Exploring the mechanism of neuronal apoptosis and brain developmental damage in the hippocampus of hypoxicischemic neonatal rats based on BDNF/TrkB/CREB pathway
2022-11-15LUTiantianZHANGYaoLIANGBinLIUMinCHENXiulingJiaYanping
LU Tian-tian, ZHANG Yao, LIANG Bin, LIU Min, CHEN Xiu-ling, Jia Yan-ping✉
1. Department of Neonatology, Haikou Maternal and Child Health Hospital, Haikou 570203,China
2. Department of Pediatrics, Haikou People's Hospital, Haikou 570208,China
Keywords:Hypoxic-ischemic brain injury Neuronal apoptosis BDNF TrkB CREB
ABSTRACT
1. Introduction
One of the leading causes of permanent neurological injury and death in neonates is hypoxic ischemic brain injury (HIBI)[1].According to the study, 0.1%~0.2% of newborns have perinatal asphyxia-related HIBI, with a 20% mortality rate, and 40% of survivors have serious complications such as intellectual disability,cerebral palsy, and epilepsy[2]. The current study's findings of HIBI may be related to various pathophysiological mechanisms such as inflammatory response, oxidative stress, energy depletion, and calcium overload, but no unified conclusion has been reached. A neurotrophic factor is brain derived neurotrophic factor (BDNF),and tyrosine protein kinase B (TrkB) is a specific functional receptor of BDNF. The combination of the two can promote brain tissue repair and regeneration following HIBI[3]. cAMP-response element binding protein (CREB) can induce BDNF gene transcription and regulate its expression, which is linked to physiological processes such as neuroregeneration and repair after HIBI[4]. This study used HIBI young mice as the research object and the BDNF/TrkB/CREB pathway as the target to investigate its role in the pathogenesis of HIBI and to provide a reference for clinical prevention and treatment of HIBI.
2. Materials and methods
2.1 Laboratory animals
The experimental animal center at Hubei Medical College provided 95 newborn 7-day-old Wistar young rats weighing (13.6±1.7) g, half male and half female. The experimental animals' certificate number was SCXK (E) 2018-0005. All of the experimental animals used in this study meet the standards of Hainan Medical College's ethics committee. The animals are allowed to complete the experiment in as comfortable an environment as possible. To reduce the pain of the experimental animals, the operation is strictly standardized during anesthesia and drug administration. Following the experiment, the animals are euthanized, and the school's animal management center is in charge of all animal corpses.
2.2 Reagent
In situ terminal transferase labeling (TUNEL) detection kit (Roche,USA); 2,3,5-triphenyltetrazolium chloride (TTC) kit (Sigma,USA); BDNF, TrkB, CREB lentiviral vector and empty vector(Shanghai Jikai gene Chemical Technology Co., Ltd.); BCA protein quantification kit and protein extraction reagent (Thermo company,USA); BDNF、TrkB、CREB、β-actin antibody (Proteintech,USA).
2.3 HIBI model development
To replicate the HIBI model in newborn rats, use the Rice-Vannucci classical method[5]. After disinfection, the young rats were anesthetized with ether inhalation and placed on the operating table.The two ends of the left common carotid artery were ligated, the middle was cut, and the skin was sutured. It was placed in a cushion containing the mother rats' feces for 2 hours before being placed in an organic glass box containing a 92% N2+8% O2mixed gas for another 2 hours. The young rats' general behavior was observed.The young mice's spontaneous left turn or tail pinching scream demonstrated that the model had been successfully replicated,and they were returned to the cage for breastfeeding. Only the left common carotid artery was isolated without ligation or hypoxia in the sham operation group. It was returned to the cage for breast feeding after suture.
