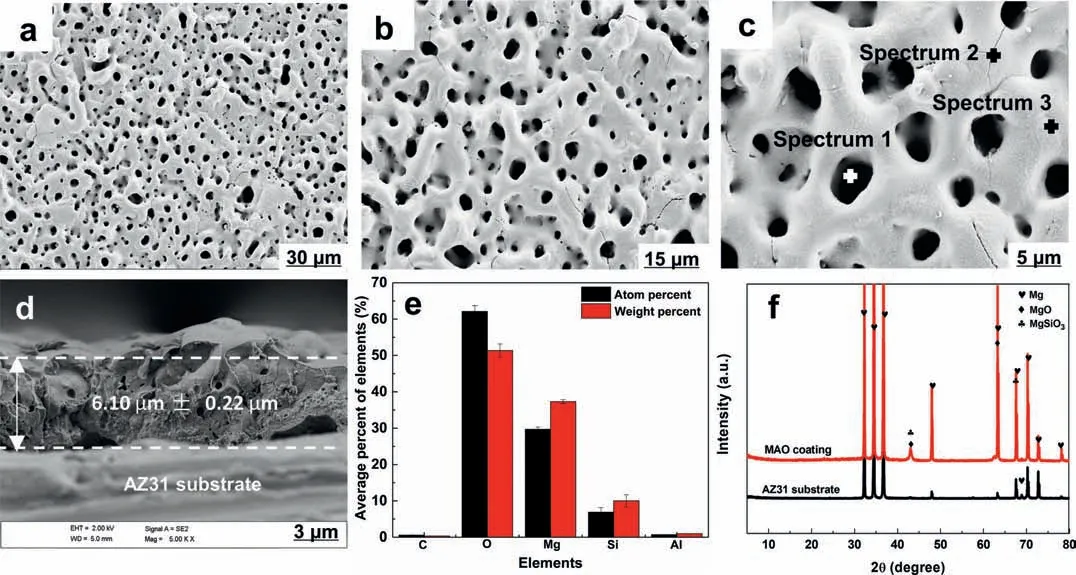
Fig.1.SEM images of morphology of MAO coating upon the surface of Mg alloy AZ31: (a–c) Plain view and (d) cross-sectional view;(e) element compositions of MAO coating and (f) XRD patterns.
The absorption peak at 3694 cm−1is attributed to -OH,which proves the formation of corrosion products (Mg(OH)2)on AZ31 substrate (Fig.2d).Moreover,the presence of -NH2group at 3440 cm−1,C = O group at 1647 cm−1and N–H group at 1548 cm−1relates to the adsorbed BSA molecules on the surface.The peak at 1060 cm−1is assigned to =C–H group,indicating that the vinyl in BSA molecules affects the substrate corrosion.Also,Wang et al.[70] showed a similar study report.For MAO coatings (Fig.2e),the band at 3440 cm−1is designated to the -NH2.In addition,the peaks at 1652 cm−1and 1548 cm−1are designated to the C = O and N–H,respectively.Results indicate that BSA molecules are adsorbed on the surface.Interestingly,the characteristic peak of Mg(OH)2is not observed on MAO coating,revealing that the MAO coating does not suffer corrosion.
XPS spectra are used to determine the initial changes in composition of MAO coating after immersion in BSA-PBS solution for 10 s (Fig.S3).With respect to C 1 s spectra(Fig.S3b),the peaks of the samples are composed of three contributions at 284.6,285.3 and 288 eV,which correspond to C–C/C–H,C–N/C–O and C = O–N,respectively.The results suggest that BSA molecules are absorbed on MAO surface.Similar results obtained from XPS spectra of N 1 s (Fig.S3c),which are composed of two peaks at 399.5 (N–O/C–N)and 400.2 eV (O = C–NH-(C,H)).[71] Interestingly,the Mg 2p spectra of MAO coating has only one contribution at 50.3 eV (Fig.S3d),corresponding to MgO,revealing that the MAO coating is not corroded in early immersion.The scenario is in pronounced agreement with Wan ’s research results [71].
