Predictors of complicated dengue infections in endemic region of Pakistan
2022-12-28IkramWaheedSamreenKhalidZubiaJamil
Ikram Waheed, Samreen Khalid, Zubia Jamil✉
1Department of Medicine, Fauji Foundation Hospital, Rawalpindi 46000, Pakistan
2Department of Medicine, Foundation University Medical College, Foundation University, DHA Phase 1 Islamabad 44000, Pakistan
ABSTRACT
KEYWORDS: Dengue fever; Severe dengue; Endemic diseases;Thrombocytopenia; Hemorrhage; Shock
1. Introduction
Dengue fever is a systemic illness caused by a mosquito-borne flavivirus, known as dengue virus[1]. Worldwide occurrence of dengue has been rapid enough to affect almost half of world’s population[2,3]. This disease is now being declared endemic in more than 100 countries in World Health Organization (WHO) regions of Africa, Latin America. Eastern Mediterranean, Southeast Asia and Western Pacific, with 70% of global disease burden only from Asia.The number of cases reported to WHO increased to 8-fold over the last two decades from around 500 000 cases in 2000, to 2.4 million in 2010, and 5.2 million in 2019[4]. In Pakistan, dengue fever is endemic with reports of seasonal surges of cases, the last notable outbreak in Autumn 2021 affecting around 50 000 cases with 183 deaths[5].
Dengue virus has 4 serotypes and humans get infected by the bite of female Aedes aegypti and Aedes albopictus mosquitoes[6]. Infecting serotype infers lifelong immunity, but no cross protection is seen among the remaining serotypes and individuals are susceptible to infection by the other 3 serotypes[7]. So, individuals with a previous history of dengue fever infected again with a second serotype are more likely to develop dengue hemorrhagic fever and dengue shock syndrome[8].
Variable clinical manifestations of dengue fever are observed, and this illness is heterogeneous in severity[9]. Almost 80% cases are asymptomatic or have mild illness with flu like symptoms while rest of the cases can progress to severe and fatal disease due to dysregulated host immunological mediators causing plasma leakage compromising hemodynamics and coagulopathy, especially the group of patients having a previous exposure to dengue virus[10].
Several determinants of dengue fever have been identified which can influence progression to severe dengue infection including secondary infections, thrombocytopenia, pre-existing comorbid conditions, dengue affecting children, bleeding from any site,maculopapular rash, raised ALT levels, low albumin, presence of ascites and pleural effusions[11].
The rapidly expanding global burden of dengue especially in an area of endemicity is a public health crisis that is difficult to be dealt without specific therapeutic agents, or efficient vector control strategies. Therefore, it is essential to predict the warning signs associated with progression to severe dengue infection to prevent potentially fatal complications[12].
We conducted a retrospective study in our tertiary care hospital to identify the determinants of severe dengue which is impeccable for patient triage and timely management to prevent mortality in successive periods of endemicity.
2. Materials and methods
2.1. Study design and settings
This single-centered retrospective cross-sectional study was done in the dengue ward of Fauji Foundation Hospital, Rawalpindi from September to November 2021. After an outbreak of dengue endemic in Mid-August 2021, hospital designated 40-bedded ward for management of dengue patients. This ward had all facilities available for patient’s management including 24-hour facility of laboratory testing, availability of ultrasonography and chest X rays as well as crystalloids and colloids fluids for management of dengue hemorrhagic fever and dengue shock syndrome. The hospital has its own facility of blood bank to meet the demand of blood or blood products for patients.
The main objective was to study the determinants that can lead to complicated dengue fever in endemic region of Pakistan.
2.2. Participants characteristics
All patients aged >18 years with dengue infection (NS1 antigen or dengue IgM antibodies positive) admitting to the dengue ward in the study period (September to November 2021) were included in this patient.
Patients with any pre-existing condition that causes or worsens the thrombocytopenia and thus altering our study results were not included. This study excluded the following patients: (1) patients with autoimmune disorder (immune mediated thrombocytopenia,systemic lupus erythematous); (2) patients who were actively infected with hepatitis C or hepatitis B virus infection identified by PCR testing; (3) patients with hypersplenism due to any cause(most common was cirrhosis of liver); (4) pregnant females (HELLP syndrome, gestational thrombocytopenia); (5) patients with bone marrow disorders (leukemia, lymphomas, aplastic anemia etc.);(6) patients on drugs that are likely to cause thrombocytopenia(anti-platelets, heparin); (7) patients in disseminated intravascular coagulation evident by elevated D-dimers (Figure 1).