2.4 Experimental design
To detect learning and memory ability, brain tissue damage, and the expression of BDNF, TrkB, and CREB proteins in the hippocampus,use 10 young rats in the sham operation group as the control group,and another 10 young rats in the HIBI model.15 young rats in the sham operation group were taken as the control group, and 60 young rats in the HIBI model were taken as the negative control group (NC), BDNF overexpression group (LV-BDNF), TrkB overexpression group (LV TrkB) and CREB overexpression group(LV-CREB), with 15 rats in each group. LV-BDNF group, LV TrkB group and LV-CREB group were injected through tail vein with titer of (1.7 × 109TU/mL) of BDNF lentiviral vector 5×107TU,titer (2.1×109TU/mL) of TrkB lentiviral vector 1×107TU, titer(1.4×109TU/mL) of CREB lentiviral vector 5×106TU, the titer of tail vein injection in NC group was (2×109TU/mL) of empty lentiviral vector 1×106TU; Once every 4 weeks for 2 consecutive times, and the experiment was completed after 8 weeks. The learning and memory ability, brain tissue damage, hippocampal neuron apoptosis and Bcl-2, Bax and NF-κB protein expression were detected.
2.5 Y-maze test memory and learning ability
The Y-maze is a reflection box of the third class with power on the bottom. Each arm has a signal lamp at the end. When the lamp is on, the arm is not powered on, and the other two arms are powered on (the voltage is 50-70 V, and the strength is able to make the mice escape). The safety zone was changed at random during the experiment. After being shocked, the young rats immediately escaped to the safety zone, which was a correct response, and 9 consecutive correct responses were learned. Each shock lasted for 5s. The training times of the young rats were recorded, and the less the training times, the better the learning ability [6]. The memory was tested 24 hours after the end of the click experiment, 20 times in total, and the memory ability was the percentage of the correct response times in the total number of tests.
2.6 The volume of cerebral infarction was measured by TTC staining
5 young rats were chosen at random from each group. The brain tissue was removed and placed in 0.15mol/L phosphate buffered saline solution before being frozen at 4 ℃ for 5 minutes and coronal sections were cut at 2 mm intervals. The sections were immersed in 5mL of 1% TTC phosphate buffer and incubated in the dark for 0.5h at 37℃. After TTC staining, red indicates normal brain tissue, white indicates infarcted tissue, brain slices were taken out, fixed in 10%formalin, and photographed layer by layer. Imagepro Plus image analysis software was used for processing and statistics, and the infarct area of each layer was calculated, and the volume of infarct was multiplied by the layer.
2.7 TUNEL was used to detect the level of neuronal apoptosis in the hippocampus
5 young rats in each group were randomly decapitated and killed.The hippocampal tissue was isolated under sterile conditions, fixed with 4% paraformaldehyde, dehydrated with ethanol, embedded in conventional paraffin, and sectioned in coronal sections with a thickness of 3-5 μm. Baking, dewaxing, ethanol hydration,proteinase K digestion for 30 min, PBS washing for 3 times, adding TUNEL reaction solution, placing in a dark temperature box at 37℃ for 60 min, hematoxylin counterstaining, ethanol dehydration,xylene transparent sealing. 5 non-repetitive visual fields were selected for each slice, and TUNEL positive neurons were counted and the average value was taken.
2.8 Western blot was used to detect BDNF, TrkB, CREB,Bcl-2, Bax and NF-κB Expression in hippocampus of young rats
5 young mice were randomly taken from each group and decapitated. The left hippocampus of the young mice was removed at low temperature,and lysed by adding RIPA. The supernatant was centrifuged at 12 000rpm for 15 min. The protein content in the supernatant was measured by BCA method.40 μg protein was taken, denaturated by adding buffer, electrophoresis, membrane transformation, blocking, adding primary antibodies BDNF, TrkB,CREB, Bcl-2, Bax, NF-κB, β-actin, incubating at 4 ℃ overnight,washing with TBST, adding secondary antibodies, incubating at room temperature for 2 h, washing with TBST. After color development with ECL chemiluminescence reagent, the image was obtained by chemiluminescence instrument.
2.9 Statistical processing
SPSS 17.0 software package was used for statistical analysis of data. The mean±standard deviation(±s ) was used for measurement data. T-test was used for comparison between two groups. One-way ANOVA was used for comparison of differences between multiple groups. P<0.05 was considered as the difference was statistically significant.