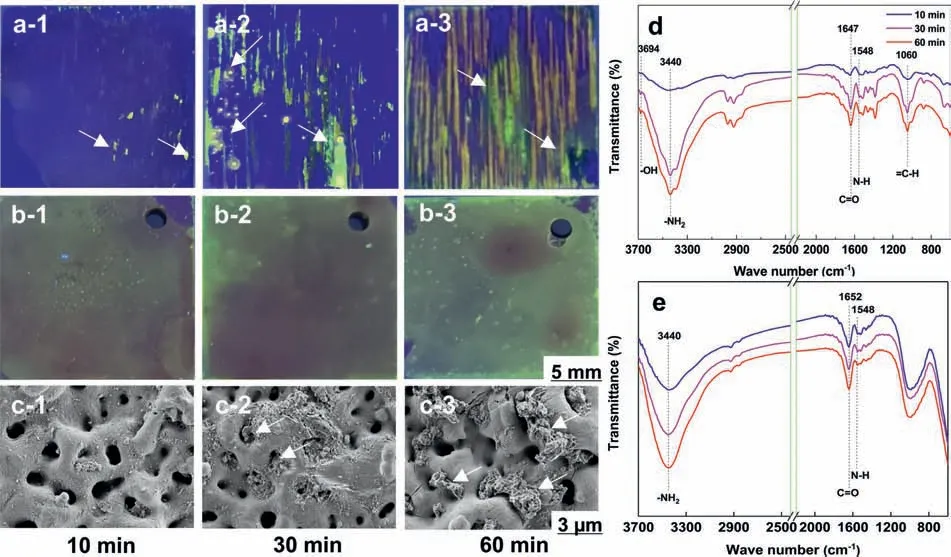
Fig.2.(a) AZ31 substrate and (b,c) MAO coating immersed in BSA-PBS solution after 10,30 and 60 min: Optical images of (a-1,2,3) AZ31 substrate and (b-1,2,3) MAO coating (labeled with FITC) were placed under UV lamp,SEM images of (c-1,2,3) MAO coating;FT-IR spectra of the samples: (d)AZ31 substrate,(e) MAO coating after an immersion.
Normally,BSA molecules belong to “soft” proteins with low internal stability,which can change their conformation upon adsorption.The fittin component peaks of amide I bands and conformational change of adsorbed BSA are shown in Fig.3a and b.For the AZ31 substrate,the percentage ofα-helix decreases while that ofβ-sheet and unordered increase with an extension of immersion time (Fig.3c).Interestingly,the conformation transition of BSA on the MAO coating displays a similar tendency.It can be seen that the conformational composition of absorbed BSA on MAO coating gradually changes fromα-helix toβ-sheet,β-turn and unordered (Fig.3d).
3.3.MD simulations of the adsorption of protein
MD simulations are applied to analyze the adsorption mechanisms and conformational change of BSA in this work.BSA molecules and substrates are displayed with a new cartoon mode and a VDW mode,respectively.Residues anchored to the surface are represented in Licorice mode.The water molecules and counterions are not showed here for clarity.In protein adsorption simulations,we speculate that the BSA molecules are absorbed on AZ31 surface through the combination with BSA molecules and Mg2+ions in the corrosion product of Mg(OH)2.As can be seen from Fig.2d,-OH groups,form on the surface of AZ31 due to the formation of Mg(OH)2,is used as the substrate in the investigation of adsorption behavior of BSA.As shown in Table 1 and Fig.1f,MgO is the main component of MAO coating.Therefore,MgO is used as the substrate for adsorption behavior of BSA on MAO coating.The initial adsorption configuration of proteins on the surfaces of two systems are shown in Fig.4.It is evident that the configuratio of BSA changes by a large extent after 20 ns MD simulation.Theα-helix components in BSA,maintained by hydrogen bonds between the carbonyl and amide groups in the backbone,shows a certain reduction;while the fl xible random coil displays a certain increase.From the distribution of BSA residues anchored to the Mg(OH)2,the O atoms with negative charges in proteins exhibit a strong interaction with Mg2+ions(Fig.4a–2 and a–3),which suggests that Mg2+ions can mediate the adsorption behavior of BSA.It can be found that a strong attraction interaction between the negatively charged O atoms in proteins with Mg atoms in MgO due to electrostatic effect (Fig.4b),which affects the distribution of the residues anchored to MgO and eventually the adsorption configuration of BSA.
To study the conformational change of the adsorbed BSA visually,the structure of the firs fi eα-helix fragments of BSA on different surface are contrasted with its crystal structure by the VMD software.As shown in Fig.4c for AZ31 substrate,the ratio ofα-helix decreases after the adsorption compared to that before adsorption,which is related to the reduction of the number of hydrogen bonds with a stabilizing effect (Fig.4c–3 and c–4).Interestingly,conformational change of absorbed BSA on the MAO coating exhibits consistency with the surface of Mg(OH)2(Fig.4d).The results of MD simulation are consistent with the fitte results of FT-IR(Fig.3).
