Ni(Ⅱ)掺杂分层多孔Cu-BTC高效去除水体中的四环素
2022-12-06陈艳霞谭力川袁光松冯思文王萃娟
陈艳霞 谭力川 王 鹏 袁光松 冯思文 王萃娟*, 童 妍 徐 敏
(1西南交通大学生命科学与工程学院化学化工系,成都 610031)
(2成都市第三人民医院,成都 610031)
Antibiotics are effective in inhibiting the growth of microorganisms and are commonly used in the pharmaceutical and livestock industries[1].However,the overuse and abuse of antibiotics have caused serious environmental pollution[2].Tetracycline(TC)is a widely used antibiotic for effective sterilization and prevention of livestock infections[3].However,its actual dosage in the livestock industry is numerous,causing a largescale TC flow into water bodies with livestock manure,polluting the environment[4].At the same time,the ingestion of TC by other organisms in the environment can pose a serious threat to human health as it accumulates in the food chain[5].Therefore,the effective removal of residual TC from the environment is very important and valuable.
In general,adsorption[6],membrane separation[7],photodegradation[8],and advanced oxidation[9]are commonly used methods for TC removal,among which,adsorption has the advantages of high adsorption,simple operation,and low cost that is widely used[10-11].Among a series of adsorbents,MOFs are a combination of inorganic-organic materials self-assembled by metal ions or clusters and organic ligands,which have attracted a lot of attention in adsorption research because of their modifiable pore size and high specific surface area[12-13]and are also used as adsorbents for antibiotic removal[14-15].However,most MOFs are microporous in structure,resulting in slow mass transfer rates and difficult access to open channels or active sites[16].Therefore,it is ideal to introduce mesopores or macropores into conventional micropores,which not only maintain the inherent advantages of MOFs in terms of components and structure to a certain extent but also have hierarchical pores to ensure the accessibility of active sites and the diffusion of molecules.
There is a large amount of research dedicated to expanding the pore size of MOFs,such as hybrid ligands[17],post-synthetic modification[18],template[19],and template-free methods[20].Among them,the templatefree method is of great interest because it minimizes the preparation cost,does not require surfactants or other special synthesis conditions,and reduces the complexity of the production process.Cao et al.[21]proposed a temperature-controlled and template-free strategy for the synthesis of hierarchical porous Cu-BTC(H3BTC=benzene-1,3,5-tricarboxylic acid)by adding small amounts of acetic acid and triethylamine as modifiers to regulate the crystallization rate of the crystals.Abdelhamid et al.[22]reported that the in situ formation of ZnO nitrate nanosheets can be promoted by adding sodium hydroxide to synthesize hierarchical porous ZIF-8 without introducing any template.
In addition,MOFs can be functionalized by various modification strategies(organic node modification,in situ doping,or loading)to enhance the adsorption capacity[23-25].Recently,various reports have shown that modification with metal doping techniques can impart additional adsorption sites to MOFs and thus improve their adsorption capacity.Yang et al.[26-27]reported that UiO-66 and UiO-67 doped Ce(Ⅲ)could significantly improve the adsorption capacity of various dyes through an adsorption drive.Sun et al.[28]reported that Cu-doped ZIF-8 used for adsorption of TC hydrochloride significantly improved the adsorption capacity,which was 2.4 times higher than that of pristine ZIF-8.
Therefore,in our study,we combined expanded pore size and metal doping techniques to synthesize hierarchical porous Cu-BTC based on the template-free and temperature-controlled methods,and added Ni(Ⅱ)to the synthetic precursor solution to synthesize metal Ni(Ⅱ)-doped hierarchical porous Cu-BTC,which was used for the first time for the adsorption of TC.We also studied the adsorption kinetics,and adsorption isotherms,and proposed a possible adsorption mechanism.