3. Results
3.1 Changes in learning and memory ability of young rats after modeling
Compared with the control group 1, the learning ability and correct response rate of memory test of the HIBI group were significantly lower (P<0.05). See Table 1.
Table 1 Correct response rate of learning ability and memory test in young rats after modeling (%,n=10, ±s )
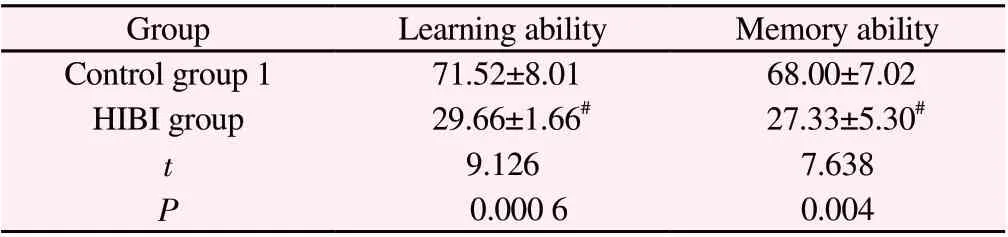
Table 1 Correct response rate of learning ability and memory test in young rats after modeling (%,n=10, ±s )
Note:#P<0.01 Compared with control group 1.
GroupLearning abilityMemory ability Control group 1 71.52±8.01 68.00±7.02 HIBI group 29.66±1.66# 27.33±5.30#t 9.1267.638 P 0.000 60.004
3.2 Brain tissue injury of young rats after modeling
Compared with control group 1, the infarct volume of brain tissue in HIBI group increased significantly (P<0.05). See Table 2.
Table 2 Infarct volume of brain tissue in young rats after modeling(n=5, ±s )

Table 2 Infarct volume of brain tissue in young rats after modeling(n=5, ±s )
Note:#P<0.01 Compared with control group 1.
GroupInfarct volume of brain tissue(mm3)Control group 10.59±0.03 HIBI group 6.64±0.59#t 11.719 P 0.0001
3.3 Expression of BDNF, TrkB and CREB protein in hippocampus of young rats after modeling
Compared with the control group 1, the expression of BDNF and TrkB in hippocampus of Hibi group was significantly higher(P<0.05) and CREB was significantly lower (P<0.05). See Table 3.
Table 3 Expression of BDNF, TrkB and CREB proteins in the hippocampus of young rats after modeling(n=5, ±s )

Table 3 Expression of BDNF, TrkB and CREB proteins in the hippocampus of young rats after modeling(n=5, ±s )
Note:#P<0.01 Compared with control group 1.
GroupBDNFTrkBCREB Control group 10.63±0.030.52±0.020.81±0.03 HIBI group 0.88±0.05# 0.78±0.06# 0.38±0.04#t 6.6825.4678.824 P 0.0060.0040.002
3.4 Effect of regulating the expression of BDNF, TrkB and CREB on learning and memory ability of young rats
Compared with control group 2, the correct response rate of learning and memory test in NC group was significantly decreased(P<0.01); Compared with NC group, LV-BDNF group, LV-TrkB group and LV-CREB group had significantly higher correct response rates in learning and memory tests (P<0.01). See Table 4.
