Diagnosis,severity stratification and management of adult acute pancreatitis-current evidence and controversies
2022-11-29KaiSiangChanVishalShelat
Kai Siang Chan,Vishal G Shelat
Kai Siang Chan,Vishal G Shelat,Department of General Surgery,Tan Tock Seng Hospital,Singapore 308433,Singapore
Vishal G Shelat,Lee Kong Chian School of Medicine,Nanyang Technological University,Singapore 308232,Singapore
Vishal G Shelat,Yong Loo Lin School of Medicine,National University of Singapore,Singapore 117597,Singapore
Abstract Acute pancreatitis (AP) is a disease spectrum ranging from mild to severe with an unpredictable natural course.Majority of cases (80%) are mild and self-limiting.However,severe AP (SAP) has a mortality risk of up to 30%.Establishing aetiology and risk stratification are essential pillars of clinical care.Idiopathic AP is a diagnosis of exclusion which should only be used after extended investigations fail to identify a cause.Tenets of management of mild AP include pain control and management of aetiology to prevent recurrence.In SAP,patients should be resuscitated with goal-directed fluid therapy using crystalloids and admitted to critical care unit.Routine prophylactic antibiotics have limited clinical benefit and should not be given in SAP.Patients able to tolerate oral intake should be given early enteral nutrition rather than nil by mouth or parenteral nutrition.If unable to tolerate per-orally,nasogastric feeding may be attempted and routine post-pyloric feeding has limited evidence of clinical benefit.Endoscopic retrograde cholangiopancreatogram should be selectively performed in patients with biliary obstruction or suspicion of acute cholangitis.Delayed step-up strategy including percutaneous retroperitoneal drainage,endoscopic debridement,or minimal-access necrosectomy are sufficient in most SAP patients.Patients should be monitored for diabetes mellitus and pseudocyst.
Key Words: Atlanta classification;Drainage;Infections;Necrosectomy;Pancreatitis;Risk stratification
lNTRODUCTlON
Acute pancreatitis (AP) is a common cause of acute abdomen,with an incidence of 50-80per100000 population[1].The common causes of AP include gallstones (range 40%-70%),alcohol (range 25%-35%),hypertriglyceridemia (range 1%-14%) and post-endoscopic retrograde cholangiopancreatogram (ERCP) (range 3%-5%)[2-5].Rarer causes include peri-ampullary tumors,autoimmune pancreatitis,hypercalcemia,medications,genetic mutationse.g.,PRSS1gene,CFTRgene,and infections[6-10].The classical description of the presentation of AP is an acute onset of severe epigastric pain radiating to the back,which worsens when in a supine position.Other accompanying symptoms include nausea,vomiting,fever,or jaundice (for those with concomitant biliary obstruction).Common biochemistry markers used in clinical practice include serum amylase and lipase.Serum amylase and lipase have comparable clinical utility provided the clinician is aware of half-life differences (amylase return to normal limits within 3 to 5 d;lipase return to normal limits within 8 to 14 d)[11,12].Thus,lipase has higher sensitivity (lipase: 82% to 100%;amylase: 67% to 83%) in patients with delayed presentatione.g.more than 24 h of abdominal pain[11].Diagnosis of AP requires at least two of the three features: (1) Classical history of acute abdominal pain as described above;(2) Serum amylase or lipase at least three times the upper limit of normal;and (3) Characteristic findings of AP on contrast-enhanced computed tomography or magnetic resonance imaging scan[13].AP is a disease spectrum ranging from mild,moderately severe,to severe AP (SAP) as stratified by the Atlanta classification[13].While most patients with AP have a mild and self-limiting disease,about 12%-20% have SAP,with high mortality ranging from 15%-30%[13-18].This editorial will discuss the controversial and emerging themes regarding AP in adults with a critical appraisal of evidence and reference to existing guidelines.
DlAGNOSlS OF AP
While the abovementioned diagnostic criteria are clear,there are inherent limitations[13].The character of epigastric pain is subject to individual judgment.Serum enzymes also have inherent limitations of half-life (as mentioned above) and clinician must rely on the accuracy of patient recall of onset of abdominal pain,which is prone to error[11,12].Furthermore,serum enzymes may be falsely elevated in other pathologies like acute cholecystitis,renal impairment,etc.Radiological investigations may not be done in a clinically stable patient,rightly so for judicious use of finite resources.Thus,it is possible that some patients may be misdiagnosed as having AP if imaging is not performed.In contrary,early imaging performed for diagnostic purposes will miss necrosis as it typically develops after 3-5 d;and patients may be wrongly stratified as mild AP in absence of evidence of radiological changes.Thus,despite the objective diagnostic criteria,clinical prudence is essential in provision of good quality patient care.
AETlOLOGY OF AP
The next step after making a diagnosis of AP is establishing the aetiology.This is generally a three-step process: (1) History taking for risk factors such as alcohol intake,trauma,medications,recent ERCP procedure,and previous history of gallstone disease[2-5];(2) Fasting serological tests for calcium and triglycerides[4];and (3) Radiological imaginge.g.abdominal ultrasound scan to look for gallstones[2].In patients with no obvious aetiology,a clinician must perform extended investigations before resorting to a diagnosis of idiopathic pancreatitis.These extended investigations include a repeat abdominal ultrasound scan,magnetic resonance cholangiopancreatography (MRCP) scan[2],endoscopic ultrasound (EUS) scan,autoimmune markers like serum immunoglobulin G 4[7],viral markers like coronavirus disease 2019 and genetic tests[10].The International Association of Pancreatology (IAP)/American Pancreatic Association (APA) guidelines in 2013 suggest that secretin-stimulated MRCP should be performed if EUS is negative for occult microlithiasis,neoplasms and chronic pancreatitis[19] (GRADE 2C evidence).Administration of secretin causes dilatation of pancreatic ducts,allowing better visualization of pancreatic duct disorders[20].If the above fail to identify a cause,a hereditary cause should be suspected in recurrent,unexplained,early onset AP.Genetic counselling should be considered in these circumstances[19].A point to note is that genetic counselling is different from genetic testing.Genetic counselling involves risk assessment (e.g.detailed past medical history and family history),patient education,psychosocial support and counselling regarding implications and need for genetic testing[21].In contrary,genetic testing involves assays for gene mutations such as mutations in thePRSS1orCTFRgene[22].There are however currently no strict recommendations on the exact indications for genetic counselling and/or testing in AP[19].