1 Experimental
1.1 Materials and reagents
Copper nitrate trihydrate(Cu(NO3)2·3H2O)was acquired from Shanghai Maclean Biochemical Technology Co.,Ltd.(Shanghai,China).1,3,5-Benzene-tri benzoic acid (H3BTC),nickel nitrate hexahydrate(Ni(NO3)2·6H2O),and TC were acquired from Tianjin Heowns Biochemical Technology Co.,Ltd.(Tianjin,China).Triethylamine(TEA),acetic acid(CH3COOH),and ethanol were purchased from Chengdu Shiyang Chemical Reagent Factory.
1.2 Synthesis of hierarchical porous Cu-BTC
The hierarchical porous Cu-BTC(HP-Cu-BTC)was synthesized based on the literature[21].Firstly,1.8 mmol Cu(NO3)2·3H2O was dissolved in 12 mL ethanol.Then,0.62 mL CH3COOH and 0.5 mL TEA were added to the solution.The mixture was ultrasonically mixed well and then stirred at room temperature.After stirring for 1 h,1.0 mmol H3BTC was added to the mixture and stirred for 2 h to form a homogeneous solution,which was poured into an autoclave and reacted at 110℃for 24 h.Finally,the obtained product was washed with ethanol,collected by centrifugation,and dried in an oven at 60℃.
1.3 Synthesis of Ni(Ⅱ)-doped hierarchical porous Cu-BTC
The method was the same as that described above for the preparation of HP-Cu-BTC,except that different masses(0.532,0.262,0.174 mg)of Ni(NO3)2·6H2O were added in the first step in molar ratios of 1∶1,1∶2,and 1∶3 with Cu(NO3)2·3H2O,respectively.The other steps were the same,and the sample powders obtained were named HP-Ni-Cu-BTC(1∶1),HP-Ni-Cu-BTC(1∶2),and HP-Ni-Cu-BTC(1∶3),respectively.
1.4 Characterizations
Scanning electron microscope(SEM)images of HP-Ni-Cu-BTC were obtained on a ZEISS Sigma 500 SEM using an acceleration voltage of 5 kV,and a working distance of 4 mm.Energy-dispersive X-ray spectroscopy(EDS)mapping was performed using X-ray transmission electron microscopy energy spectrometer(Apollo XLT SDD)with an acceleration voltage of 15 kV,and a working distance of 5.8 mm.Powder X-ray diffraction (XRD) patterns were obtained using EMPYREAN diffractometer(Netherlands,PANalytical)in a 2θ range from 5°to 80°with a step size of 0.02°and a test speed of 0.1 s·step-1,with Cu Kα ion source radiation(λ=0.154 06 nm),a voltage of 40 kV,a current of 40 mA.Fourier transform-infrared(FT-IR)analyses were obtained using KBr tablets in a range of 4 000-400 cm-1on an infrared spectrometer(Thermo Nicolet 5700,America).N2adsorption-desorption isotherm measurements were acquired using an automatic gas adsorption analyzer at 77 K(ASAP2020,America).The specific surface areas were calculated using the Brunauer-Emmett-Teller(BET)method,and pore size distribution was based on a nonlocalized density functional theory(NLDFT)model.Thermal gravimetric analyses(TGA,Mettler-Toledo TGDSC3+)were measured from 30 to 600℃under a nitrogen atmosphere at 10℃·min-1.
1.5 Adsorption experiments
The adsorption performance of HP-Ni-Cu-BTC on TC was investigated by batch adsorption experiments.Specifically,a certain amount of TC was dissolved in deionized water respectively,thereby preparing a solution with different concentrations(10,30,50,70,and 100 mg·L-1).Then,30 mL of the known concentration of TC solution was added to a conical flask and 5 mg of adsorbent was added to the TC solution.Next,the flask was shaken in a shaker at room temperature,and 3 mL of the sample was taken out and filtered by centrifugation at a set time.Finally,the absorbance of the supernatant was measured in a spectrophotometer(UV-1800PC)at 357 nm.The adsorption capacity was calculated by the following Equation 1:

Where q was the adsorption capacity of TC,mg·g-1;c0was the initial concentration of the TC solution,mg·mL-1;ctwas the concentration of the supernatant at moment t,mg·mL-1;V represented the volume of the antibiotic solution,mL;m represented the mass of the adsorbent,mg.