Table 4 Learning ability and correct response rate of memory test in young rats after regulation of BDNF, TrkB and CREB expression(%,n=15, ±s )
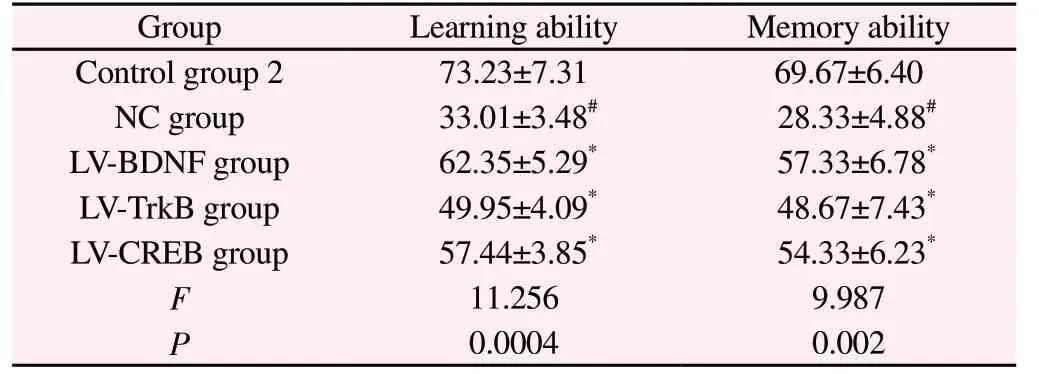
Table 4 Learning ability and correct response rate of memory test in young rats after regulation of BDNF, TrkB and CREB expression(%,n=15, ±s )
Note: #P<0.01 Compared with control group 2; *P<0.01 Compared with NC group.
GroupLearning abilityMemory ability Control group 273.23±7.3169.67±6.40 NC group 33.01±3.48# 28.33±4.88#LV-BDNF group 62.35±5.29* 57.33±6.78*LV-TrkB group 49.95±4.09* 48.67±7.43*LV-CREB group 57.44±3.85* 54.33±6.23*F 11.2569.987 P 0.00040.002
3.5 Effect of regulating the expression of BDNF, TrkB and CREB on brain tissue injury in young rats
Compared with control group 2, the infarct volume of brain tissue in NC group was significantly increased (P<0.01); Compared with NC group, the infarct volume of brain tissue in LV-BDNF, LV-TRKB and LV-CREB groups was significantly reduced (P<0.01). As shown in Table 5.
Table 5 Infarct volume in young rat brain tissue after regulation of BDNF, TrkB and CREB expression (n=5, ±s )
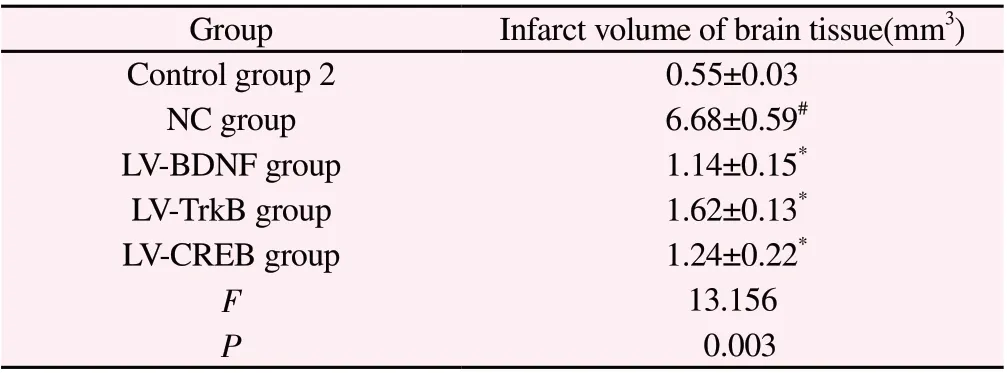
Table 5 Infarct volume in young rat brain tissue after regulation of BDNF, TrkB and CREB expression (n=5, ±s )
Note: #P<0.01 Compared with control group 2; *P<0.01 Compared with NC group.
GroupInfarct volume of brain tissue(mm3)Control group 20.55±0.03 NC group 6.68±0.59#LV-BDNF group 1.14±0.15*LV-TrkB group 1.62±0.13*LV-CREB group 1.24±0.22*F 13.156 P 0.003
3.6 Effect of regulating BDNF, TrkB and CREB expression on apoptosis of hippocampal neurons in young rats
Compared with control group 2, the number of TUNEL positive cells in the hippocampus of young rats in NC group increased significantly (P<0.01); Compared with NC group, the number of TUNEL positive cells in hippocampus of LV-BDNF group, LV-TrkB group and LV-CREB group decreased significantly (P<0.01). See Table 6 and Figure 1.