2.3. Methodology

Figure 1. Flow chart showing the selection and distribution of dengue infected patients.
The ethical acceptance was taken from research committee of Fauji Foundation Hospital, Rawalpindi on August 15th, 2021 with registration number (559/RC/FFH/RWP) before conducting the study. This tertiary care teaching hospital has developed the policy that at the time of admission, informed written consent would be signed by every patient or their relative, in which clinical, laboratory and biochemical data of patients can be utilized for research purposes with complete confidentiality of patient’s personal profile.Clinical and laboratory test results were retrieved by specific medical record number (MR number) allocated to every patient at the time of admission.
To confirm the diagnosis of dengue infection, NS1 antigen was done if illness was <5 days and anti-dengue antibodies IgM by enzyme-linked immunoassay (ELISA) was done if illness was >5 days[13]. Secondary dengue infection was confirmed by anti-dengue antibodies IgG by ELISA in the presence of positive anti-dengue antibodies IgM or NS1 antigen.
Detailed profile and history (age, gender, symptomology, duration of illness, previous symptomatic dengue fever) were recorded. At the time of admission, all vital signs (blood pressure, pulse pressure,pulse and temperature) was recorded along with detailed physical examination.
All laboratory parameters were also noted. About 2-2.5 mL of blood was collected in EDTA tubes and an automated hematology analyzer was used to do complete blood picture (automated hematology analyzer XT-2000i by Sysmex Corporation; Japan). Liver function tests and renal function tests were done by chemistry analyzer (max chemistry analyzer by Dimensions RxL, Siemens Healthineers Laboratories; USA). Ultrasound abdomen was done in all patients at the time of admission when patient got afebrile (leakage phase) and 6-hourly during leakage phase up to 48 hours.
Duration the hospital stay, the number of patients survived and died was also noted. Patients were classified into three groups according to disease severity: (1) dengue fever (DF, uncomplicated dengue infection); (2) dengue hemorrhagic fever (DHF, complicated dengue infection); (3) dengue shock syndrome (DSS, complicated dengue infection).
DF: Person with acute febrile illness of >2 days but <10 days with two or more than two manifestations including retrobular pain,severe headache, severe muscular pain, severe backache, myalgias/arthalgias, or joint pain and platelets <150 000[13].
DHF: All of the features mentioned below must be present for DHF[14]: (1) platelet ≤100 000/μL; (2) acute febrile illness of >2 days but <10 days; (3) at least one of the following features as evidence of hemorrhage phenomenon: (a) purpura, ecchymoses or petechiae; (b)bleeding from gastrointestinal tract, injection sites, mucosa or other sites; (c) tourniquet test is positive; (d) melena or hematemesis. (4)At least one of the following features as evidence of plasma leakage:(a) pleural effusion or ascites, or evidence of hypoalbuminemia(plasma leakage signs); (b) an increase in hematocrit 20% from previous hematocrit (hemoconcentration).
DSS: above mentioned features for DHF plus evidence of circulatory failure: pulse pressure ≤20 mmHg + feeble and weak pulse, or manifested by cold and clammy body, hypotension or irritablility[14].
2.4. Statistical analysis
Statistical analysis was done with the help of MedCalc Statistical Software 19.6.4 (MedCalc software, Ostend, Belgium) and version 26 of SPSS. The percentages were used for qualitative variables.Ranges, means and standard deviation were used for quantitative variables. The patients were classified into three categories; DF, DHF and DSS. The comparison between qualitative variables were done by Chi-square testing while comparison of quantitative variables was done by one-way ANOVA. In the end, predictors of complicated dengue infection were first determined by univariate regression analysis and then confirmed by multi-variate regression analysis.
3. Results
Totally, 129 patients were admitted to the dengue ward of the tertiary care hospital from September to November 2021 and they were included in this study. The main aim of our study was to study determinants that complicate the disease course of dengue infection in endemic region of Pakistan.
The mean age was (40.05 ± 19.07) (range: 18-90) years. Among 129 patients, 63.6% (n=82) were females and 36.4% (n=47) were males.
The mean day of illness at the time of admission was (4.61 ±1.62) (range: 2-11) days. The most common presenting symptom was fever that was present in 96.9% (n=125) patients. The second common symptom was body aches 76.0% (n=98), followed by nausea 73.6% (n=95), abdominal pain 66.7% (n=86), headache 65.9% (n=85) and vomiting 65.1% (n=84). Maculopapular rash(diffuse or localized) was present in 54.3% (n=70) of patients.Around 45.0% (n=58) patients presented with diarrhea. Conjunctival hemorrhage was present in 38.0% (n=49) patients. A total of 35.7%(n=46) patients presented with bleeding from any site of body and 35.7% (n=46) patients presented with right hypochondria tenderness;throat congestion was presenting symptom in 33.3% (n=43) dengue patients.