In our opinion,a multidisciplinary discussion alongside genetic counselling should definitely be offered when extensive evaluation fails to identify an aetiology for AP.A patient should never be diagnosed with idiopathic pancreatitis without a multidisciplinary team discussion and endorsement.Establishing aetiology is important as this guides management[13].For example,patients with mild to moderate acute biliary pancreatitis (ABP) will be advised to undergo index admission laparoscopic cholecystectomy to reduce future recurrent biliary events.Also,abstinence from alcohol drinking,omission of the culprit medication,and pharmacological management of hypercalcemia or hypertriglyceridemia can prevent recurrent AP episodes[3,4].In patients with autoimmune pancreatitis,the immune-mediated pathology affects multiple organs like salivary and lacrimal glands,kidneys,retroperitoneum,lungs,and bile ducts.In addition,autoimmune pancreatitis is implicated in pancreas carcinogenesis[23].Thus,diagnosis and management of this pathology is unique and requires detailed assessment as well as long-term follow up.Genetic testing however,may be considered only after detailed discussion between clinicians and patients and/or family members due to potential psychosocial impact of results[21].
SEVERlTY STRATlFlCATlON OF AP
Severity stratification is done concurrently with aetiologic determination.There are three broad systems of severity stratification: (1) Two risk categories;(2) Three risk categories;and (3) Four risk categories.The two risk categories include mildvsSAP.This is the traditional and time-tested approach that is guided by various scoring systems like the Ranson’s score[24],and the Glasgow-Imrie score[25].Other newer approaches like the Acute Physiology and Chronic Health Evaluation II (APACHE-II) score[26,27],the Bedside Index of Severity in Acute Pancreatitis (BISAP) score[28],computerized tomography scan severity index (CTSI)[29,30],etc.continue to provide binomial severity risk stratification.This is important as patients with mild AP have almost no morbidity and mortality.The three-risk category system is proposed by the 2012 revised Atlanta classification system[13].Here,patients without organ failure or radiological changes are graded as mild,while patients with persistent organ failure (defined as > 48 h) are graded as SAP.The in-between risk category defined as moderately-SAP includes patients having radiological changes or transient organ failure (defined as ≤ 48 h).This system has limitations as some clinically stable patients might not have an imaging performed to assess morphological changes,thus categorized as mild AP.The four-risk category system is widely known as determinant based classification[31].This system is similar to the Atlanta classification;however,it includes a fourth risk category of “critical AP”.This is defined as patients with persistent organ failure and infected (peri)pancreatic necrosis.It is intuitive that these group of patients will be at highest risk of poor clinical outcomes.
Regardless of the type of system used,it is essential to risk stratify to allocate resources,counsel patients and family,and guide clinical care.The presence of many systems itself is a testament that none of them is perfect and their accuracy is not too far apart.The most commonly validated systems include the Ranson’s score[24],the Glasgow-Imrie score[25],APACHE-II[26,27,32],BISAP[28],Harmless Acute Pancreatitis Score (HAPS)[33],and Sequential Organ Failure Assessment (SOFA) score[34,35].We have summarized the abovementioned scoring systems and their respective advantages and disadvantages from the information obtained from recent meta-analyses in Table 1[36-39].Computed tomography severity index;FiO2: Fraction of inspired oxygen;HAPS: Harmless Acute Pancreatitis Score;Hct: Hematocrit;ICU: Intensive care unit;LDH: Lactate dehydrogenase;MCTSI: Modified computed tomography severity index;NPV: Negative predictive value;PaO2: Partial pressure of oxygen;PPV: Positive predictive value;SAP: Severe acute pancreatitis;SIRS: Systemic inflammatory response syndrome;SOFA: Sequential Organ Failure Assessment;U/L: Unitsperlitre;WBC: White blood cell.
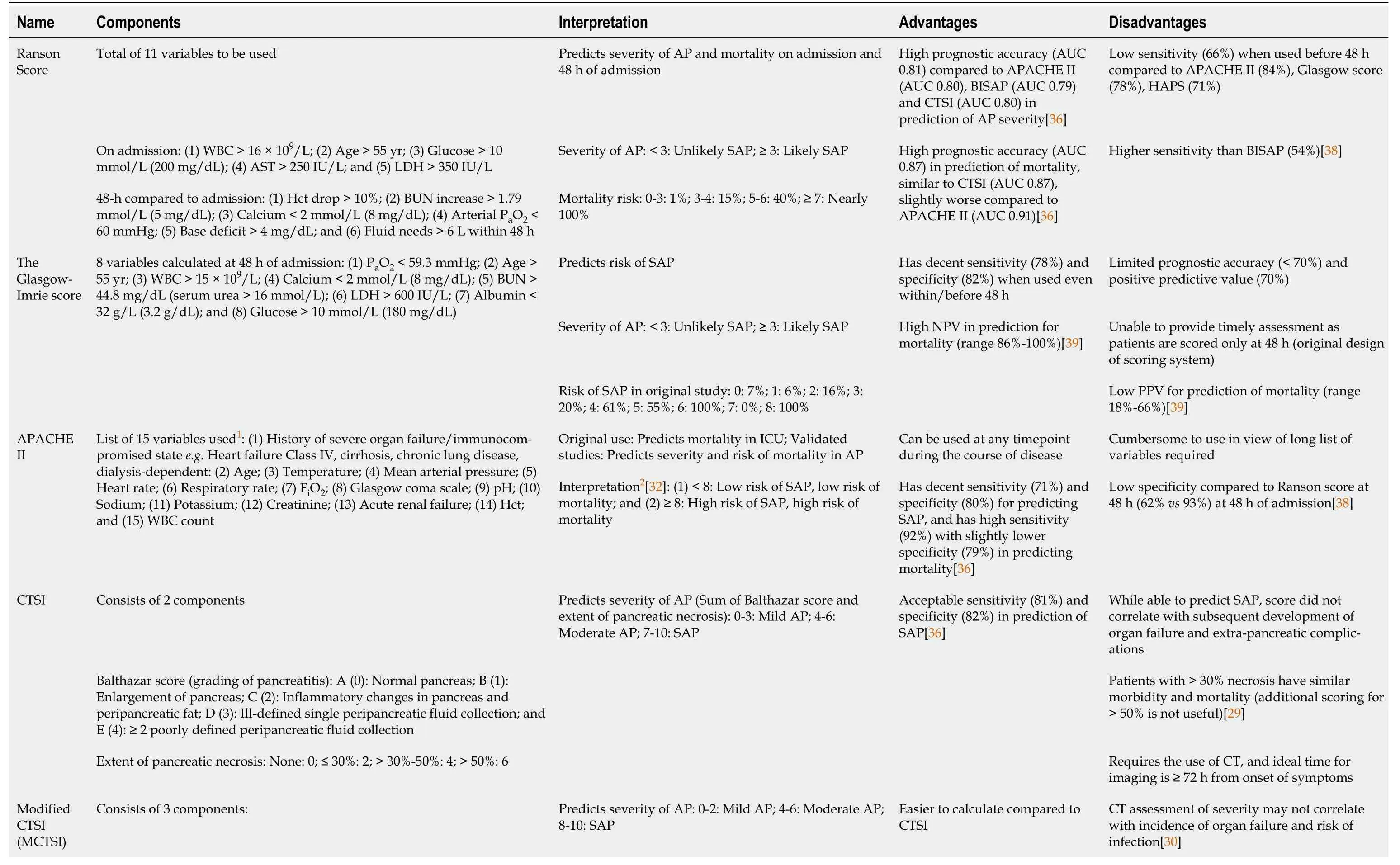
Table 1 Summary of various scoring systems which has been developed and/or validated for use in acute pancreatitis

1The APACHE II score and SOFA score are detailed scoring systems which take into account patients’ acute and chronic disease,signs,and laboratory values.Each variable consist of multiple components for which a score will be allocated for different range of values.The exact breakdown and scoring of each variable will not be included in this table due to its complexity.2The original Atlanta classification in 1992 defined severe acute pancreatitis as APACHE II ≥ 8.AP: Acute pancreatitis;APACHE: Acute Physiology and Chronic Health Evaluation;AST: Aspartate transaminase;AUC: Area under curve;BISAP: Bedside Index of Severity in Acute Pancreatitis;BUN: Blood urea nitrogen;CTSI:
The traditional 11-variable Ranson’s score is validated over five decades and has high prognostic accuracy in the prediction of severity and mortality[24,40].The main criticism of requiring to wait for 48 h for complete scoring is misplaced,as this need for 48 h is indeed the inherent strength[35,40].The APACHE-II is a 15-variable scoring system which has high accuracy in predicting severity and mortality and may be used at any time point in the disease[36].However,it is cumbersome for bedside clinical use.Easier to use scoring systems include the BISAP score and the HAPS[28,33].These are 5-variable and 3-variable scoring systems respectively with external validation.The BISAP score includes altered mental state and requires a chest x-ray to ascertain pleural effusion.Assessment of mental state could be subjective and pleural effusion may not manifest in the early phase of AP.Similarly,serological markers (hematocrit and creatinine) used in the HAPS may be misleading during the early phase of AP.Serum creatinine may take 24 to 36 h to rise after acute kidney injury[41].This may mis-stratify patients as mild AP which can progress to moderately-severe or SAP.This phenomenon is opposite to Ranson’s score which is shown to over-stratify patients as high risk.In our opinion,it is safer to risk stratify patients as having high risk and then use clinical judgment for resource allocation than to stratify patient wrongly as having low risk.With the revised Atlanta classification,organ failure-based scoring systems are increasingly used.The SOFA score is a 5-variable scoring system used to predict severity and mortality in AP[42].This can be completed within 24 h and has high accuracy (Table 1)[35].
Age and obesity
Age is a common variable used in traditional as well as modern systems.Elderly patients have reduced physiological reserves,more co-morbidities and are at increased risk of severity and mortality[43].However,there is a different extent of impact of age across various scoring systems.Liet al[44] analyzed Ranson’s score,APACHE-II and BISAP scores in elderly patients[44].They compared the traditional cut-off with an additional point added for elderly patients: ≥ 4 compared to ≥ 3 for Ranson’s score,≥ 9 for compared to ≥ 8 for APACHE-II score and ≥ 3 compared to ≥ 2 for BISAP score.Ranson’s score and APACHE-II score were accurate for the prediction of SAP and mortality in younger patients,while BISAP score was accurate in both elderly and young patients.However,recent propensity-score matched studies have shown that outcomes in elderly patients are comparable to younger patients in biliary sepsis[45];more evidence is necessary,especially to identify the risk into tertiles or quartiles,if not the cut-off value.Nevertheless,AP is a sterile process to begin with.Majority of mortality risk is in the late phase of illness on a background of sepsis-related complications.Thus,it is possible that the impact of age is a surrogate of underlying co-morbidities.In our opinion,patient co-morbidities as assessed by objective scoring systems like Charlson’s co-morbidity index may be more accurately associated with risk stratification than age alone.Furthermore,there is emerging data to suggest that obesity and increased body mass index are predictors of severity and mortality in AP[46].Obese individuals pose significant challenges in bedside clinical care and these issues are not reported in literature.For example,there is added difficulty in intravenous cannulation,insertion of intra-arterial and central venous lines,mobilisation and interpretation of chest X-ray findings.Use of ultrasonography is also limited by the increased abdominal fat and reduces sensitivity in diagnosis of gallstones.To add on,obese individuals are at increased risk of ventilatory problems and have higher risk of abdominal compartment syndrome[47].Individual units must locally audit various scoring systems and use the most accurate system to guide clinical decisions.
MANAGEMENT OF MlLD AP
Mild AP is self-limiting and emphasis should be placed on symptom control and managing the aetiology to prevent future recurrences.Pain control has been emphasised in several guidelines[48,49].Use of non-steroidal anti-inflammatory drugs (NSAIDs) has been shown to be equally effective as opioids in reducing the need for rescue analgesia in mild AP[50].In our opinion,analgesia should be administered and escalated according to the World Health Organization pain ladder[51],and patient’s co-morbidities (e.g.elderly patients with renal impairment should not be given NSAIDs).Patients with ABP should be advised index admission (or within 2 wk) laparoscopic cholecystectomy,provided there is no suspicion of bile duct stone[19].Patients with alcohol abuse should be provided psychological support and enrolled in de-addiction initiatives alongside social support.Lifestyle modifications (e.g.diet control,weight loss) should be made in hypertriglyceridemia-induced AP[4].First-line medications with fibrates should also be started with an aim for triglyceride level to be <500 mg/dL (5.65 mmol/L)[52].Patients with idiopathic AP belong to a special group where a discussion for EUS and/or laparoscopic cholecystectomy for possibility of underlying microlithiasis is important for informed decision making[19,53].In patients with pancreas divisum,multidisciplinary team collaboration is essential to discuss the role of sphincterotomy to ameliorate intraductal hypertension and recurrent AP[54].