2.7 Effects of regulating the expression of BDNF, TrkB and CREB on the expression of Bcl-2, Bax and NF-κB in hippocampus of young rats
Compared with the control group 2, the expressions of apoptosispromoting proteins Bax and NF-κB in the hippocampus of NC group were significantly increased (P<0.05), while the expression of Bcl-2 protein was significantly decreased (P<0.05). Compared with NC group, the expressions of Bax and NF-κB protein in hippocampus of young rats in LV-BDNF, LV-TrkB and LV-CREB groups were significantly decreased (P<0.05), the protein expression of Bcl-2 was significantly increased (P<0.05). As shown in Table 7.

Figure 1 The apoptosis of hippocampal neurons in young rats after regulation of BDNF, TrkB and CREB expression(×100)
Table 6 The apoptosis of hippocampal neurons in young rats after regulation of BDNF, TrkB and CREB expression(n=5,±s )
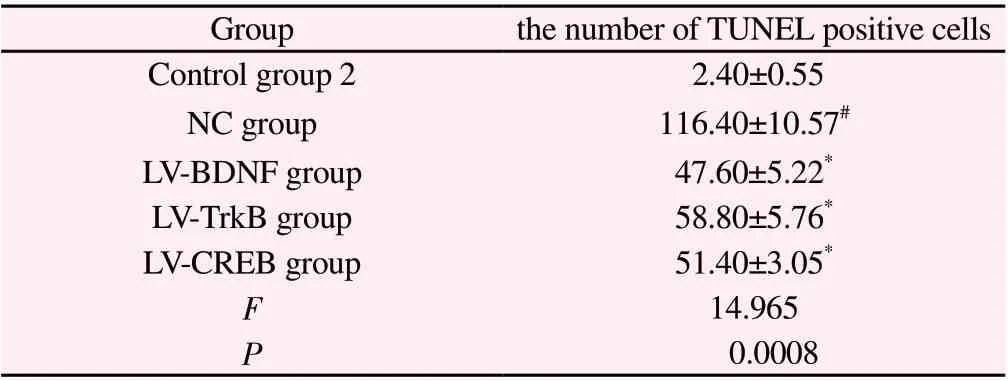
Table 6 The apoptosis of hippocampal neurons in young rats after regulation of BDNF, TrkB and CREB expression(n=5,±s )
Note: #P<0.01 Compared with control group 2; *P<0.01 Compared with NC group.
Groupthe number of TUNEL positive cells Control group 2 2.40±0.55 NC group116.40±10.57#LV-BDNF group47.60±5.22*LV-TrkB group58.80±5.76*LV-CREB group51.40±3.05*F 14.965 P 0.0008
Table 7 Bcl-2, Bax and NFκB protein expression in hippocampal tissue of young rats after regulation of BDNF, TrkB and CREB expression (n=5,±s )

Table 7 Bcl-2, Bax and NFκB protein expression in hippocampal tissue of young rats after regulation of BDNF, TrkB and CREB expression (n=5,±s )
Note: #P<0.01 Compared with control group 2; *P<0.01 Compared with NC group.