At the time of admission, mean systolic blood pressure of dengue patients was (116.81 ± 15.15) (range: 90-180) mmHg and diastolic blood pressure was (73.95 ± 10.11) (range: 50-100) mmHg with pulse pressure of (42.68 ± 11.06) (range: 10-90) mmHg. Mean pulse was (89.09 ± 15.11) (60-150) bpm and mean temperature was(100.89 ± 9.50) (range: 98.5-104) degrees Fahrenheit.
Among 129 patients, 62.8% (n=81) had only simple DF while 31.0% (n=40) presented as DHF. Trivial number of patients 6.20%(n=8) developed dengue shock syndrome.
When we compared the clinical parameters of three groups of dengue patients, we found that median age of patients developing DSS was 25 years compared to median age of 38 years in patients who had uncomplicated DF (P<0.05); while median age of patients with DHF was 35 years, indicating that young patients develop complicated dengue (DHF and DSS) as compared to old age patients. Similarly, patients with complicated dengue infection had multiple family members (>5) affected.
Interesting results were obtained regarding the history of previous symptomatic dengue infection. Only 28.4% patients developing uncomplicated DF were previously infected with dengue compared to DSS and DHF in which 100% and 72.5% respectively had previously symptomatic dengue infection (P<0.05), indicating that patients with secondary dengue infection are more prone to develop DHF and DSS.
Statistically significant symptoms present in complicated dengue fever were maculopapular rash, diarrhea, conjunctival hemorrhages,bleeding from any site of body and right hypochondrial tenderness(P<0.05). As complicated dengue fever was more pronounced in young group, we didn’t find any association of co-morbidities with complicated dengue fever. The comparison of clinical parameters,presence of co-morbidities in three stages of dengue patients is shown in Table 1 and comparison of symptomology in these stages of dengue patients is shown in Table 2.
When we compared the laboratory parameters of three stages of dengue, we found that the patients with DSS had more leucopenia, thrombocytopenia and elevated ALT compared to DF(P<0.05) (Table 3).
Interesting results were obtained regarding positivity of IgG levels among these three groups. Complicated dengue infection (DHF and DSS) had positivity percentages of 77.5% and 100%, respectively(P<0.05) showing that secondary dengue infection plays a critical role in complicating the disease course of dengue infection.
Radiological findings (ascites, gall bladder wall thickness and pleural effusion) were mostly present in complicated dengue fever.Patients with DSS had prolonged hospital stay compared to patients with DF (P<0.05).
Overall mortality rate in the study cohort was 3.9% (5/129). When we compared mortality percentages among three groups of dengue infection, we found that 50.0% of patients with DSS died while mortality percentages of DHF were 2.5% and none of the patient with simple DF died (P<0.05). Table 3 showed the comparison of laboratory parameters, radiological findings and clinical outcomes among three stages (DF, DHF and DSS) of dengue patients.
In the end, regression analysis was used to determine the determinants influencing the severity of dengue infection. All variables were tested with univariate regression analysis, then only those variables found to be significant were further tested bymultivariate analysis. Omnibus tests showed that the model was statistically fit for analysis (Chi-square test=43.18, P<0.01). The model covered 31% to 41% variation of variables (Cox and Snell pseudo R2and Nagelkerke pseudo R2respectively) and classified 80.5% of cases. At time of admission, severe body aches, bleeding from any site of body, conjunctival hemorrhage, presence of maculopapular rash and right hypochondrial tenderness were found to be predictors of complicated dengue among symptomology.Secondary dengue infection (previous symptomatic dengue infection or IgG positive levels), low platelet count, raised ALT levels, presence of ascites and pleural effusion were found to be independent predictors of complicated dengue in these patients. The various predictors that significantly influence the severity of dengue infection among the study cohort is shown in Table 4.

Table 1. Comparison of clinical parameters and presence of co-morbidities among three stages (dengue fever, dengue hemorrhagic fever and dengue shock syndrome) of dengue patients.
