Content Determination of Cistanches Herba in Zhenrongdan Mixture by HPLC
2022-11-28JianpingZHANGXuemeiZHOUHanGAODongWANG
Jianping ZHANG, Xuemei ZHOU, Han GAO, Dong WANG
Inner Mongolia Autonomous Region Institute for Drug Control , Huhhot 010020, China
Abstract [Objectives] To establish a HPLC-UV method for the determination of Cistanches Herba in Zhenrongdan mixture. [Methods] According to the content determination method of Cistanches Herba in the 2020 edition of Chinese Pharmacopoeia, using HPLC-UV method, the content determination method of Cistanches Herba in Zhenrongdan mixture was established, and the content of echinacoside was determined. The related chromatographic conditions were explored, and the methodological investigation was carried out. [Results] The chromatographic conditions were determined as follows: octadecylsilane bonded silica gel as filler (C18); 80% acetonitrile solution (containing 0.2% phosphoric acid)-0.2% phosphoric acid aqueous solution (15∶85) as mobile phase; detection wavelength was 330 nm. The number of theoretical plates should not be less than 3 000 according to the peak of echinacoside. The methodological investigation showed that the method had better precision, accuracy and repeatability. Under the conditions of this study, echinacoside had better linear relationship in the range of 74.9-1 498 ng, and the average recovery was 99.1%, RSD=0.5% (n=6). [Conclusions] A method for the determination of Cistanches Herba in Zhenrongdan mixture by HPLC-UV was established. The method is sensitive, rapid, accurate and suitable for the determination of Cistanches Herba in Zhenrongdan mixture.
Key words HPLC-UV, Zhenrongdan mixture, Cistanches Herba, Echinacoside
1 Introduction
Zhenrongdan mixture is an exclusive Chinese patent medicine produced by Hebei Kangzhi Pharmaceutical Co., Ltd. It was approved by the State Food and Drug Administration in 2005 and has been produced since then. Raw medicinal materials mainly contain Rehmanniae Radix Praeparata, Paeoniae Radix Alba, Angelicae Sinensis Radix, Chuanxiong Rhizoma, Moutan Cortex, Salviae Miltiorrhizae Radix Et Rhizoma, Spatholobi Caulis, Cistanches Herba, and Ligustri Lucidi Fructus. It is mainly used for nourishing blood, yin and spirit, and tonifying liver and kidney. It is applicable to middle-aged and elderly women who suffer from waist and knee soreness, fatigue, irritability, insomnia, forgetfulness, listlessness, dizziness and tinnitus due to deficiency of liver and kidney and disharmony of qi and blood[1]. Cistanches Herba is one of the main drugs in the prescription, and its main functions are nourishing kidney yang, replenishing blood essence, moistening intestines, and relieving constipation, and it plays an obvious role in tonifying and smoothing. Its quality shall be controlled. In content determination of Cistanches Herba in the 2020 edition ofChinesePharmacopoeia, the total amount of echinacoside and acteoside is controlled[2]. Since the Chinese patent medicine contains Rehmanniae Radix Praeparata, which also contains acteoside, it is unreasonable to carry out quality control of Cistanches Herba in the Chinese patent medicine by using the total amount of echinacoside and acteoside. Therefore, content determination of echinacoside is used to control the quality of Cistanches Herba in Zhenrongdan mixture.
2 Instruments and reagents
2.1 InstrumentsHigh performance liquid chromatography: Shimadzu LC-20A, Waters e2695, both diode array detector; Sartorius electronic balance (1/10 000); Sartorius electronic balance (1/1 000 000).
2.2 ReagentsEchinacoside reference substance (111670-201505, purity: 92.5%) was bought from China Institute for Food and Drug Control. Acetonitrile was chromatographically pure, and other reagents were all AR, and water was high purity. Zhenrongdan mixture was produced by Hebei Kangzhi Pharmaceutical Co., Ltd., and sample information was shown in Table 1.