MANAGEMENT OF NON-MlLD AP
In patients with non-mild AP,radiological changes and/or organ dysfunction are evident.Some patients with moderately-SAP may clinically improve and potentially can qualify for index admission cholecystectomy.The remaining moderately-SAP patients are managed according to SAP due to inherent risk of mortality and unpredictable natural disease course[15,16].We shall discuss the controversies related to fluid management,role of antibiotics,indications for intensive care unit (ICU) admission,mode of nutrition,role of ERCP,and indications for invasive (endoscopic and/or surgical) interventions.
Fluid management
The inflammatory cascade in AP may result in persistent organ dysfunction lasting > 48 h,resulting in SAP.Patients with SAP often present with cardiovascular compromisee.g.hypotension and are kept nil by mouth during the acute presentation (refer to sub-section on mode of nutrition for further discussion).Prompt intravenous fluid resuscitation is key for initial cardiovascular support in SAP.Two common questions need to be addressed: (1) Choice of fluid;and (2) Amount/rate of fluid administration.
While colloids have the advantage of more efficient replacement of intravascular loss (1:1 replacement compared to 3:1 replacement for crystalloids),there is risk of acute kidney injury requiring renal replacement therapy (RRT) with starch,and risk of allergic reactions.A Cochrane review on the use of crystalloids and colloids in critically ill patients (69 studies with 30020 patients) found no difference in all-cause mortality[55].However,there was moderate certainty evidence of slight increase in need for RRT when starches were used.Use of hydroxyethyl starch (HES) in severe sepsis has also been shown to increase mortality compared to ringer’s lactate[56].The American Gastroenterological Association (AGA) guidelines on the initial management of AP similarly recommends against the use of HES due to the lack of mortality benefits[57],and a study which showed increased multi-organ failure with HES[58].In our opinion,in a condition like SAP which already bears high mortality on its own,measures should be taken to minimise further insult.Crystalloids should be the choice of fluids.When comparing between type of crystalloids,the IAP/APA guidelines recommend ringer’s lactate due to reduced incidence of systemic inflammatory response syndrome compared to normal saline in AP[19,59].However,the AGA guidelines make no recommendations on whether ringer’s lactate or normal saline should be used as clinical outcomes such as organ failure,necrosis or mortality were not investigated[57].In patients with AP secondary to hypercalcemia,normal saline should be used instead as ringer’s lactate contains 3 mEq/L calcium.While different guidelines make conflicting recommendations over the choice of crystalloids,normal saline is considered “less physiological” due to high sodium and lack of potassium[60].Over-administration of normal saline may also lead to normal anion gap hyperchloremic acidosis in cases of persistent hypotension.Therefore,we believe that ringer’s lactate should be considered first.
Secondly,how fast and how much fluids should be given? Like any resuscitation,this should be goaldirected with an initial rate of 5-10 mL/kg/h[19,61].However,excessive fluid replacementi.e.overresuscitation may do more harm than goode.g.dilutional coagulopathy,fluid overload and re-perfusion mediated injury.Additionally,in AP,faster rate of infusion at 10-15 mL/kg/h has been shown to increase the need for mechanical ventilation,abdominal compartment syndrome,sepsis and mortality[61].The definition of “goal-directed” is similar to the management of hypotension or shock,where vital parameters are used to trend clinical response,such as fall in heart rate,mean arterial pressure ≥ 65 mmHg and urinary output > 0.5 mL/kg/h.Invasive methods may also be used,but clinicians are to be cognisant that central venous pressure monitoring is a static marker.Stroke volume variation is a better marker of fluid responsiveness as it allows dynamic monitoring of fluid responsiveness.
Role of antibiotics in SAP
Sequelae of SAP include (peri) pancreatic necrosis with or without infection.A meta-analysis by Wergeet al[62] on 71 studies with 6970 patients showed that patients with infected necrosis had higher mortality than those with sterile necrosis [Odds ratio (OR): 2.57,95% confidence interval (CI): 2.00-3.31][62].Organ dysfunction with concomitant infection in SAP was also associated with higher mortality compared to organ dysfunction with sterile necrosis (35.2%vs19.8%).This raises the question on the role of antibiotics in SAP and its impact on clinical outcomes: (1) Prophylactic antibiotics in SAPvsantibiotics for infected necrosis only;and (2) Choice and/or duration of antibiotics.
Older guidelines,for instance the Japanese Guidelines 2015,recommend prophylactic antibiotics administration in SAP and acute necrotizing pancreatitis (ANP) as its use may improve prognosis if carried out early within 72 h from onset of disease (level 2B evidence)[63].However,the 2019 World Society of Emergency Surgery (WSES) guidelines do not recommend the routine use of prophylactic antibiotics for all AP as there is no significant reduction in morbidity or mortality[49].
There have been several systematic reviews and meta-analyses on this topic.Ukaiet al[64] in 2015 analysed 6 randomized controlled trials (RCTs) with 397 ANP patients and showed that early prophylactic antibiotics (within 72 h from onset of symptoms or 48 h after admission) was associated with lower mortality (prophylactic antibiotics: 7.4%vsno antibiotics: 14.4%,OR: 0.48,95%CI: 0.25-0.94) and reduced incidence of infected pancreatic necrosis (prophylactic antibiotics: 16.3%vsno antibiotics: 25.1%,OR: 0.55,95%CI: 0.33-0.92) compared to no antibiotics use[64].However,a recent meta-analysis on the use of prophylactic carbapenem antibiotics by Guoet al[65] on 6 studies (5 RCTs,1 retrospective observational study) showed similar mortality (prophylactic antibiotics: 11.0% (n= 29/264)vsno prophylactic antibiotics: 15.4% (n= 38/246),OR: 0.69,95%CI: 0.41-1.16,P= 0.17) and incidence of infected pancreatic necrosis [prophylactic antibiotics: 12.5% (n= 33/264)vsno prophylactic antibiotics: 15.9% (n= 39/246),OR: 0.74,95%CI: 0.44-1.23,P= 0.24][65].Guoet al[65] included studies with heterogeneity in the timing of prophylactic antibiotics administration: One study started antibiotics within 48 h of symptom onset[66],three studies within 72 h of symptom onset[67-69] and one study within 120 h of symptom onset[70].Unlike Guoet al[65] who analysed only patients with prophylactic carbapenem,Ukaiet al[64] included studies with cefuroxime[71],and ciprofloxacin[72].In addition,while the populations examined are similar between the two studies,ANP (study by Ukaiet al[64]) is not synonymous with SAP (study by Guoet al[65]).Moderately-SAP is defined as presence of local complications which include acute necrotic collection (ANC),peri-pancreatic collection,or walled-off necrosis (WON).SAP is defined as presence of persistent organ dysfunction > 48 h.While ANP may result in systemic inflammation,infection,and subsequent organ dysfunction,not all cases of ANP qualify for SAP as determined by the revised Atlanta classification.Though Guoet al[65] did not show any statistically significant improvement in mortality or reduced infected pancreatic necrosis[65],there was an absolute unadjusted difference of 4.4% in mortality,which in our opinion is clinically meaningful and should not be dismissed as insignificant.