In addition,the effects of adsorption performance were investigated at different pH values(4,5,6,7,8),and adsorbent addition amounts(5,8,10,12,15 mg),respectively.The adsorption kinetics and adsorption isotherms were calculated based on the adsorption capacity at different TC concentrations(10,30,50,70,100 mg·L-1).
After performing adsorption experiments,the recoverability of the adsorbent was evaluated.Specifically,20 mg of adsorbent was dispersed in 30 mL TC solution,the adsorbent was separated by centrifugation,washed by ultrasonication in ethanol,cycled three times,dried,and reused in the next adsorption process.
2 Results and discussion
2.1 Characterizations
The morphology of the HP-Cu-BTC and HP-Ni-Cu-BTC(1∶2)were photographed by SEM(Fig.1),which showed that the hierarchical pores were formed by the accumulation of nanoscale Cu-BTC crystals and that the Ni(Ⅱ)ion doping had no significant effect on the size and morphology of the obtained particles.For the synthesized HP-Ni-Cu-BTC(1∶2),the Ni/Cu molar ratio in the framework was 0.42,confirming the presence of Ni(Ⅱ),and its EDS spectrum(Fig.2)showed a uniform distribution of each element,further indicating the successful synthesis of HP-Ni-Cu-BTC(1∶2).
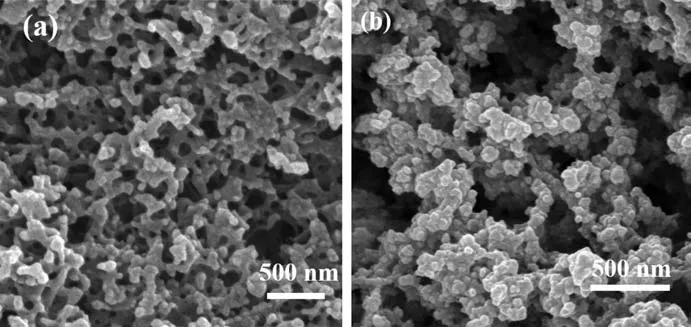
Fig.1 SEM images of(a)HP-Cu-BTC and(b)HP-Ni-Cu-BTC(1∶2)
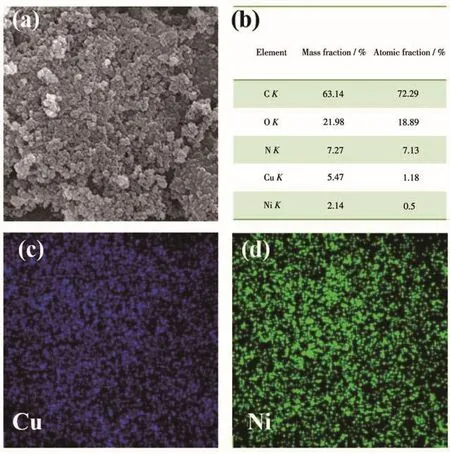
Fig.2 (a)SEM image,(b)EDS elemental contents,and elemental distribution of(c)Cu and(d)Ni of HP-Ni-Cu-BTC(1∶2)
The compositions and crystal structures of the synthesized samples were characterized by FT-IR and XRD(Fig.3).FT-IR patterns showed that the characteristic peaks of Ni(Ⅱ)-doped Cu-BTC and HP-Cu-BTC were consistent with those of undoped microporous Cu-BTC and HP-Cu-BTC,demonstrating that the skeletal structure of Cu-BTC was not impacted by Ni(Ⅱ).XRD patterns also showed that all samples had similar diffraction peaks and no extra additional diffraction peaks were noticed after doping with Ni(Ⅱ),indicating that the doping of Ni(Ⅱ)did not affect the crystal structure of the material.All the above characterizations proved the successful synthesis of the samples.
