Multiple hepatic infarctions secondary to diabetic ketoacidosis: Acase report
2022-11-29VitoriaMikaellydaSilvaGomesGustavodeSousaArantesFerreiraLuiseCristinaTorresRubimdeBarrosBarbaraMoreiraRibeiroTrindadedosSantosLorennaPaulinelliBahiaVieira
Vitoria Mikaelly da Silva Gomes,Gustavo de Sousa Arantes Ferreira, Luise Cristina Torres Rubim de Barros,Barbara Moreira Ribeiro Trindade dos Santos,Lorenna Paulinelli Bahia Vieira
Abstract
BACKGROUND
Hepatic infarctions (HI) are ischemic events of the liver in which a disruption in the blood flow to the hepatocytes leads to focal ischemia and necrosis. Most HI are due to occlusive events in the liver’s blood vessels, but non-occlusive HI may occur. They are associated with disruption of microvasculature, such as in diabetic ketoacidosis. While HI usually presents as peripheral lesions with clear borders, irregular nodular lesions may occur, indistinguishable from liver neoplasms and presenting a diagnostic challenge.
CASE SUMMARY
We report a case of multiple extensive HI in a patient with poorly controlled diabetes mellitus, who first presented to the emergency room with diabetic ketoacidosis. He then developed jaundice, thrombocytopenia, and a marked elevation of serum aminotransferases. An ultrasound of the liver showed the presence of multiple irregular lesions. Further investigation with a computerized tomography scan confirmed the presence of multiple hypoattenuating nodules with irregular borders and heterogeneous appearance. These lesions were considered highly suggestive of a primary neoplasm of the liver. While the patient was clinically stable, his bilirubin levels remained persistently elevated, and he underwent an ultrasound-guided percutaneous biopsy of the largest lesion. Biopsy results revealed extensive ischemic necrosis of hepatocytes, with no signs of associated malignancy. Three months after the symptoms, the patient showed great improvement in all clinical and laboratory parameters and extensive regression of the lesions on imaging exams.
CONCLUSION This case highlights that diabetic ketoacidosis can cause non-occlusive HI, possibly presenting as nodular lesions indistinguishable from neoplasms.
Key Words: Hepatic infarction; Non-occlusive infarcts; Diabetic ketoacidosis; Pseudotumor of the liver;Liver infarcts; Case report
INTRODUCTION
Hepatic infarctions (HI) are ischemic events of the liver in which a disruption in the blood flow to the hepatocytes leads to focal ischemia, necrosis, and, in severe cases, hepatocellular dysfunction[1]. Due to the dual blood supply that the liver receives from the hepatic artery and the portal vein, HI occurs less commonly than infarctions in other abdominal organs[2]. Most HI is a consequence of occlusive events in either blood vessels supplying the liver. Common causes are portal vein thrombosis, hepatic artery thrombosis, trauma, pancreatitis, surgery (liver transplantation in particular), or hilarious neoplasms[1,3-5]. However, non-occlusive HI may rarely occur[3,4,6]. These uncommon events are associated with disruption of the liver microvasculature and can be secondary to rheumatologic diseases (polyarteritis nodosa, scleroderma, systemic lupus erythematosus, Churg-Strauss syndrome), infection, polycythemia vera, hemodynamic shock, and severe preeclampsia, among other causes[6].
Diabetic ketoacidosis (DK) has been described as a potential cause of non-occlusive HI in a limited number of cases reported in the medical literature[3,6-8]. The pathophysiology of HI in patients with DK is not completely understood but is thought to be multifactorial. Elevated levels of catecholamines released in DK might induce vasoconstriction and liver ischemia[3]. Dehydration and hypotension often present in DK decrease blood flow to the liver, further contributing to ischemia[3]. The low levels of 2, 3-diphosphoglycerate in patients with DK may affect hepatocyte oxygenation, and widespread atherosclerosis, endothelial dysfunction, and hypercoagulability–that are commonly found in patients with diabetes–can also play a role in the occurrence of HI[3,6,7]. Abdominal pain, nausea, jaundice, and fever are the most common symptoms of HI[3,6]. Transaminase levels are elevated, and hyperbilirubinemia, leukocytosis, and disorders of hemostasis are also frequent findings in HI[3,6,8].