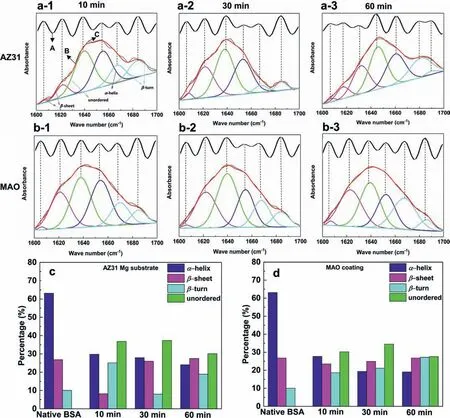
Fig.3.Amide I bands with fitte component peaks of (a) the AZ31 substrate,and (b) MAO coating.A: FT-IR spectra after band narrowing by Fourier deconvolution (black wavy line);B: fitte curve absorbance spectra (red curve);C: original absorbance spectra (black curve).Conformational changes of adsorbed BSA on the samples compared with native BSA after immersion of 10,30 and 60 min: (c) the AZ31 substrate,and (d) its MAO coating in BSA-PBS solution.(For interpretation of the references to colour in this figur legend,the reader is referred to the web version of this article.).
The radius of gyration (Rg) define as the mass-weighted root-mean-square average distance of all atoms in a protein from its center of mass,demonstrating the overall size of a protein.Rgis a critical parameter to assess the conformation of BSA.Fig.S4 showsRgof BSA as a function of simulation time.After the firs 6 ns of simulation process,theRgvalue of BSA begins to sharply change,which represents the touch of BSA to the surfaces.The slightly decrease ofRgvalues at 6 ns implied that the sizes of BSA are cut down due to compression [49],indicating that the structure of adsorbed BSA is more stable.
3.4.Cytocompatibility tests
Our previous study indicated that MAO coating can improve the cell viability of Mg substrate due to a higher available area [31].Hence,the cell compatibility of MAO coating with or without BSA adsorption is further evaluated,as shown in Fig.5.The OD values and cell viability of MAO and MAO-BSA slightly decrease after 1 d of incubation (Fig.5a and b),which is related to the influenc of the residual electrolyte in the micropores of MAO coating.[19] When cultured for 3 d,the MAO and MAO-BSA show a significantl increased osteogenesis effect.Interestingly,the MAO-BSA exhibits the better cell viability than MAO,indicating that the adsorption of BSA on the MAO coating can improve the cell compatibility.Moreover,Live/Dead staining of MC3T3-E1 cells cultured for 72 h is shown in Fig.5c-d.The cultured cells for MAO and MAO-BSA possess healthy spindle shapes,and there are almost no dead (red) cells in Fig.5d and e.Also,Wang et al.