In our opinion,the role of antibiotics is absolute in patients with concomitant acute cholangitis (AC) (biliary sepsis) and in selected patients where intestinal bacterial translocation has ensued due to prolonged duration of hypoperfusion.Future studies should consider evaluating the role of prophylactic antibiotics in high-risk patientse.g.elderly with multiple co-morbidities.If prophylactic antibiotics are started,then one must titrate according to the results of fluid cultures and clinical response to reduce risk of resistant strains or fungal superinfection in vulnerable SAP patients.
Apart from prophylactic antibiotics,other adjuncts have been considered in improving outcomes of SAP.Selective decontamination of the digestive tract (SDD) is a prophylactic strategy to reduce exogenous and endogenous infection consisting of a course of parenteral and enteral antibiotics,topical antibiotics (for patients on tracheostomy),good hygiene and surveillance throat and rectal cultures[73].SDD has been shown to reduce multi-organ dysfunction in critically ill patients (meta-analysis on 7 RCTs with 1270 patients)[74].Mortality was also shown to be reduced in another meta-analysis[75].However,evidence is scarce on the utility of SDD in SAP.To date,only 1 RCT in 1995 reported reduction in mortality[76],while 1 retrospective study in 2007 reported non-statistically significant reduction in organ dysfunction (70% to 59%) and mortality (40% to 28%) with SDD[77].Further studies are required to validate these findings before definitive conclusion can be made on recommendations.In contrary,probiotics have been shown to have no benefits in preventing infections in AP[78].
Until more evidence is reported,we endorse the 2019 WSES and the IAP/APA that there should not be a recommendation for the use of prophylactic antibiotics nor probiotics in SAP[19,49].SDD may have benefits in reducing organ dysfunction and mortality in SAP.However,further well-designed RCTs are required to fill in this knowledge gap.This also draws attention for the need of an umbrella review to summarize findings from existing systematic reviews and meta-analysis on the use of prophylactic antibiotics in SAP.
Indications for ICU admission
By definition,all cases of SAP will require at least high dependency unit (HDU) monitoring in view of persistent organ failure lasting > 48 h.This aids continuous vital chart assessment,invasive haemodynamic monitoring,accurate fluid balance charts documentation,round the clock nursing and medical attention for timely escalation of care in event of deterioration.The escalation of care is determined by clinical judgement and use of surrogate markers to assess the severity of AP and physiological disturbance.Prediction and prognostic scores serve as useful adjuncts to guide clinicians,but do not replace the need for continuous vigilant monitoring and reliance on one’s judgment to detect early warning signs of clinical deterioration so as not to miss the golden window of opportunity for timely care.Point of care tests like arterial blood gas analysis are integral to early recognition of deterioration.The 2021 joint guidelines by the French Society of Anaesthesia and Intensive Care Medicine also strongly recommends for intra-abdominal pressure monitoring for diagnosis and rapid treatment of intra-abdominal hypertension (IAH)[79].SAP and large administration of fluids are risk factors for IAH[80],which bears significantly higher mortality than those without[81].In rare instances,an astute family member may highlight certain cues which suggest patient’s clinical deterioration,and those should not be dismissed.For example,they may highlight to medical staff “today he/she looks more tired”,“yesterday he/she could open eyes and could talk to me for xx minutes,but not today”etc.The HDU team should have a seamless access to the ICU team.Communication or personal egos have no place in timely escalation and expeditious transfer for airway management or ventilatory support.It is our view that even patients with non-invasive ventilation should be under the care of the ICU outreach team even though they are physically nursed in HDU.In our institution,HDU is able to support continuous vitals monitoring,invasive lines (e.g.arterial line and central venous pressure line),support patients on one vasopressor (e.g.noradrenaline);and has a nurse to patient ratio of 1 to 2 or 1 to 3.
Furthermore,various tiers of ICU have also been defined: (1) Level 1 ICU: Capable of providing oxygen,non-invasive monitoring,and more intensive nursing care than in normal ward;(2) Level 2 ICU: Capable of providing invasive monitoring and basic life support for a short period;and (3) Level 3 ICU: Capable of providing full spectrum of monitoring and life support[82].Ohbeet al[83] defined ICU as availability of physician on-site 24 hperday,at least 2 intensivists working full-time,around-theclock nursing and nurse-to-patient ratio of 1 to 2.HDU was defined as similar capabilities compared to ICU,without requirement for intensivists and reduced nurse-to-patient ratio of 1 to 4 or 1 to 5[83].In our institution,ICU has capabilities of supporting patients on mechanical ventilation,invasive life supporte.g.extracorporeal membrane oxygenation and support dual or triple vasopressors and/or inotropes.Interestingly,Ohbeet al[83] showed that ICU (i.e.with availability of intensivists and better nurse-to-patient ratio) decreased 30-d mortality by 7.2% in patients with pneumonia on mechanical ventilation[83].The authors attributed this to better nurse-to-patient ratio,especially in the context of high workload with critically ill patients[84].Patients with SAP may also present with acute respiratory distress syndrome or severe metabolic acidosis requiring mechanical ventilation[85,86].Such patients should be directly admitted to an ICU.
Additionally,the IAP/APA guidelines state that all patients with SAP should be managed at a specialist centre (defined as a high-volume centre)[19].Improved morbidity and/or mortality have been reported for pancreas resection (pancreatectomy or pancreaticoduoenectomy) when performed at highvolume centres[87,88].However,what is defined as “high-volume”? Even for oncological surgeries,“high-volume” has been variable,with studies reporting 20-35 cases annually as cut-off for pancreas resection[89,90].In contrary,studies which reported on outcomes of out-of-hospital cardiac arrest defined high-volume as ≥ 40-100 cases annually[91].For AP,there is no literature on what defines “high-volume”.In our opinion,there is no real “cut-off” for what defines a high-volume centre in AP.We believe that SAP should be managed in a specialist centre,which should be defined as the availability of specialised round-the-clock services for radiological imaging,interventional radiology,endoscopic interventions and surgical capabilities.