CASE PRESENTATION
Imaging examinations
An ultrasound of the liver with doppler evaluation of the hepatic vessels showed multiple heterogeneous nodular lesions in both lobes, with no signs of the hepatic artery or portal vein thrombosis. He then underwent a computerized abdominal tomography (CT) scan on the same date, which revealed the presence of multiple heterogenous lesions in both lobes of the liver, which were hypoattenuating with slight peripheral enhancement in the late phase of the study (Figure 1A and B). Of note, there was a clear wedge-shaped delineation between affected parenchyma and normal areas in the periphery of the liver (Figure 1C and D). The largest lesions were located on liver segments IV and VI, measuring 127 mm and 95 mm, respectively. Based on the imaging exams, primary metastatic neoplasm of the liver (most likely cholangiocarcinoma) or multiple liver abscesses were considered the most likely diagnoses. However, given the lack of clinical and laboratory markers of infection and the sudden onset of symptoms associated with elevation of transaminases, HI was also considered a differential diagnosis. The patient was discharged from the intensive care unit (ICU) 6 d after admission. A control CT scan was obtained 10 days after admission, with no difference in the aspect of the liver lesions but an additional finding of subsegmental pulmonary thromboembolism in the right lung. Anticoagulation with therapeutical doses of enoxaparin was initiated while the patient remained asymptomatic. A magnetic resonance imaging (MRI) scan 16 d after admission showed the same irregular nodular lesions, with a slight peripheral enhancement of the lesions by the contrast medium (gadolinium). As the patient remained clinically well but with significant cholestasis, the decision was made to perform an ultrasound-guided liver biopsy to determine the lesions’ definitive diagnosis, which was made 20 days after patient admission.

Figure 1 Computed tomography scan of the liver. A: Coronal view of the portal phase, showing multiple nodular lesions in both lobes of the liver; B: Axial view of the portal phase, showing multiple nodular lesions in both lobes of the liver, with discrete peripheral enhancement; C: Coronal view of the portal phase,showing the marked and linear transition from the wedge-shaped area of infarction and adjacent liver parenchyma; D: Axial view of the portal phase, showing the marked and linear transition from the wedge-shaped area of infarction and adjacent liver parenchyma.
Chief complaints
A 57-year-old male patient was transferred to the ICU of a tertiary hospital due to a diagnosis of DK with hemodynamical instability. He had first presented to an emergency medical service complaining of diffuse abdominal pain.
Laboratory examinations
Blood and urine exams obtained at arrival at the emergency department (Table 1) showed marked ketonuria, hyperglycemia (470 mg/dL), acidosis (pH of 7.27 and bicarbonate of 15 mEq/L), the elevation of aminotransferases [aspartate aminotransferase (AST) of 2356 U/L and alanine aminotransferase (ALT) of 2438 U/L], and thrombocytopenia (9380 platelets/mcL). At admission to the ICU, there was a decrease in aminotransferase levels (AST of 1121 U/L and ALT of 1546 U/L) but an increase in bilirubin levels (total bilirubin of 1.59 mg/dL). A serological panel for viral hepatitis, dengue fever, and yellow fever yielded negative results. While the patient remained hemodynamically stable, he developed jaundice as his bilirubin levels steadily increased, and he underwent an abdominal ultrasound 2 d after admission.

Table 1 Laboratory data
Physical examination
On arrival at the ICU, physical examination was unremarkable, except for light tenderness on deep palpation of the right upper quadrant during the abdominal exam. Vital signs were within the normal range of values, and the patient was afebrile.
Personal and family history
The patient suffered from hypertension and poorly controlled diabetes mellitus, with irregular use of metformin. He had a previous history of smoking tobacco but was abstinent for more than 20 years and ingested small amounts of alcohol once per week.
History of past illness
At the moment of his arrival in the emergency room, the patient was noticed to be tachycardic and hypotensive. He was placed in close monitoring and was diagnosed with monomorphic ventricular tachycardia, being subject to successful synchronized electrical cardioversion, improving his hemodynamical condition. Treatment with ceftriaxone was started and intravenous insulin, as he had significant hyperglycemia (470 mg/dL). After this procedure, he was transferred to the ICU of a tertiary hospital for stabilization and further investigation.