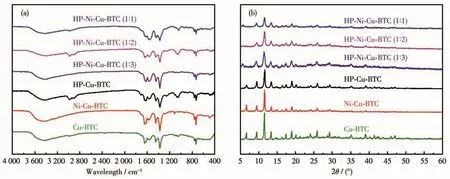
Fig.3 (a)FT-IR spectra and(b)XRD patterns of Cu-BTC,Ni-Cu-BTC,HP-Cu-BTC,and HP-Ni-Cu-BTC
The thermal stability of Ni(Ⅱ)-doped HP-Cu-BTC(1∶2)was investigated by TGA curves.Fig.4a showed some differences between the TGA curves of undoped and doped Ni(Ⅱ),but the doping of Ni(Ⅱ) did not affect the thermal stability of HP-Cu-BTC.The first weight loss of HP-Ni-Cu-BTC(1∶2)(90%)was smaller than that of undoped HP-Cu-BTC(80%),which was due to the mass loss in the first stage mainly attributed to the loss of guest molecules,and the smaller pore volume in the MOF after Ni(Ⅱ)doping leading to the reduction of guest molecules in its pores.The mass loss of HP-Ni-Cu-BTC(1∶2)in the second stage was greater than that of HP-Cu-BTC,indicating that the residual solids of undoped Ni(Ⅱ)were higher and further indicating that Ni(Ⅱ)was successfully doped into the backbone of HP-Cu-BTC[29].
The N2adsorption-desorption isotherms were further researched(Fig.4b).Cu-BTC and Ni-Cu-BTC showed Ⅰ-type adsorption isotherms,indicating typical microporous structures,while HP-Cu-BTC and HP-Ni-Cu-BTC(1∶2)had obvious hysteresis loops at high partial pressure,suggesting the presence of mesoporous structures.Table S1(Supporting information)lists some pore parameters.The results showed that the specific surface area and pore volume decreased after doping Ni(Ⅱ).
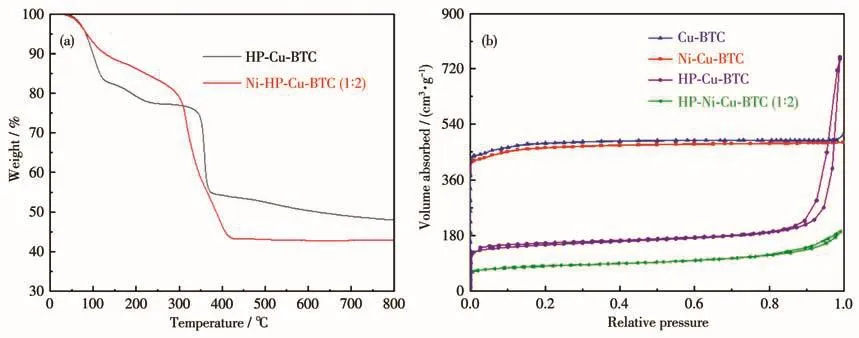
Fig.4 (a)TGA curves of HP-Cu-BTC and HP-Ni-Cu-BTC(1∶2);(b)N2 adsorption-desorption isotherms of Cu-BTC,Ni-Cu-BTC,HP-Cu-BTC,and HP-Ni-Cu-BTC(1∶2)
2.2 Adsorption experiment
To investigate the effect of Ni(Ⅱ)-doped HP-Cu-BTC on the adsorption of TC,we also synthesized Cu-BTC,Ni-Cu-BTC,and HP-Cu-BTC,which were used to adsorb TC under the same conditions as a comparison.Fig.5a showed their adsorption capacities were(52.4±2.5)mg·g-1,(69.4±3.3)mg·g-1,(96.0±3.1)mg·g-1,and(171.8±1.6)mg·g-1,respectively.The adsorption capacity of the Ni(Ⅱ)-doped samples was larger than that of the undoped samples.In addition,the hier-archical porous structure also facilitated the improvement of the adsorption capacity.Meanwhile,we also investigated the effect of Ni(Ⅱ)addition on adsorption,Fig.5b demonstrated that the adsorption capacities of Ni(Ⅱ) doped with different ratios were(116.3±0.7)mg·g-1,(171.8±1.6)mg·g-1,and (134.4±2.6)mg·g-1,respectively.As the addition of doped Ni(Ⅱ)increased,it contributed to improving adsorption,but the adsorption capacity decreased when too much was added,probably because more Ni(Ⅱ)was introduced into the adsorbent,which may block the pores and lead to increase diffusion resistance in the mass transfer reaction and reduce the accessible active sites.Therefore,in the subsequent optimization experiments,we chose an adsorbent with a molar ratio of 1∶2 as the adsorbent model for the following experiments.