[72] found that a sodium montmorillonite(MMT) coating modifie by BSA significantl improves cytocompatibility of the AZ31 Mg alloy.However,the mechanisms by which BSA coating enhances the cytocompatibility of MMT coating have not been explained.Herein,the results of cell viability and live/dead assay implied that the MAO-BSA has acceptable cytocompatibility to osteoblasts.
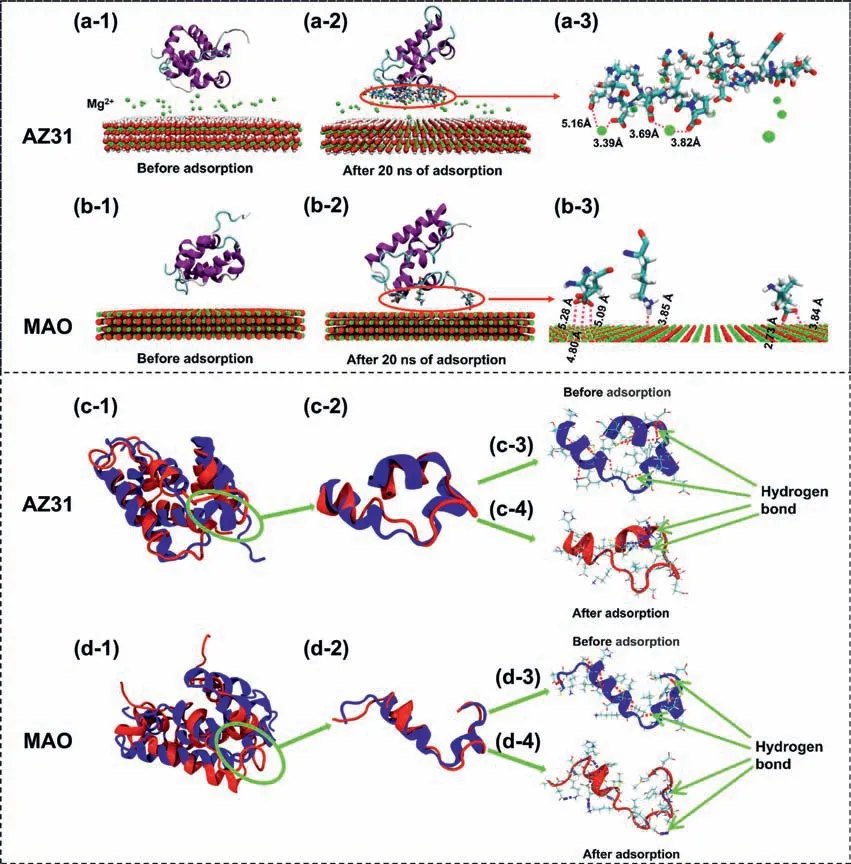
Fig.4.The adsorption of protein on (a) the AZ31 substrate with Mg2+ ions and (b) MAO coating;Conformational changes of BSA on (c) the AZ31 substrate and (d) MAO coating.The pink and blue dotted lines represent hydrogen bonds between H atoms and O atoms or H atoms and N atoms in protein.The parameters of hydrogen bonds are R = 3.5 °A and β = 30° (For interpretation of the references to colour in this figur legend,the reader is referred to the web version of this article.)
4.Discussion
Mg alloys are attractive materials for medical implants because of well-known biodegradable and biocompatible properties [73].However,Mg alloy is not suitable for use as biodegradable implants due to its rapid degradation rate to be functionally applied in bone implantation [74].MAO coating is one of the most simple and effective method to enhance the corrosion resistance as well as biocompatibility of Mg alloy.The main composition of MAO coating is MgO,which is the result of plasma chemical oxidation reactions between the Mg substrate and the electrolyte in the discharging channels produced by the sparks.Theα-Mg phase is the major components in the AZ31 alloy substrate.During the sparking process,the dissolving Mg2+migrates outward from the substrate,whereas OH¯migrates inward into the channels under the electrical field As a consequence,the MgO phase in the coating is formed through the following reactions 1–(6) [27]:
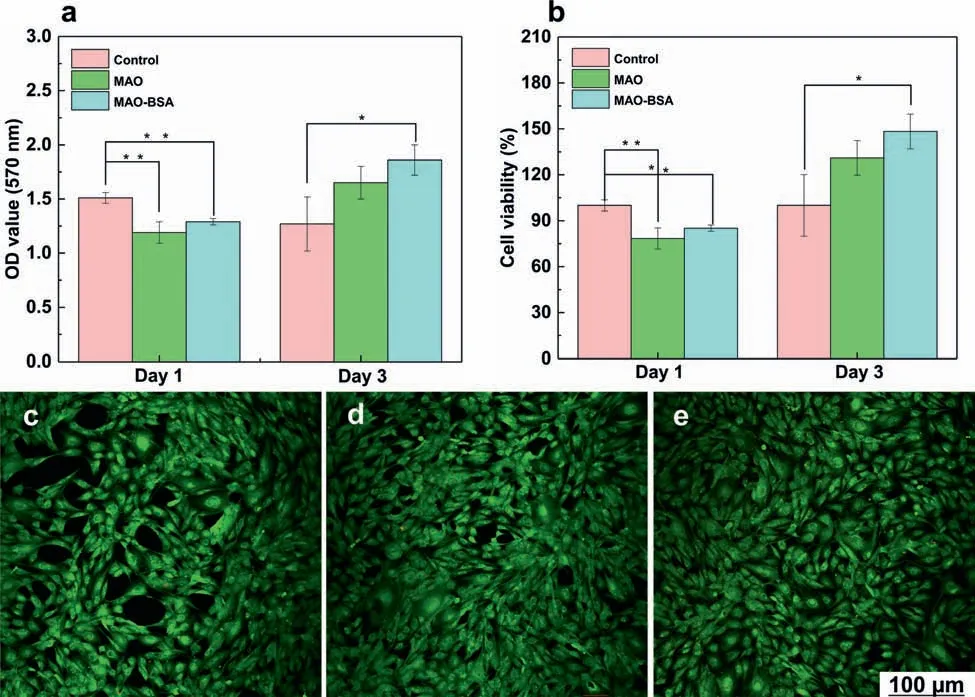
Fig.5.(a) OD values and (b) cell viability of MC3T3-E1 cells incubated in the extracts of each samples prepared with control,MAO,and MAO-BSA for 1 and 3 d Statistically significan differences (∗∗p < 0.01,∗p < 0.05.);Live/dead cells of MC3T3-E1 cells after incubating for 72 h of the (c) control,(d)MAO and (e) MAO-BSA.