History of present illness
The patient complained of diffuse abdominal pain that had started 2 d prior and progressively worsened, associated with malaise, asthenia, nausea, and vomiting.
FINAL DIAGNOSIS
Histology of the liver biopsy showed extensive mononuclear infiltration of the liver, associated with intracellular cholestasis, and areas of ischemic necrosis, with no signs of associated malignancy (Figure 2). Tissue cultures obtained at the same moment showed no signs of bacterial growth. These results confirmed the diagnosis of non-occlusive HI, secondary to DK.
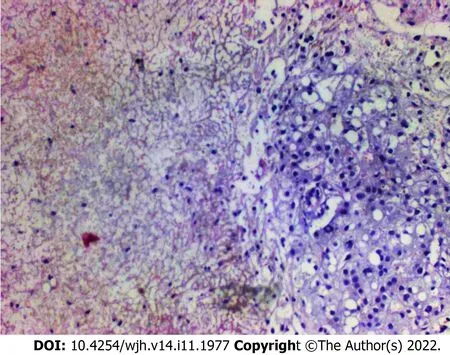
Figure 2 Liver biopsy. Histological analysis of the liver biopsy, showing extensive mononuclear infiltration of liver tissue, associated with intracellular cholestasis,and areas of ischemic necrosis. Hematoxylin and eosin staining, magnification 40 ×.
TREATMENT
The patient was discharged from the hospital 21 days after admission, with optimized control of diabetes and anticoagulation with oral rivaroxaban.
OUTCOME AND FOLLOW-UP
While the patient still had significant cholestasis at the moment of discharge, his jaundice began to improve 1 mo after the onset of the symptoms, and bilirubin levels returned to normal after another month. The patient remains asymptomatic and well during two months of outpatient follow-up, and an ultrasound scan obtained 3 mo after the onset of the symptoms revealed small, focal areas of heterogeneity on the right lobe of the liver, measuring no more than 4 cm, therefore showing significant regression of the lesions.
DISCUSSION
Non-occlusive HI secondary to DK is a rare occurrence, with a small number of cases reported in the literature (Table 2). Its correct diagnosis depends on a high index of clinical suspicion during the evaluation of diabetic patients presenting with abdominal pain and elevation of aminotransferases. While imaging exams can usually correctly determine the presence of HI, atypical presentations may pose a diagnostic challenge. Prolonged hypotension, as described in the case reported, can be a significant factor in the occurrence of HI[8]. CT scan is the most commonly used imaging exam in the diagnosis of HI. While findings of peripheral lesions with clearly limited borders are characteristic of HI, with triangular or wedge-shaped areas of low attenuation, irregular nodular lesions of central location may be present in extensive infarction, indistinguishable from liver neoplasms[3,9,10]. These central parenchyma pseudo nodular lesions are found in about 25% of HI[10]. Enhancement of HI by the contrast medium is generally patchy and heterogenous, with areas of more extensive necrosis remaining hypoattenuating in all phases, while areas that remain isoattenuating in the portal venous phase are suggestive of viable liver tissue[1,10]. A high attenuation, thin subcapsular rim may be present in some cases, which must be distinguished from liver abscesses[9]. Gas formation has been described in both sterile and infected infarcts, and the presence of gas is not an unequivocal marker of infected necrosis of the liver[1,10]. Bile lakes may be present as a late complication of large infarcts due to ischemic necrosis of bile duct epithelium, with jaundice persisting for several weeks[1].
In some cases reported in the literature, diagnosis of hepatic infarction was only established postoperatively, with resection being performed due to the aspect of the lesion being highly suggestive of a liver neoplasm in the imaging exams[3,6]. MRI can be helpful in the diagnosis of HI, showing lesions of heterogeneous intensity, with the center of the lesion being more apparent than the rim, restricted diffusion, no significant enhancement, and little or no mass effect, which helps in differentiating HI from liver neoplasms[3,11,12]. Using a gadoxetate disodium contrast medium may further increase specificity in the differential diagnosis of HI[5].