The effects of adsorbent dosage and pH on the adsorption performance were investigated.With the increase of adsorbent addition from 3 to 15 mg,the adsorption efficiency gradually increased,while the adsorption capacity decreased,because the equilibrium concentration of TC would be lower when more adsorbent was added(Fig.5c).Therefore,based on the principle of economy,5 mg of adsorbent was chosen to in the later experiments to obtain higher adsorption capacity and removal efficiency.The adsorption capacity increased with increasing pH from 4 to 6,and reached the maximum at pH 6(Fig.5d).It may be because at pH 4-7,both the adsorbent and TC are heterogeneously charged and electrostatic attraction occurs to increase the adsorption capacity.For comparison purposes,Table 1 summarizes several recent reports on the adsorption of TC by different adsorbents and their adsorption capacities.Compared with other materials,the Ni(Ⅱ)-doped hierarchical porous Cu-BTC exhibited excellent adsorption capacity.2.2.1 Adsorption kinetics

Table 1 Comparison of the adsorption performance of different adsorbents on TC
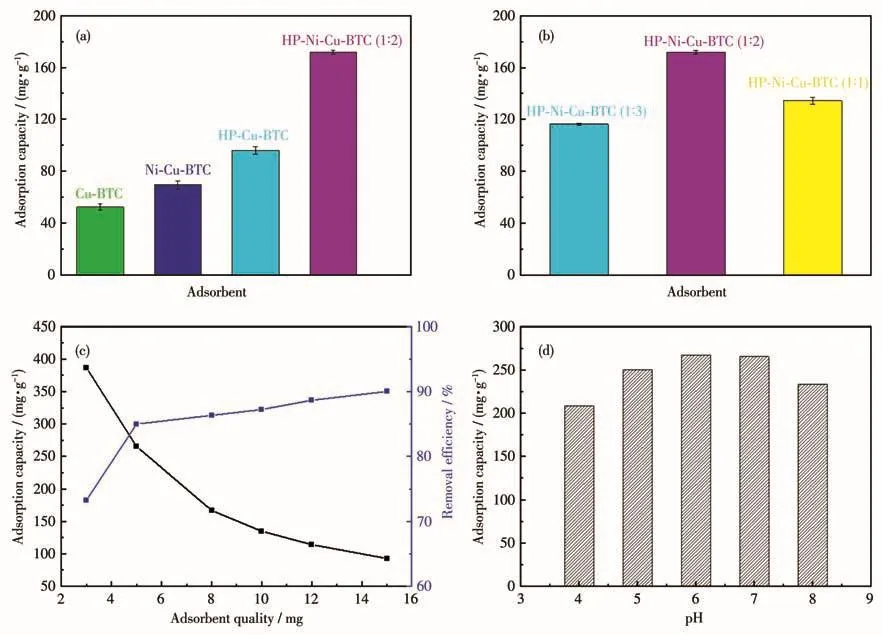
Fig.5 (a)Adsorption capacities of different adsorbents on TC(m=5 mg,cTC,0=30 mg·L-1,V=30 mL);(b)Adsorption capacities of HP-Cu-BTC doped with different molar ratios of Ni(Ⅱ) on TC(m=5 mg,cTC,0=30 mg·L-1,V=30 mL);Effect of(c)adsorbent addition(cTC,0=50 mg·L-1,V=30 mL)and(d)pH(m=5 mg,cTC,0=50 mg·L-1,V=30 mL)on the adsorption capacity of TC
To further investigate the relationship between adsorption capacity and time,the pseudo-first-order model and pseudo-second-order model were used to describe the adsorption behavior of HP-Ni-Cu-BTC(1∶2)on TC.Formulas 2 and 3 were as follows:

Where qtwas the adsorption capacity of the adsorbent for TC at t time,mg·g-1;qewas the equilibrium adsorption capacity,mg·g-1;k1was pseudo-first-order model adsorption rate constant,min-1;k2was the pseudosecond-order model adsorption rate constant,g·mg-1·min-1.The corresponding fitted curves and parameters of the experimental results were shown in Fig.6 and Table 2(qe,calwas the calculated equilibrium adsorption capacity after fitting,mg·g-1).It is shown that the adsorption dates of TC on HP-Ni-Cu-BTC(1∶2)were more consistent with the pseudo-second-order,which had a higher linear R2and calculated the theoretical values closer to the experimental values.In the adsorption process,the TC molecules were adsorbed from the solution to the outer surface of HP-Ni-Cu-BTC(1∶2)and diffused into the pore,and the rate-limiting step was a chemisorption process between HP-Ni-Cu-BTC(1∶2)and TC molecules.