Mode of nutrition
While almost all patients with mild AP will be allowed to maintain oral nutrition,patients with SAP may have associated nausea or vomiting,gastrointestinal ileus with nasogastric tube in-situ,or are on mechanical ventilatory support.The traditional belief that feeding stimulates the release of cholecystokinin,causing the secretion of proteolytic enzymes that results in autodigestion and further damage to the pancreas is unfounded[92].Furthermore,enteral feeding has been shown to maintain bowel mucosa integrity and prevents intestinal bacterial translocation,thus reducing risk of pancreatic necrosis with superadded infection and systemic sepsis[93].Evidence has also shown that early oral feeding reduces length of stay (LOS)[94].To add on,SAP is a catabolic process which results in loss of nutrients,water,electrolytes and protein[95,96].Thus,early and optimal caloric formula feeds considering “stress factor multiplication” should be commenced early in the journey of SAP.
Enteral nutrition has been recommended over total parenteral nutrition (TPN) in SAP;Yiet al[97] in 2012 who analyzed 8 RCTs (381 patients) showed reduced infective complications [Risk ratio (RR): 0.46,95%CI: 0.27-0.78],organ failure (RR: 0.44,95%CI: 0.22-0.88) and mortality (RR: 0.37,95%CI: 0.21-0.68) with enteral nutrition[97].However,evidence is lacking regarding the mode of enteral nutrition: Peroralvsnaso-enteric feeding tube.As mentioned above,patients with SAP have physiological compromise and may not be able to tolerate per-oral intake.A RCT comparing early nasoenteric tube feeding (within 24 h from randomization) and delayed oral feeding (initiated 72 h after presentation) did not show superiority of early nasoenteric tube feeding in reducing infections and mortality[98].Another RCT (110 patients) compared hunger-based feeding (commencement of oral feeding once patients felt hungry)vsconventional feeding (commencement of oral feeding after normalization of biochemical parameters and resolution of symptoms) in moderate AP and SAP[99].Compared to conventional feeding,hunger-based feeding allowed for earlier feeding (mean fasting duration 1.6 dvs2.7 d,P= 0.001) and was also associated with shorter LOS (6.3 dvs7.3 d,P= 0.041).However,incidence of infection and mortality was comparable between both feeding regimes.Results from this study suggest that “hunger” reflects recovery of gastrointestinal dysfunction.Benefits of earlier feeding and ensuring return to their baseline status therefore allows for earlier discharge.
The type of diet is also an important consideration.The revised Clinical Practice Guidelines of the Korean Pancreatobiliary Association for Acute Pancreatitis recommend for low-fat diet as long as tolerated in AP (level B evidence)[48].High fat diet has been shown to increase oxidative stress and enhance inflammation in animal studies[100].Human studies also show increase in pancreatic secretion after fat-rich diet[101],which may worsen pain.Use of low-fat diet has been shown to be safe compared to clear liquid diet with provision of more calories[102].Tolerating low-fat diet and solid diet early may expedite discharge and reduce LOS.
Apart from the timing of feeding,the mode of nasoenteral (NG) feedingi.e.nasogastricvsnasojejunal (NJ) feeding should also be considered.Insertion of NJ tube requires fluoroscopic guidance and technical expertise,while NG tube insertion is a simple bedside procedure.It has been postulated that NJ tube reduces pancreatic stimulation and risk of aspiration pneumonitis[103,104].A Cochrane review on 5 RCTs (220 patients) showed similar mortality between NJ and NG feeding,and no studies reported any incidence of aspiration pneumonia[105].After review of all the above evidence,per-oral or nasoenteric feeding should be used over TPN unless contraindicated.The mode of feeding,per-oralvsfeeding tube,should be determined by clinical wisdom and earlier enteral nutrition should be advocated,especially if it is driven by “hunger” sensation.If enteral feeding is planned,NG tube insertion should be attempted first due to ease of insertion and lack of benefits of NJ tube insertion.
Role of ERCP for gallstone pancreatitis
Gallstone is the most common cause of AP and it is possibly lodged into the common bile duct for it to cause AP.Thus,ERCP for biliary decompression and/or stone removal is an integral consideration in AP management.The 2019 WSES guidelines recommend against routine ERCP for acute gallstone pancreatitis (AGP) (Level 1A evidence)[49].However,the American College of Gastroenterology guidelines recommend urgent ERCP within 24 h for severe AGP complicated by organ failure[106],and the United Kingdom practice guidelines similarly advocate early ERCP (within 72 h) for predicted or severe AGP[107].The 2012 Cochrane review which compared early routine ERCPvsconservative management in AGP (5 studies with predicted mild AP,7 studies with predicted SAP) showed comparable mortality and local complications[108].Subgroup analysis was also performed for studies with predicted mild AP and SAP;similarly there was no significant differences in outcomes: (1) Mortality (early routine ERCP in mild AP: RR: 4.53,95%CI: 0.22-92.88,P= 0.33;early routine ERCP in SAP: RR: 0.64,95%CI: 0.20-2.04,P= 0.45);and (2) Local complications (early routine ERCP in mild AP: RR: 0.99,95%CI: 0.52-1.90,P= 0.99;early routine ERCP in SAP: RR: 0.70,95%CI: 0.36-1.39,P= 0.31).
While ERCP is minimally invasive compared to surgery,ERCP still bears the risk of sedation and post-ERCP complications.This is added onto the physiological insult during AP.Hence,there needs to be a clear benefit before attempting ERCP in AGP.No benefit has been shown for early ERCP compared to conservative management for both mild AP and SAP in AGP[108].However,in the same metaanalysis,the authors showed significantly lower local complications in patients who had biliary obstruction (without cholangitis)[108].No analysis was done for mortality.For patients with concomitant cholangitis,there was reduced mortality,local and systemic complications in patients who received early ERCP compared to conservative management[108].
Biliary obstruction leads to bile stasis and in presence of stone,this is considered infected until proven otherwise.Bactibilia in patients with biliary obstruction leads to cholangio-venous reflux and spillover of gram negative endotoxins into systemic circulation with downstream injury to organ systems[109].ERCP reverses the pathophysiology of cholangitis and thus the maximal utility is in SAP patients with concomitant cholangitis[108].