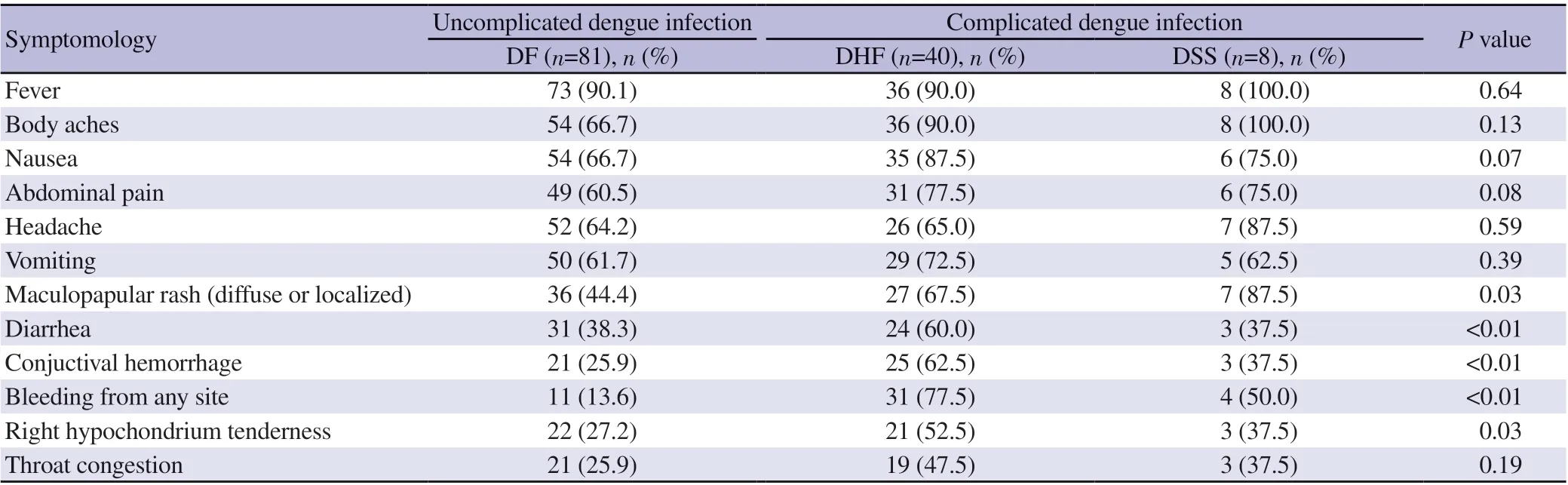
Table 2. Comparison of symptomology among three stages (dengue fever, dengue hemorrhagic fever and dengue shock syndrome) of dengue patients.

Table 3. Comparison of laboratory parameters, radiological findings and clinical outcome among three stages (dengue fever, dengue hemorrhagic fever and dengue shock syndrome) of dengue patients.

Table 4. Cox regression analysis showing variables in predicting the severity of dengue infection among the study cohort.
4. Discussion
Life threatening complications of dengue fever can be prevented if several determinants are identified early in the disease course. In our study, we compared the three sets of patients presented to us: simple DF, DHF and DSS. Then we identified the factors that can lead to progression of disease severity and affect the mortality.
In a total of 129 patients admitted to our tertiary care hospital,62.8% had simple DF while 31.0% presented as DHF and only 6.2% developed DSS. Many patients had multiple family members affected following incubation period of 1-2 weeks.
Secondary infection by a second heterologous dengue virus is identified as a major risk factor and predictor of severity in dengue fever in our study. In DSS group, 100% patients had prior history of symptomatic dengue infections while 72.5% of patients with DHF had been previously infected with dengue. Only 28.4% patients with uncomplicated DF are found to have history of dengue fever before.Supporting this evidence, IgG levels were found to be positive in 100% and 77.5% of patients with DSS and DHF respectively,indicating that individuals with secondary infection are more prone to develop complicated dengue fever as analyzed by other studies[7,15-17].
Several studies have acknowledged a second heterologous dengue virus infection as a principal risk factor for severe dengue infections[18]. Antibody dependent enhancement of dengue virus infection has been proposed as the early mechanism in dengue hemorrhagic fevers[19]. Dengue cross reactive antibodies raised following a first dengue infection combining with a second infecting virus to form infectious immune complexes that enter host cells resulting in increased number of infected cells. In the later stage,high levels of cytokines result in vascular permeability leading to shock and death[8].
Our study showed an overall mortality of 3.9% (5/129) which is similar to the mortality rate of 3% in a study conducted by Ahmad et al.[20] and 3.2% in severe dengue patients observed in a study by Lee et al[21]. However, another study showed a higher overall mortality rate of 6%[22].