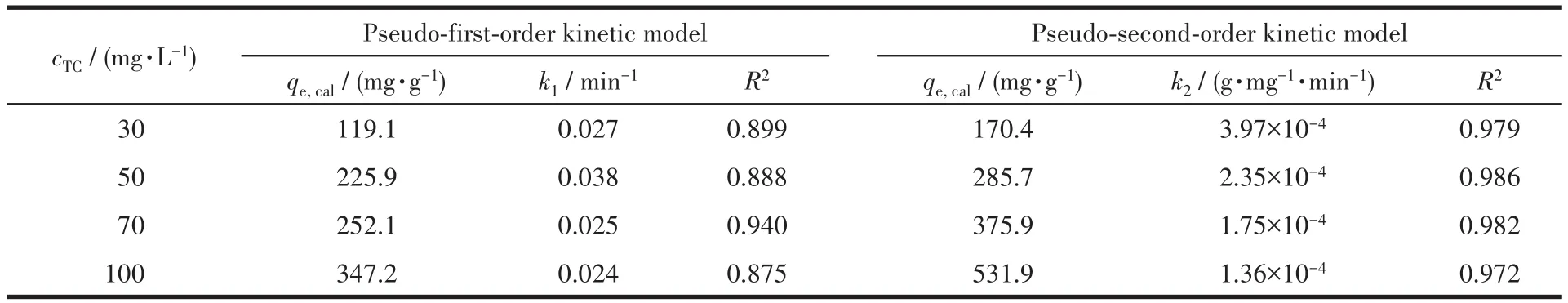
Table 2 Pseudo-first-order model and pseudo-second-order model parameters of TC adsorption on HP-Ni-Cu-BTC(1∶2)
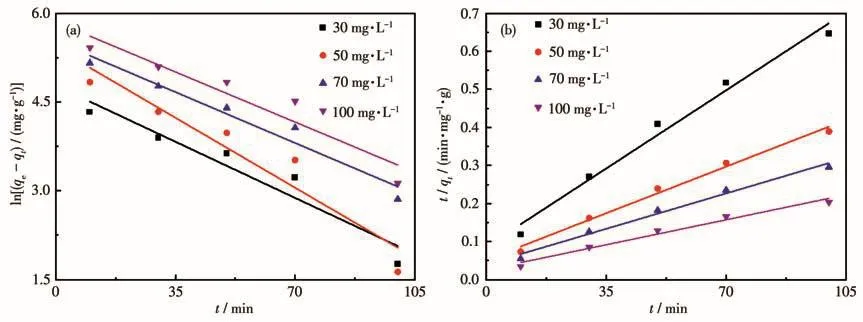
Fig.6 Dynamic simulation for TC adsorption onto HP-Ni-Cu-BTC(1∶2):(a)pseudo-first-order model and(b)pseudo-second-order kinetic model
2.2.2 Adsorption isotherms
Adsorption isotherms were described using Langmuir,Freundlich,and Temkin models.Therein,the Langmuir model was used in the ideal case of monolayer adsorption and Freundlich isotherms are usually suitable for non-ideal adsorption on heteroge-neous surfaces[32].The equations for the three models were as follows:
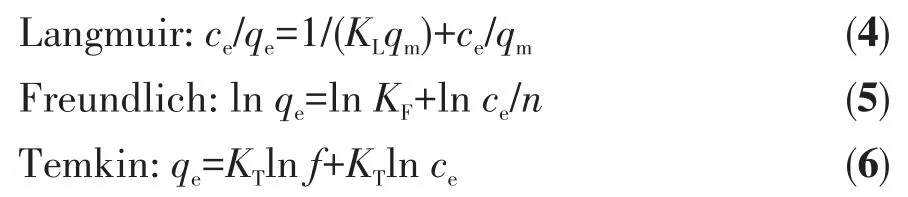
Where cewas the concentration at equilibrium,mg·L-1;KLwas the adsorption constant of Langmuir,L·mg-1;qmwas the fitted maximum adsorption capacity(mg·g-1);KF(mg·g-1-1/n·L1/n)and n were the adsorptions constant of Freundlich;KTand f(L·mg-1)were Temkin constant.Fig.7 and Table 3 showed the fitting results of the Langmuir,Freundlich,and Temkin isotherm adsorption models.R2values demonstrated that the Freundlich model was best fitted in comparison to the Langmuir and Temkin models.It showed that the adsorption of TC by HP-Ni-Cu-BTC(1∶2)was more consistent with the Freundlich equation for multilayer adsorption.These results were consistent with the kinetic fitting results,suggesting that the adsorption mechanism involved chemisorption processes.
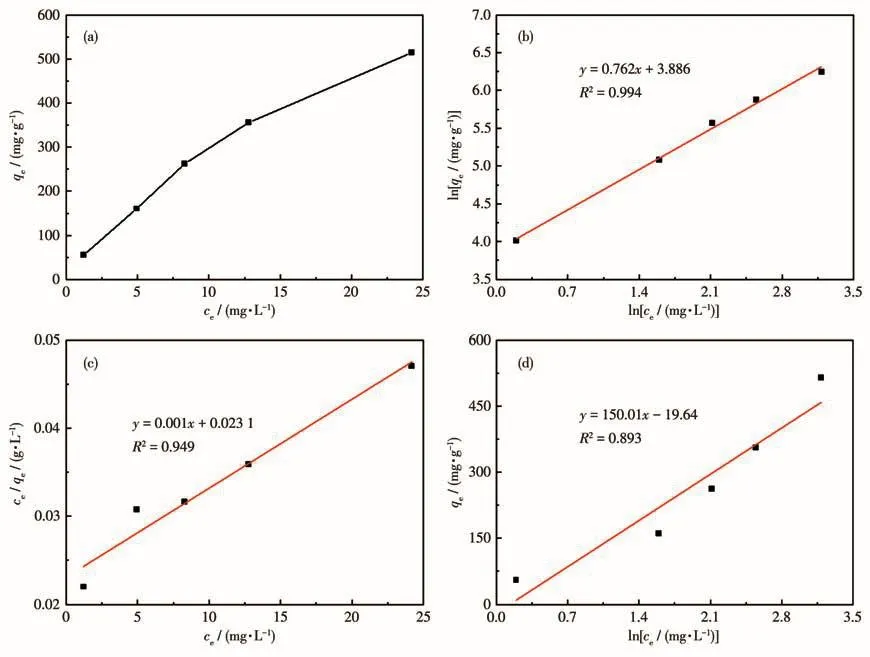