However,diagnosis of concomitant AC is challenging in AP.Both AC and AP present with acute epigastric and/or right hypochondrium pain and fever;AP may present with jaundice in the presence of biliary obstruction.This essentially fulfils the Charcot’s triad,the traditional method of diagnosis for AC.Commonly used biochemistry markers includes white blood cell count,C-reactive protein (CRP) and liver function test.Both AC and AP result in systemic inflammation and subsequent leukocytosis and raised CRP.Presence of biliary obstruction will result in an “obstructive pattern” of liver function test,with raised alkaline phosphatase and γ-glutamyl transferase.The Tokyo Guidelines 2018 (TG18) guidelines require the presence of (1) Systemic inflammation: Fever and/or chills,laboratory data with evidence of inflammatory response;(2) Cholestasis: Jaundice,abnormal liver function tests;and (3) Biliary dilatation and evidence of etiology on imaging (e.g.stricture or stone)[110].AP with biliary obstruction without AC will fulfil all the criteria for the diagnosis of AC.A study by Weilandet al[111] showed that the TG18 fairs poorly in the diagnosis of AC with suspected biliary obstruction (sensitivity 82%,95%CI: 74-88%;specificity 60%,95%CI: 56-63%)[111].
Procalcitonin (PCT) is a trending biomarker which may be used to distinguish between AP alonevsAP with concomitant AC.PCT has higher sensitivity (88%vs75%) and specificity (81%vs67%) than CRP for discriminating bacterial infections from non-infective causes of inflammation[112].Albertiet al[113] did a prospective study on 152 patients on the use of PCT and showed that PCT > 0.68 mg/dL had higher incidence of AC,infected necrosis and need for urgent ERCP in patients with AP[113].Similarly,a RCT on 260 patients with AP was conducted to compare PCT-guided care (antibiotics administration if PCT ≥ 1.0 μg/L,and to withhold antibiotics if PCT <1.0 μg/L)vsstandard care (asperIAP/APA guidelinesi.e.antibiotics administration if clinical suspicion of infection or proven infected WON)[114].They showed that PCT-guided care resulted in fewer administration of antibiotics (risk difference: -15.6%,95%CI: -27.0,-4.2,P= 0.0071),with similar number of clinical infections,hospital-acquired infections,mortality and adverse events.While PCT may not be able to differentiate infected pancreatic necrosisvsAC,its use is promising and may prove as a useful adjunct alongside other investigations for starting empirical antibiotics.
After review of the above evidence,early ERCP should not be performed for all AGP.However,in the presence of biliary obstruction and/or AC,early ERCP should be performed.There is difficulty in the differentiating ACvsbiliary obstruction in AP.Nevertheless,early ERCP should still be performed in biliary obstruction as benefits have been shown compared to conservative management alone.
Indications for invasive (endoscopic and/or surgical) intervention in SAP
In general,interventions in SAP patients should be performed on-demand and not by-the-clock.Also,interventions should be delayed as much as possible and the least invasive modality should be selected due to the high physiological insult in SAP.Open necrosectomy (ON) is rarely performed due to high morbidity and mortality[115-119].Advances in endoscopic and minimally invasive techniques have shifted the approach towards minimally invasive necrosectomy (MIN).Several meta-analyses showed no difference in short-term mortality,but has reduced incidence of serious adverse events (rate ratio: 0.41,95%CI: 0.25-0.68,only 1 study was included) and multiple organ failures (OR: 0.16,95%CI: 0.06-0.39,P<0.0001) in MIN patients compared to ON[120,121].
The 2019 WSES guidelines recommend a step-up approach for infected pancreatic necrosis with initial treatment with percutaneous drainage (Level 1A evidence)[49].The TENSION (Transluminal endoscopic step-up approachvsminimally invasive surgical step-up approach in patients with infected necrotising pancreatitis) trial is a RCT which was published in 2018 (Endoscopic step-up approachn= 51,surgical step-up approachn= 47)[122].They compared the use of endoscopic step-up approach (initial treatment with EUS-guided transluminal drainage (EUS-TD) with placement of two stents,with subsequent endoscopic transluminal necrosectomy if no clinical improvement)vssurgical step-up approach (initial treatment with radiologically-guided percutaneous drainage through the left retroperitoneum,with subsequent video-assisted retroperitoneal debridement (VARD) if drainage was clinically unsuccessful) in patients with high suspicion of infected pancreatic or extra-pancreatic necrosis.Endoscopic step-up approach was associated with reduced LOS {median 35 [interquartile range (IQR) 19-85] dvsmedian 65 (IQR: 40-90) d,P= 0.014} and reduced pancreatic fistula [5%vs32%,RR: 0.15 (95%CI: 0.04-0.62),P= 0.0011] compared to surgical step-up approach.Major complications and mortality were comparable between endoscopic and surgical step-up approach.Similar results were noted in a meta-analysis comparing endoscopicvsminimally invasive techniques (laparoscopic cystogastrostomy,VARD,or step-up approach to VARD following radiologically guided percutaneous drainage);incidence of pancreatic fistula,new-onset multiple organ failure (5.2%vs19.7%,RR: 0.34,P= 0.045) and LOS were lower in endoscopic techniques[123].However,mortality was comparable.Percutaneous drainage and surgical step-up approach may cause external extravasation of pancreatic exocrine exudates resulting in pancreatic fistula[124].To add on,pro-inflammatory response of pancreatic enzymes may result in systemic inflammation resulting in new-onset organ failure[125].These result in longer LOS for surgical step-up approach compared to the endoscopic step-up approach.
Apart from the advantages endoscopic approach offers,it is however important to consider the technical challenges of endoscopic drainage.Endoscopic techniques include conventional direct transluminal drainage (CTD) by forward viewing endoscopy,transpapillary drainage (TPD) and EUSTD.CTD offers drainageviaa blind approach (identified through luminal bulging of peripancreatic collection) which presents risk of bleeding,perforation,and oversight of main pancreatic duct (MPD) abnormality.TPD requires communication between the peripancreatic collection with the MPD to allow for drainage.EUS-TD is the safest with visual guidance,but fluid collections must be within 1cm of gastric or duodenal walls[126].Anatomical location of ANC or WON may render difficulty for endoscopic drainage and hence,radiologically guided percutaneous drainage should still be considered first in these circumstances.
Apart from short-term outcomes,studies have evaluated long-term patient-related outcome measures.A recent systematic review by Psaltiset al[127] in 2022 included 11 articles which assessed the quality of life (QOL) after endoscopic and/or surgical management of SAP[127];literature was heterogenous which rendered inability for pooled analysis.However,the authors suggested that endoscopic management may confer better QOL compared to surgical management based on current literature.A RCT comparing endoscopicvsMIN showed significantly higher physical component scores for endoscopic necrosectomy at 3 mo following intervention (P= 0.039)[128].Mental health was also reported to be better following minimally invasive drainage (consisting of percutaneous catheter drainage,negative pressure irrigation and endoscopic necrosectomyviaan artificial sinus tract) compared to ON[129].It is noteworthy that the studies included in the review did not include laparoscopic or minimally invasive retroperitoneal pancreatic necrosectomy.