Table 1 Sample information of Zhenrongdan mixture
3 Methods and results
3.1 Selection of chromatographic conditions
3.1.1Chromatographic column. Chromatographic column: octadecylsilane bonded silica gel as filler. SHMADZU InertSustain C18250 mm×4.6 mm 5 μm column and Alltima C18250 mm×4.6 mm 5 μm column were used in the experiment.
3.1.2Selection of mobile phase. Referring to the content determination method of Cistanches Herba in the 2020 edition ofChinesePharmacopoeia, echinacoside reference substance was taken as control, and content determination conditions of Zhen-rongdan mixture (Z-1) were groped. Because there were too many interfering components in the test sample, it was impossible to obtain a good separation effect. After exploring the conditions of mobile phase, 80% acetonitrile solution (containing 0.2% phosphoric acid) -0.2% phosphoric acid aqueous solution (15∶85) was used as the mobile phase. The results showed that the separation effect was good, and the retention time was moderate, so it was determined as the mobile phase conditions for this test.
3.1.3Selection of column temperature. 35 ℃ and 40 ℃ of column temperature was compared in the experiment. The results showed that there was little difference in retention time, theoretical plate number and resolution. So, column temperature was selected as 35 ℃.
3.1.4Selection of detection wavelength. Diode array detector was used for spectral scanning of echinacoside reference substance in 190-800 nm (Fig.1). The results showed that echinacoside had the absorption at middle wavelength. After investigation, 330 nm was selected as detection wavelength.
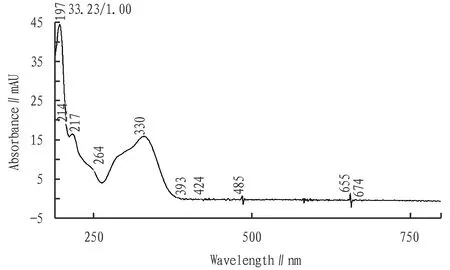
Fig.1 Ultraviolet spectrum of echinacoside
3.1.5Determination of theoretical plate number. The determination results of reference substances with different concentrations and multiple lots of test samples showed that the theoretical plate number of echinacoside peak was more than 3 000, which can achieve good results, and the resolution also met the requirements (R>1.5), shown in Fig.2. So, the number of theoretical plates specified by the standard should not be less than 3 000 according to echinacoside peak.

Fig.2 Chromatogram of test sample under detection conditions (2.3 of resolution)
3.2 Preparation of test solutionsPreparation of reference substance solution: appropriate amount of echinacoside reference substance was precisely weighed, and 80% methanol was added to prepare the solution containing 0.075 mg/mL echinacoside.
Preparation of test sample solution: 5 mL of solution was precisely weighed and set in 100 mL of measuring flask. 80% methanol was added to dilute to the graduate, and then it was shaken evenly.
Preparation of negative sample solution: negative sample (not adding Cistanches Herba) not containing echinacoside was prepared according to the prescription process, and the solution was prepared according to the method of above test sample solution.
3.3 Specificity inspectionAccording to the determined chromatographic conditions, 10 μL of reference substance solution, 10 μL of test sample solution and 10 μL of negative control solution were precisely weighed to inject into liquid chromatograph, and the results were shown in Fig.3. It was clear that in the chromatogram of the test sample, there was chromatographic peak at the same position as the retention time of the reference substance, and the separation effect was good, while the negative sample had no chromatographic peak at the same position as the retention time of the reference substance. It showed that the content determination method had no interference and better specificity.

Note: a. Echinacoside reference substance; b. Test sample; c. Negative sample.
3.4 Peak purity check10 μL of test sample solution was precisely weighed and injected into liquid chromatograph, and diode array detector was used for purity verification of echinacoside peak, and detection results were shown in Fig.4. The results showed that echinacoside peak in the measured sample was single component.

Note: A. Purity. Impurity: not detected; peak purity index: 0.999 991; single-point threshold: 0.995 805; minimum peak purity index: 4 186. B. Outline.
3.5 Linear investigation8.104 mg of echinacoside (92.5% of purity) was set in 100 mL of measuring flask. 80% methanol was added to dissolve it and dilute to the graduate. After shaken evenly (containing 0.074 9 mg/mL of echinacoside), 1, 2, 5, 8, 10, 15 and 20 μL of solution was precisely injected into liquid chromatograph. Measured by above chromatographic conditions, regression analysis of injection volume by peak area was conducted. The results showed that echinacoside had good linear relationship in the range of 74.9-1 498 ng. Regression equation was as below:Y=0.000 9X-27.002,r2=0.998.
3.6 PrecisionRepeatability test: 6 test samples with the same lot number (Z-1) were precisely weighed, each 5 mL. They were measured according to above mentioned chromatographic conditions, and injection volume was 10 μL (Table 2). The results showed that the method had good repeatability.

Table 2 Repeatability test results of echinacoside
3.7 Accuracy6 test samples from the same lot number (Z-1) were precisely weighed, each 2.5 mL, and 4 mg of echinacoside was precisely added. They were were measured by above mentioned chromatographic conditions, and injection volume was 10 μL (Table 3). The results showed that the accuracy of the method was good.
3.8 Durability testChanging to different models of instruments and chromatographic columns from different manufacturers, content determination of Z-1 sample was conducted according to the determined chromatographic conditions (Table 4). The results showed that the method had better durability.