Fig.7 (a)Adsorption isotherms of TC adsorption on HP-Ni-Cu-BTC(1∶2)and the corresponding(b)Freundlich fit,(c)Langmuir fit,and(d)Temkin fit

Table 3 Corresponding three model parameters of TC adsorption on HP-Ni-Cu-BTC(1∶2)
2.2.3 Adsorption mechanism
Possible mechanisms were proposed based on the available experimental results(pH,kinetics,isotherms),as shown in Fig.8.It is well known TC is a ternary acid and appears as differently charged ions in different pH environments,and its three acid dissociation constants are 3.3,7.7 and 9.7,respectively[33-35].Fig.S1 showed that the ζ potential of the adsorbent was negatively charged in the pH solution from 4 to 8.When pH from 4 to 7,the adsorbent and TC were heterogeneously charged and electrostatic attraction enhanced the adsorption performance.For pH>7.7,adsorbent and TC were negatively charged and electrostatic phase repulsion weakened the adsorption.Furthermore,in kinetic experiments,it was shown that chemisorption dominated the process of TC adsorption by HP-Ni-Cu-BTC(1∶2),suggesting TC may also interact with functional groups on the adsorbent,with π -π bonds and hydrogen bonds[36].In addition,from the results of TC adsorption by different adsorbents,it can be concluded that the adsorption capacity of Ni(Ⅱ)doped HP-Cu-BTC was significantly higher than the other three adsorbents,indicating that the hierarchical porous structure facilitates the diffusion of TC to the active sites inside the material and that the doping of Ni(Ⅱ)can provide valence electrons and increase the adsorption sites,thus improving the adsorption capacity.