Considering all available evidence on endoscopic,MIN and ON,there is no mortality benefits between the choice of intervention.This is in line with the WSES 2019 guidelines[49].However,endoscopic step-up approach confers additional benefits such as reduced incidence of pancreatic fistula,lower new-onset organ failure,and shorter LOS compared to surgical step-up approach.It is important to note that while mortality has been shown to be comparable,existing studies did not evaluate longterm mortality.Organ failure has been demonstrated to be an important case of long-term morbidity and mortality[15,130].Therefore,endoscopic step-up approach should be used for infected ANP if technically feasible.
Summary of the management of SAP
While there are several controversies surrounding the abovementioned areas discussed,there are also several guidelines,such as the IAP/APA guidelines,2019 WSES guidelines and the revised Clinical Practice Guidelines of the Korean Pancreatobiliary Association for Acute Pancreatitis[19,48,49].Guidelines serve as recommendations for clinical practice.However,compliance is equally,if not more important.Results however have been disappointing.A large multi-center international audit showed poor compliance to clinical guidelines in the management of ABP[131].For instance,there were 53.4% of patients who received prophylactic antibiotics for mild ABP,and 83.4% who received prophylactic antibiotics for severe ABP.Similarly,only 44.7% with ABP (all severity) had early enteral feeding,and 47.7% with mild ABP had early enteral feeding.An international survey on 1054 participants from 94 countries similarly showed that 15.5% of participants administer routine prophylactic antibiotics for AP,and only 26.6% will start patients who did not vomit on early enteral feeding[132].As discussed above,there are currently no recommendations for prophylactic antibiotics,and early enteral feeding is recommended due to its protective effect on bowel mucosa integrity and prevents intestinal bacterial translocation.Possible explanations for the lack of compliance may be due to traditional beliefs clinicians have,reluctance for compliance to guidelines or a delay of translation of evidence into personal or institutional protocols[133].Hirotaet al[134] in 2014 extracted 10 statements from the Japanese guidelines on AP and classified them into 10 AP bundles for SAP;they showed that patients who had ≥ 8 bundles implemented had lower mortality compared to <8 bundles (overall 505 patients with SAP,mortality 13.7%vs7.6%,P= 0.042)[134].This reinforces that while guidelines help shape clinical practice,what is more important is compliance to guidelines and not more guidelines.Clinicians need to be up-to-date with evidence and guidelines,and integrate them into personal and/or institutional practices and protocols to optimise clinical outcomes.
MANAGEMENT OF RECURRENT AP
In some patients,AP recurs or relapses,especially when the initial aetiology is not treated or removed.In patients with AGP,this means that cholecystectomy is essential.In patients with hypercalcemia or hyperlipidemia,appropriate management of underlying aetiology is essential.In patients with druginduced pancreatitis,the culprit drug should be avoided and substituted with an alternative medication[9].However,sometimes the underlying etiology may be multifactorial or idiopathic.The International State-of-the-Science conference defined recurrent AP as two or more well-documented separate attacks of AP with complete resolution for more than 3 mo between attacks[135].Recurrent AP is a complex pathology with possible anatomic,environmental,and genetic causal interplay.Thus,the diagnostic work-up should include EUS,autoimmune serological tests,and genetic studies.In rare situations,ERCP during the acute episode of abdominal pain may be necessary to identify and treat the causative aetiology[136].Biliary and pancreatic ductal manometry and biliary sphincterotomy can potentially reduce recurrent AP rates in patients with anomalous pancreato-biliary junction,choledochocele,ampullary neoplasms,biliary parasitosis,and sphincter of Oddi dysfunction[137].Empiric trial of steroids without compelling evidence of autoimmune pancreatitis is not advised[135].Similarly,empiric cholecystectomy is not advised in patients with no evidence of gallbladder disease on EUS and other imaging modalities and with normal liver function tests[135].About one-quarter of patients with recurrent AP may progress to chronic pancreatitis,and a diagnosis of chronic pancreatitis does not preclude a future diagnosis of AP or recurrent AP[138].It is essential that patients with recurrent AP are managed by physicians with special interest in pancreatology and its management should be guided by local multidisciplinary teams to not only reduce progression to chronicity,but also to maintain good QOL in patients.
CONCLUSlON
AP is a disease spectrum where majority of patients present with mild disease.However,in the minority with non-mild AP,mortality is high.Proper risk stratification using a conglomerate of clinical judgement and predictive scores for proper resource allocation and care is integral of any health system to deliver good outcomes.Early goal-directed fluid resuscitation with crystalloids should be carried out.Prophylactic antibiotics have yet to show any clear morbidity or mortality benefits in SAP.Enteral nutrition is recommended over parenteral nutrition,if not contraindicated.Timing of starting enteral nutrition is still unclear,but should not be delayed until complete resolution of disease.Decision for higher intensity monitoring should also be based on clinical status and ICU capabilities of respective institutions.Early ERCP should be performed for concomitant biliary obstruction or AC.Endoscopic step-up approach is the preferred choice in the management of infected pancreatic necrosis.
FOOTNOTES
Author contributions:Chan KS is involved in the conceptualization and drafting of the initial manuscript;Shelat VG is involved in the conceptualization,supervision and revision of the manuscript.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Singapore
ORClD number:Kai Siang Chan 0000-0001-9533-801X;Vishal G Shelat 0000-0003-3988-8142.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Preoperative blood circulation modification prior to pancreaticoduodenectomy in patients with celiac trunk occlusion:Two case reports
- Observational Study Development of a warning score for early detection of colorectal anastomotic leakage: Hype or hope?
- Disturbed passage of jejunal limb near esophageal hiatus after overlapped esophagojejunostomy following laparoscopic total gastrectomy
- Assessment of tumor markers CA 19-9,CEA,CA 125,and CA 242 for the early diagnosis and prognosis prediction of gallbladder cancer
- Recombinant human thrombopoietin treatment in patients with chronic liver disease-related thrombocytopenia undergoing invasive procedures: A retrospective study
- Comprehensive abdominal composition evaluation of rectal cancer patients with anastomotic leakage compared with body mass indexmatched controls