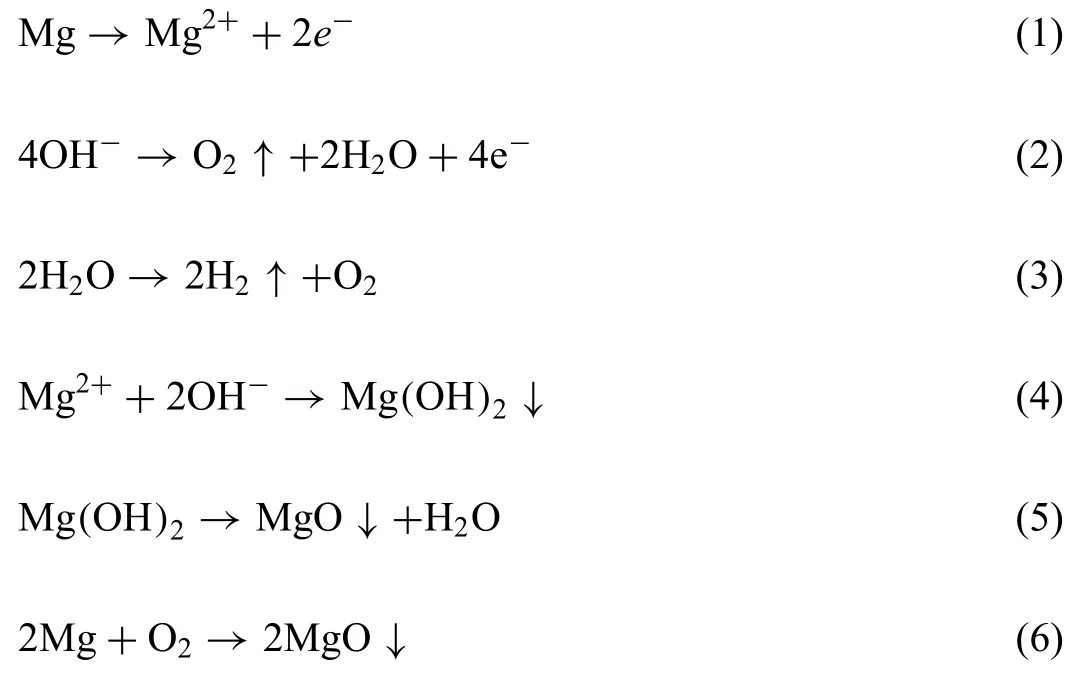
According to the formation mechanism,MAO coating is composed of a porous outer layer with loose structure and a thin inner layer with dense structure.It can be seen from the cross-sectional view that the outer layer of MAO coating has channels,while the inner layer is dense (Fig.1d).
The presence of proteins in blood plasma can critically affect the biodegradability and biocompatibility of implants.However,the interactions between proteins and Mg-based materials are still unclear.As shown in Table 2,an overview of studies on surface interactions between proteins and Mgbased implants is summarized.It can be found that most of the literatures investigate the interaction mechanism through experimental methods,and the interactions between proteins and Mg-based implants are mainly focused on adsorption and chelation [3].Unfortunately,the adsorption mechanism proposed by experimental methods in the literature does not directly show the adsorption process of protein onto material from the molecular and atomic levels.MD simulations,a computer molecular simulation method,is particularly suitable for providing detailed information about complex molecular processes at the molecular and atomic levels.However,only a few literatures apply MD simulation to study the interaction between protein and Mg alloy surface.Wang et al.[46] indicated that the electronegativity of added alloy elements in Mg alloy has a substantial effect on the adsorption of protein by means of MD simulation.However,the effect of corrosion products on protein adsorption is not explored in the MD simulation.In this paper,the interaction mechanisms between BSA molecules and Mg alloys and its MAO coating is investigated through a combination of experimental method and MD simulation.