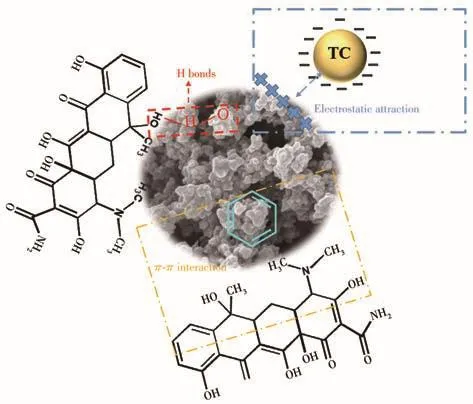
Fig.8 Main adsorption mechanism of TC on HP-Ni-Cu-BTC(1∶2)
In actual wastewater,TC molecules may coexist with other inorganic ions.Therefore,we investigated the effect of HP-Ni-Cu-BTC(1∶2)on the adsorption of TC in the presence of 40 mmol·L-1Cl-and CO2-3(Fig.9a).Among them,Cl-ions had a slight effect on the adsorption performance,while the effect of CO2-3ions was obvious,indicating that CO32-may compete with TC molecules for adsorption sites,leading to a decrease in the adsorption capacity of TC[37].
Recoverability is another essential factor to evaluate the adsorption performance of the adsorbent.In the experiment,HP-Ni-Cu-BTC(1∶2)was collected by centrifugation and soaked in ethanol after the first adsorption,and the adsorption efficiency was 71.45% in the 4th cycle(Fig.9b),showing good reproducibility of HP-Ni-Cu-BTC(1∶2).In addition,Fig.S2 showed the XRD patterns of HP-Ni-Cu-BTC(1∶2)before and after the adsorption reaction.After adsorption,the positions of the characteristic diffraction peaks of the adsorbent were basically the same as before.It showed that HP-Ni-Cu-BTC(1∶2)had good stability during the adsorption of TC.

Fig.9 (a)Effect of coexisting anions(Cl-,CO32-)on TC adsorption by HP-Ni-Cu-BTC(cTC,0=50 mg·L-1,m=5 mg,V=30 mL);(b)Reusability of HP-Ni-Cu-BTC(1∶2)
3 Conclusions
In conclusion,Ni(Ⅱ)-doped hierarchical porous Cu-BTC was successfully constructed in this study.Compared with microporous Cu-BTC,HP-Ni-Cu-BTC not only had hierarchical pores structure but also provided additional valence electrons to bring more active sites,which together significantly improved the adsorption capacity with 2.28 times higher adsorption capacity for TC.In addition,various characterizations showed that the Ni(Ⅱ)doping modification did not change the morphology,crystal structure,and functional groups of the HP-Cu-BTC.The adsorption kinetics and adsorption isotherm studies showed that the adsorption process was following the pseudo-second-order and Freundlich models.The adsorbent showed good reusability in the recyclability experiments.We believe that Ni(Ⅱ)-doped hierarchical porous Cu-BTC can be used as a multifunctional adsorbent with great potential for environmental remediation.
Supporting information is available at http://www.wjhxxb.cn