In the case we reported, both CT and MRI were unable to differentiate the lesions from the liver's primary neoplasms or liver abscesses. Besides the clinical history of acute onset of symptoms with no signs of infection, there was also one finding in the imaging exams that were suggestive of HI: The wedge shape marked delimitation between the areas affected by the infarction and normal liver parenchyma, which was visible in the peripheral areas of the liver and coexisted with the nodular areas which were more centrally located. The use of percutaneous biopsy to confirm the diagnosis of HI is a novel aspect in this case report, as in previously reported cases, HI was diagnosed either by imaging exams or surgical exploration (Table 2). Since correct diagnosis could not be confirmed by imaging exams and considering a high clinical suspicion of HI, liver biopsy was seen as the next step in the investigation to avoid unnecessary surgical exploration with significant morbidity to the patient and also to avoid missing a diagnosis of liver neoplasm, which could coexist or even be the cause of a liver infarction. Histological analysis of HI is characterized by the presence of a centrilobular zone of parenchymal necrosis, in contrast to a peripheral zone with relative preservation of portal tracts, hepatic veins, and intralobular stroma[2,9].

Table 2 Cases of hepatic infarction secondary to diabetic ketoacidosis reported in the literature
Non-occlusive liver infarcts usually regress after a while as regeneration of the liver occurs. While the necrotic tissue present at the site of a HI is usually sterile, an infection may occur due to biliary tract or hematogenous dissemination of bacteria, with progression to a liver abscess that may require treatment with antibiotics and/or percutaneous drainage[6]. In the case we reported, the patient showed no signs of infection, and tissue cultures obtained at the moment of the liver biopsy showed no signs of bacterial growth. His persistently elevated bilirubin levels may be attributed to the formation of bile lakes in the central areas of necrosis and the significant disruption of the biliary drainage of the areas of liver parenchyma adjacent to the areas most affected by the HI. The benefits of anticoagulant therapy in the management of HI are uncertain, and unless the infarction is associated with vascular occlusion or a thrombotic etiology, the use of anticoagulants is not generally recommended[13]. In this case, the patient received anticoagulation due to concomitant pulmonary thromboembolism. This thromboembolic event raises the question of whether a hypercoagulable state may also play a role in the genesis of HI associated with DK, as microvascular thrombosis of the liver may aggravate the ischemic insult already present due to the other mechanisms of aggression in DK that were previously discussed.
CONCLUSION
DK is a rare cause of non-occlusive HI that must be remembered in diabetic patients with abdominal pain and elevated markers of hepatic injury. While in the imaging exams, HI usually presents itself as wedge-shaped areas of hypoattenuation on the periphery of the liver, atypical presentations with irregular nodular areas of central location may occur, which are indistinguishable from liver neoplasms. Using ultrasound-guided percutaneous biopsy may provide the correct diagnosis in these cases, avoiding unnecessary surgical exploration.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the important contribution of Dr. Neto RT and the CONLAB laboratory in the diagnostic investigation of the case reported and in the elaboration of this manuscript.
FOOTNOTES
Author contributions: Barros LCTR and Santos BMRT designed the report; Gomes VMS and Ferreira GSA collected the patient’s clinical data, analyzed the data and wrote the paper; Barros LCTR, Santos BMRT and Vieira LPB reviewed the paper.
Informed consent statement: Consent was obtained from the patient, and the signed Informed Consent Form was provided to the publisher.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin: Brazil
ORCID number: Vitoria Mikaelly da Silva Gomes 0000-0003-3785-7115; Gustavo de Sousa Arantes Ferreira 0000-0002-2225-9190; Luise Cristina Torres Rubim de Barros 0000-0001-5499-8548; Barbara Moreira Ribeiro Trindade dos Santos 0000-0002-7792-5920; Lorenna Paulinelli Bahia Vieira 0000-0002-5727-6396.
S-Editor: Xing YX
L-Editor: A
P-Editor: Xing YX
杂志排行
World Journal of Hepatology的其它文章
- Haemochromatosis revisited
- Current status of disparity in liver disease
- Liver test abnormalities in asymptomatic and mild COVID-19 patients and their association with viral shedding time
- Elevated calprotectin levels are associated with mortality in patients with acute decompensation of liver cirrhosis
- Current management of liver diseases and the role of multidisciplinary approach