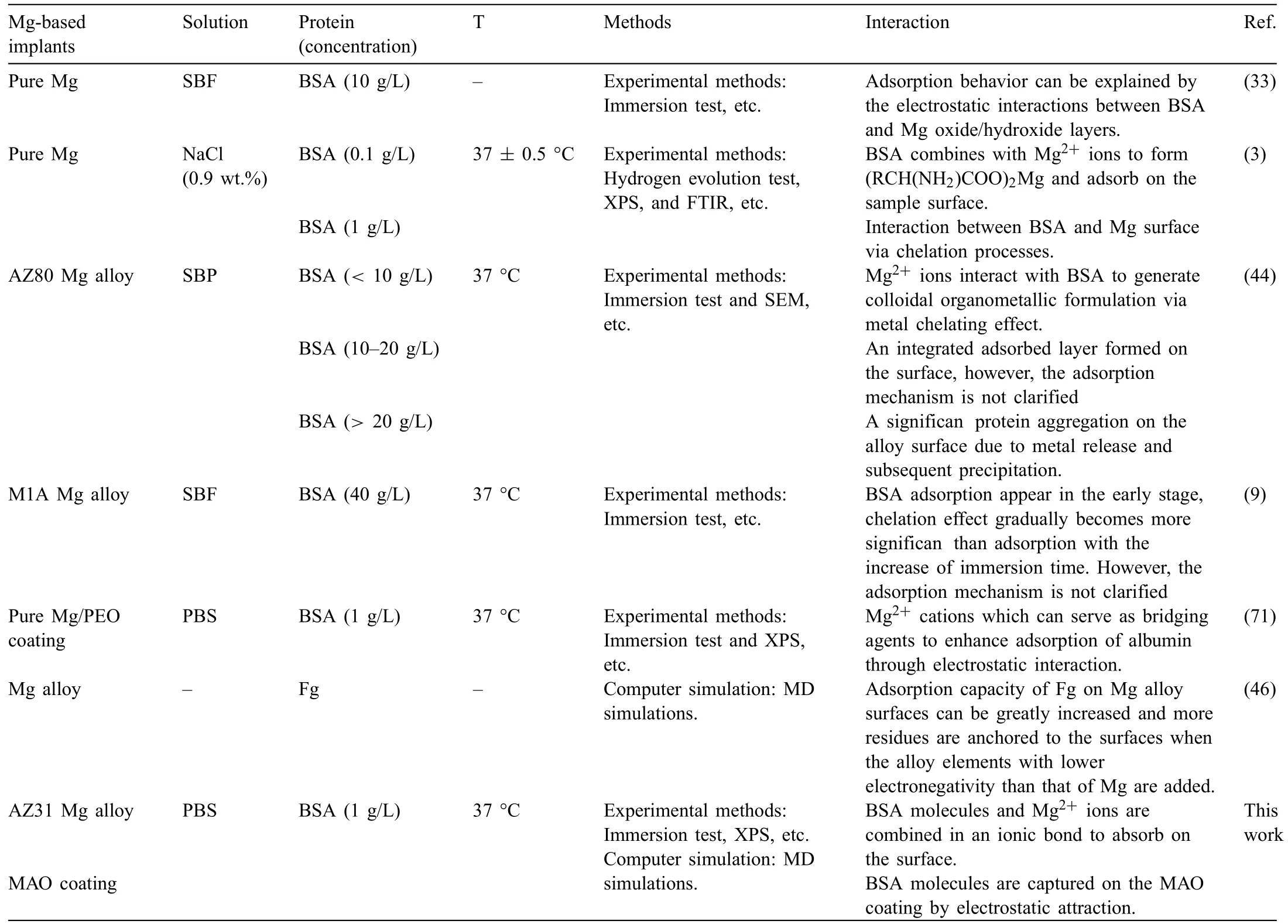
Table 2 An overview of studies on surface interactions between proteins and Mg-based implants.
In PBS solution (pH 7.4),BSA molecules (pI4.7) experience a neutral-acidic transition and become negatively charged,suggesting that the -COO¯ groups are exposed to the outside of BSA molecules (Fig.6) [75].Firstly,Mg2+ions slowly form on the surface when AZ31 substrates are immersed into BSA-PBS solution (Fig.6a–2) [76].Then,BSA molecules combine with Mg2+ions to generate(RCH(NH2)COO)2Mg and are chemically adsorbed on the AZ31 substrate (Fig.6a–3) through ionic bonds (reaction (7))[31]:
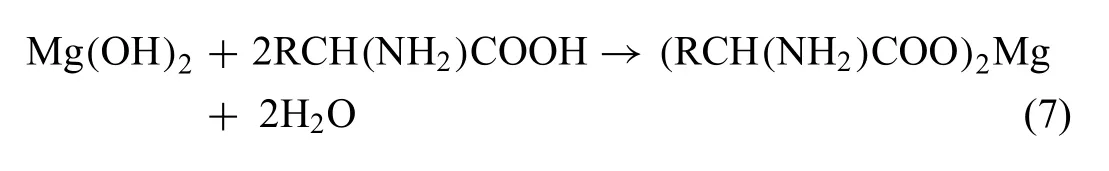
Yan et al.[3] reported similar research results.This result is a good explanation for the adsorption of proteins in the corrosion product (Mg(OH)2) area.As time proceeds,a large amount of Mg2+ions are produced during the corrosion of metallic Mg piece,which increases free binding sites to attract BSA molecules.