Table 3 Standard adding recovery test

Table 4 Durability test of different instruments and chromatographic columns
3.9 Stability testThe same test sample solution was taken for determination at 0, 2, 5, 10, 15 and 24 h. Integral value of peak area of echinacoside was 1 036 158, 1 034 095, 1 022 337, 1 027 918, 1 011 163 and 1 011 870, andRSDwas 1.1%. Within 24 h, integral value of peak area of echinacoside was basically stable.
3.10 Determination method for echinacoside content in Rongzhendan mixtureChromatographic conditions and system suitability test: octadecylsilane bonded silica gel as filler (C18); 80% acetonitrile solution (containing 0.2% phosphoric acid)-0.2% phosphoric acid aqueous solution (15∶85) as mobile phase; detection wavelength was 330 nm. The number of theoretical plates should not be less than 3 000 according to the peak of echinacoside. Preparation of reference substance solution: appropriate amount of echinacoside reference substance was taken, and 80% methanol was added to prepare the 50 μg/mL of solution. Preparation of test sample solution: 5 mL of solution was precisely weighed and set in 100 mL of measuring flask, and 80% methanol was added to dilute to the graduate. After shaken evenly, it was filtered. Determination method: 10 μL of reference substance solution and 10 μL of test sample solution were precisely weighed and injected into liquid chromatograph, and then it was determined.
4 Content determination results of test sample
14 lots of samples were taken for treatment and determination according to above method, and determination results of multiple lots of samples were shown in Table 5.
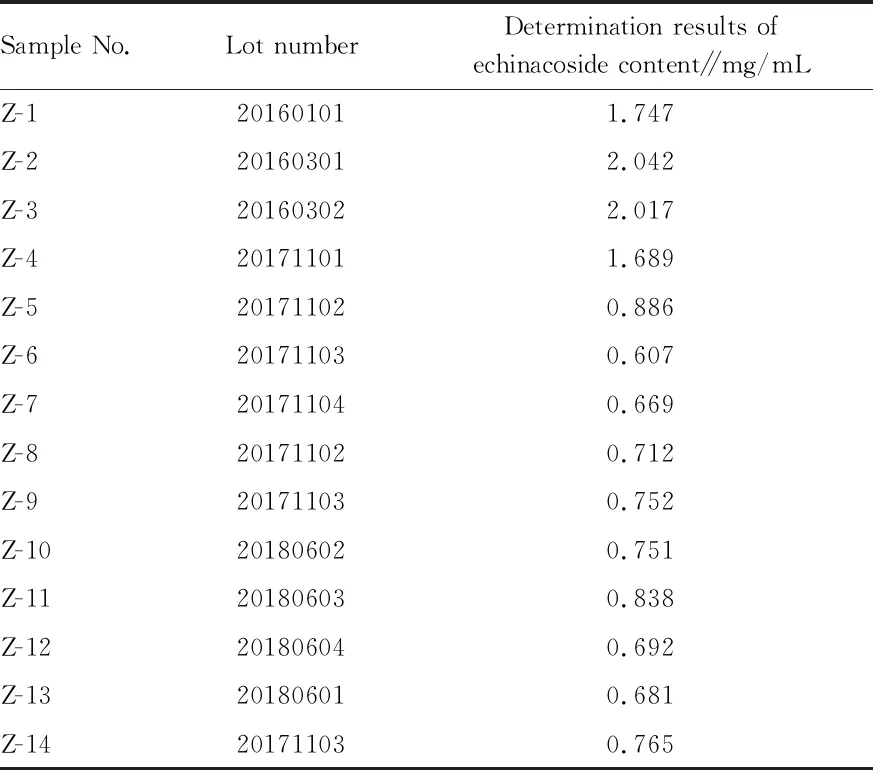
Table 5 Content determination results of echinacoside in the sample
5 Discussion
Cistanches Herba in Zhenrongdan mixture is used as medicine by decocting, and one of its effective components, echinacoside, can be better transferred to the finished medicine. Therefore, this study developed a highly targeted HPLC-UV method to determine its content. In the experiment, acetonitrile-phosphoric acid water was taken as mobile phase, and echinacoside was separated and determined by C18column. By adjusting chromatographic conditions, better separation effect could be obtained. From the chromatograms and results, the method has high sensitivity, less interference, good peak type of echinacoside, good stability, repeatability and feasibility. The test sample solution had good chromatographic performance, and this study could provide a scientific basis for the quality study of echinacoside.
Since the content of Cistanches Herba in theChinesePharmacopoeiais calculated by the total amount of acteoside and echinacoside, the limit of echinacoside cannot be determined by calculating the transfer rate. Considering that the content of echinacoside from different sources of Cistanches Herba is quite different, the limit of echinacoside content in Zhenrongdan mixture should fully consider the impact of its difference on the content, so as to ensure the scientific and reasonable limit designation.
杂志排行
Medicinal Plant的其它文章
- Establishment of Quality Standard for Freeze-dried Tablets of Polygonatum sibiricum and Study on Anti-tumor Activity of Diosgenin
- Herbal Textual Research of Inulae Flos in Chinese Classic Prescription
- Quality Standard of Zijinbiao
- Quality Standard of Yi Medicinal Material Anaphalis margaritacea
- Serum Metabolomic Characteristics of Primary Dysmenorrhea Rat Model Induced by Estradiol Benzoate Combined with Oxytocin
- Prescription Design and Preparation Process of Paeonol Bead Popping Gum with Hypoglycemic Effect