Among the groups, high mortality of 50% is observed in DSS patients (4 out of 8 died), comparable to mortality of 42% in DSS group in a similar study[20] and a prolonged hospital stay of 9 days as compared to 3-4 days of admission in other groups. A similar study showed a high mortality of 71.4% in DSS patients[23]. However,only 2.5% (1/40) patients died in DHF group probably because of worsening renal failure. No mortality is observed in simple DF group.
In our study, apart from secondary infections as a major risk factor for developing DSS and DHF, most common symptoms observed were diarrhea, maculopapular rash, conjunctival hemorrhages,bleeding from any site of body, and right hypochondrial tenderness which were statistically significant. These findings are consistent with a large meta-analysis which showed similar symptoms and risk factors for severity of dengue fever[11,24]. These warning signs should alert the physicians for early admission to hospital, close observation, and prompt but judicious use of intravenous hydration therapy which is the current mainstay therapy for most symptomatic patients.
In addition, complicated dengue patients admitted with us had marked leucopenia, thrombocytopenia, elevated ALT levels. This is similar to a few case studies which have predicted raised ALT as a marker of severity before the development of acute liver failure in dengue patients[25,26]. There is evidence of increased vascular permeability showing ascites, pleural effusion and gall bladder wall thickness in patients radiological findings. All these clinical, biochemical and radiological findings contribute and act synergistically to cause severe infections which should be identified early to improvise immediate indoor management and reduce mortality.
We also studied the independent predictors of severity in dengue fever in our case study and found that among symptoms, severe body aches, bleeding from any site of body, conjunctival hemorrhages,right hypochondrial tenderness and maculopapular rash were statistically significant determinants influencing complications in dengue fever. Deranged liver function tests with right hypochondrial tenderness and hepatomegaly are seen in many patients with DHF in another study[27]. Similarly, bleeding from any site most commonly gastrointestinal tract is a significant predictor of adverse outcomes in dengue in another study[27].
In addition, our study showed that secondary dengue infections,severe thrombocytopenia (platelet count <50 000/uL), raised ALT levels, and presence of ascites and pleural effusions were independent predictors that can affect survival outcomes in complicated dengue fever. Identical risk predictors for morbidity and mortality in dengue fever are observed in a case study by Badreddine et al[28]. All these warning signs in our study are consistent with the WHO alarming signs in severe dengue as well as a few recently observed factors such as secondary infection, gastrointestinal and mucosal bleeding and gall bladder wall thickening as risk predictors of severe dengue as studied in a meta-analysis by Htun et al[29].
Although single-centered, retrospective nature of study and nonavailability of serotyping of dengue virus are the limitations of this study, yet it is unique in this aspect that it encompasses various parameters (clinical signs, symptomology, laboratory, radiological findings) to assess severity of dengue fever in the single study.
In conclusion, all these alarming clinical and laboratory determinants associated with progression to severe dengue infection are of paramount importance in aiding physicians in patients’ triage to decrease mortality especially in endemic seasons of dengue fever(spring and autumn) in Pakistan. Efficient vector control strategies and development of effective dengue vaccine are an urgent need of recent times to reduce the globally expanding health burden posed by dengue fever.
Conflict of interest statement
The authors have no conflict of interest to disclose.
AcknowledgementsThe authors would like to acknowledge house officers and post graduate trainees who helped in the data collection. The authors are thankful to MedCalc software limited, Ostend, Belgium for providing free MedCalc software that helped a lot in statistical analysis.
Funding
The authors received no extramural funding for the study.
Authors’contributionsBoth ZJ and SK contributed to the study concept, design, analysis and interpretation of data. IK conducted investigations. Both IK and SK carried out data curation and wrote the original manuscript. ZJ contributed to the final version of the manuscript. All listed authors contributed significantly to the creation of this manuscript, each having fulfilled criteria as established by the ICMJE and all who meet the four criteria are identified as authors. All authors have read and approved the final manuscript.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Etiology of granulomatous inflammation: A retrospective study of 174 children in a tertiary care center
- Detection of SARS-CoV-2 Eta VOI among international travelers using COVIDSeq-NGS
- COVID-19-induced anxiety, depression and stress among healthcare professionals in Sri Lanka
- Prevalence and risk factors associated with Japanese encephalitis virus infection in swine population of Assam, India
- Intestinal parasitic infections in Aetas and domesticated swine in Pampanga,Philippines
- Post-discharge mortality in the first wave of COVID-19 in Turkey