Unlike the bare AZ31 substrate,MAO coating possesses positive charges over surface (pIMAO12.4>pH 7.4) [77],whereas BSA molecules show negative charges(Fig.6b).According to the results of XPS data (Fig.S3),the adsorption of protein on MAO coatings surface is earlier than the corrosion.Hence,some BSA molecules are adsorbed on MAO surfaceviacharge attraction (Fig.6b–2 and b—3).The experimental results are in good agreement with the MD simulation results.Although the inner layer of MAO coating is dense,a trace of BSA molecules may penetrate through the through-pores of MAO coating and directly contact Mg alloy substrate,the adsorption mechanism of BSA molecules is the same as that on the uncoated Mg alloy substrate discussed above.
BSA molecules are promptly adsorbed on the surface or micro-pores of MAO coating (0.5 ˜5 μm) given their ellipsoidal dimensions of ˜14 × 4 nm (Fig.S5a) [78].Moreover,the adsorbed multilayer BSA molecules are observed on the MAO coatings (Fig.S5b),which can be attributed to the BSA molecules interacted with MAO coatings by an adsorption affinit superior to the intermolecular electrostatic repulsion.Until attractive forces(F3)of the MAO coating and the outermost BSA molecules are equal to the repulsive forces(F1+F2) between the BSA molecules,the adsorption of BSA molecules on the MAO coating reach equilibrium (Fig.S5c).Mori and Imae [79] found that the interaction forces between BSA molecules and mica surfaces can induce the multilayer adsorption.The research results are consistent with our experimental conclusion.Moreover,MAO coating with higher roughness can adsorb more BSA molecules due to its larger specifi surface area [80].
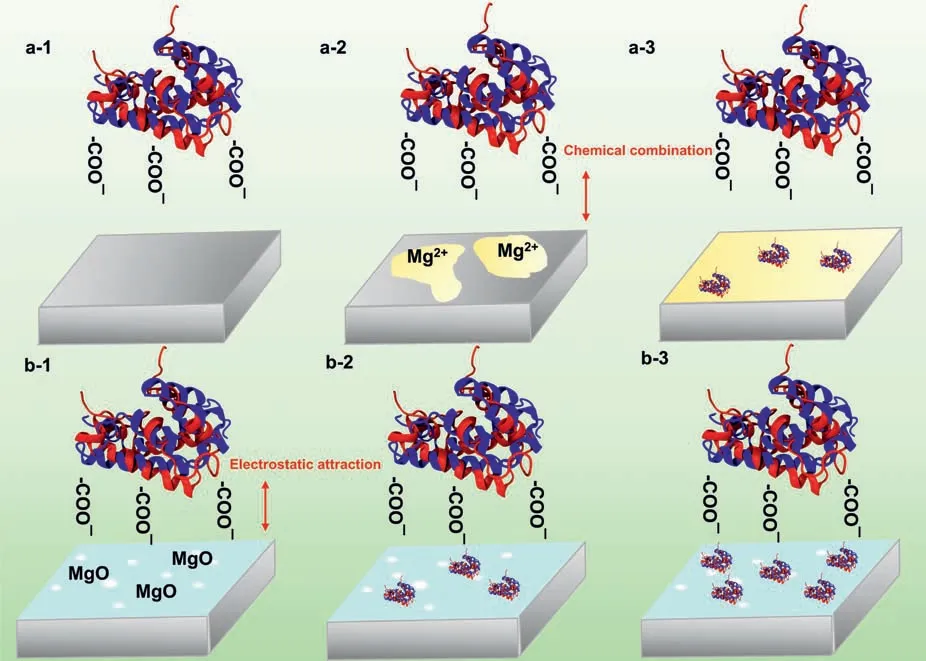
Fig.6.The adsorption behavior of protein on the (a) the AZ31 substrate and (b) MAO coating.
Cell adhesion and proliferation behavior are significantl improved on MAO coating and MAO-BSA coating compared with untreated Mg alloy AZ31.MAO coating can enhance the biocompatibility of Mg alloy substrate because MAO coating mainly composed of MgO means that no toxic elements are introduced.Moreover,MAO coating with porous microstructure provides a high available area for cell adhesion[31].Currently,literature has shown that proteins adsorbed on the surface of the materials can interact with the cell and thus affect the subsequent cell behavior [81,82].In this study,cytocompatibility tests imply that the adsorption of BSA molecules further enhances the cells viability of MAO coating (Fig.5).The functional groups and conformational structures of the adsorbed proteins are expected to mediate the cell behavior.On the one hand,the charge of cell membrane can exert cell behavior,including cell adhesion,locomotion,and cell communication.The absorbed BSA molecules can induce the cells cell migration to the MAO surfaces due to the electrostatic attraction between the exposed -NH3+group with positive charge and the cell membrane with negative charge (Fig.7b).Moreover,the -COO¯group of BSA molecules can promote cell attachment,proliferation and differentiation by playing roles for ligand immobilization with cell membrane surface[56].
On the other hand,the rearrangement of secondary structures of proteins post adsorption has remarked influenc on the cell adhesion.Hence,cell recognition mechanisms can also be explained from the conformational perspective.Grohmann et al.[83] reported thatβ-sheet secondary structures enhance cell adhesion and proliferation over unordered structures becauseβ-sheet provides more rigidity and space for cells to spread.In the study,a higher proportion ofβ-sheet may significantl improve the cell adhesion and spreading on the surface through a strong interaction betweenβ-sheet with positive charge and the charged cell membrane [56].Moreover,theα-helix content of the adsorbed protein molecules plays a key role in regulating cell adhesion;the exposedαhelix of the absorbed BSA molecules can fi the cells on the MAO surface [84].Also,a consistent report has been given by Hasan et al.[85].In summary,the synergistic effect of the functional groups and conformational components of the adsorbed BSA molecules improves the cytocompatibility of MAO coating by mediating cell attachment,proliferation and differentiation.
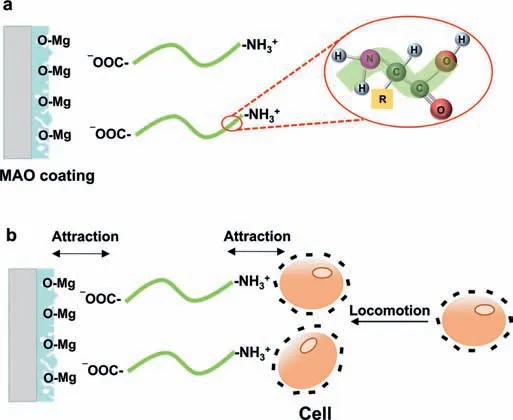
Fig.7.Cell recognition mechanisms of MAO-BSA coating.
5.Conclusions
Adsorption mechanism and conformation change of BSA molecules on the AZ31 Mg substrate and its MAO coatings are elucidated through combining experimental methods and MD simulations,the summarization of results as follows:
1 Negatively charged O atoms in BSA molecules have a strong interaction with Mg2+ions in corrosion product of Mg(OH)2,indicating that Mg2+ions and BSA molecules are combined in an ionic bond to generate(RCH(NH2)COO)2Mg,and then mediate the adsorption of BSA molecules on the AZ31 substrate.
2 MAO coating can capture BSA molecules by electrostatic attraction because of the difference ofpIs(pIMAO12.4>pH 7.4;pIBSA4.7 3 The conformational changes of absorbed BSA molecules on the AZ31 substrate and its MAO coating show a similar trend: a change fromα-helix intoβ-sheet,β-turn and unordered,which relates to the reduction in hydrogen bonds. 4 The synergetic effect of functional groups (-NH3+) and conformational components (β-sheet) of absorbed BSA molecules promotes the adhesion of osteoblasts by immobilizing the charged cell membrane,thus improving the cell compatibility of MAO coating. Declaration of Competing Interest The authors declare that they have no conflic of interest. Acknowledgments This work was supported by the National Natural Science Foundation of China (52071191). Supplementary materials Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2021.04.005.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Grain refinemen of Mg-alloys by native MgO particles: An overview
- Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis
- In silico studies of magnesium-based implants: A review of the current stage and challenges
- Investigating local corrosion processes of magnesium alloys with scanning probe electrochemical techniques: A review
- A tightly bonded reduced graphene oxide coating on magnesium alloy with photothermal effect for tumor therapy
- Nucleation of recrystallization in magnesium alloy grains of varied orientation and the impacts on texture evolution