GroupBcl-2BaxNFκB control group 2 0.85±0.05 0.69±0.040.91±0.06 NC group 0.25±0.05# 1.67±0.09# 2.21±0.11#LV-BDNF group 0.59±0.05* 0.95±0.06* 1.44±0.08*LV-TrkB group 0.69±0.06* 0.85±0.08* 1.36±0.06*LV-CREB group 0.61±0.04* 0.82±0.06* 1.25±0.07*F 9.98712.36515.015 P 0.005 0.0007 0.0002
4. Discussion
The physiological tissue state of rats at birth is equivalent to the premature birth stage of human newborns. However, the development speed of brain tissue after birth is much faster than that of human newborns. The degree of cell proliferation and differentiation of 7-day-old rats and the situation of periventricular hair matrix are basically equivalent to that of newborns. At present,the most classical method for preparing the hypoxic-ischemic brain injury model is the Rice modified method, which is simple to operate and has a high success rate. The time and symptom degree are easy to grasp and the histopathological changes are obvious.Therefore, 7-day-old young mice are selected in this experiment to replicate the HIBI model of newborn mice by the Rice modified method. The hippocampus is the most sensitive part of HIBI model to injury. Studies have shown that ischemia and hypoxia can cause a large number of apoptosis of neurons in the hippocampus of newborn rats[7,8]. After TTC staining, it was found that there were large white infarct areas in the cerebral cortex and hippocampus of the young rats in the model group, and the infarct volume was significantly increased, indicating that the experimental model was successfully replicated. It has been reported that HIBI has a great impact on the body cognition and neuronal function of CA1 area in the hippocampus of young rats[9,10]. The results of this study showed that the learning and memory abilities of HIBI young mice were significantly weakened, which was consistent with the literature reports. In conclusion, HIBI can induce apoptosis of hippocampal neurons in newborn rats, and further promote the decline of learning and memory ability in rats.
BDNF is a member of neurotrophic factor family, which is widely distributed in brain tissue and has a large content in the hippocampus. It can promote the reproduction, development,differentiation and regeneration of neurons, and enhance the nutritional support, protection and function expression of the central nervous system[11]. Under the stimulation of ischemia and hypoxia stress, highly expressed BDNF can protect neurons. Some studies have shown that injection of BDNF into lateral ventricles of HIBI newborn rats can significantly inhibit neuronal death in hippocampal CA1 area[12]. TrkB is a specific receptor of BDNF, which can transmit signals, reactively cause the activation of various signal pathways in cells, and promote the development of nerve cells,repair and regeneration after injury[13]. When HIBI occurs, BDNF and TrkB proteins are highly expressed in the damaged area. A large amount of combination of the two can promote the activation of CREB, maintain the survival of nerve cells and promote their repair[14,15]. Studies have shown that after overexpression of BDNF in HIBI mice, the expression of TrkB mRNA and protein in brain tissue is enhanced, which strengthens the repair effect of nerve cells[16]. CREB is an important transcription factor nuclear protein,which can regulate the expression of BDNF and inhibit neuronal apoptosis through Ca2+pathway and regulating the expression of some nutritional factors[17]. This study found that the expression of CREB protein in the hippocampus of young rats in the model group was significantly decreased, and the expression of BDNF and TrkB protein was significantly increased. In order to further explore its mechanism, we used tail vein injection of BDNF, TrkB and CREB lentiviral vectors to intervene the young mice. The results showed that after overexpression of BDNF, TrkB and CREB, the learning ability and memory ability of the young mice were significantly enhanced, the infarct volume of brain tissue was significantly reduced, and the number of TUNEL positive cells was significantly reduced, indicating that the neuronal function and cognitive function of the young mice were improved.
Bcl-2 is an important gene that inhibits apoptosis. Studies have confirmed that down-regulation of Bcl-2 expression can lead to apoptosis[18,19]. Ischemia and hypoxia can promote the release of Bax from the anti apoptotic factor complex, and then enter the mitochondria to activate apoptosis[20]. NF-κB is an important immune response factor in the process of cell signal transduction,and participates in the regulation of cellular immune response, cell proliferation, apoptosis and other cellular stress behaviors. NFκB can promote apoptosis by regulating the expression or signal transmission of anti apoptotic factors and apoptosis inducing ligands.It was found that after ischemia and hypoxia in neonatal rats NF-κB expression will be upregulated and NF-κB can effectively reduce the level of neuronal apoptosis in HIBI rats[21]. In this study, Bcl-2,Bax and NF-κB in hippocampus of young rats were detected. The results showed that the expressions of Bax and NF-κB protein were significantly increased in the model group, while the expression of Bcl-2 protein was significantly decreased, which indicated that HIBI could induce apoptosis of hippocampal neurons, which was consistent with literature reports. However, the protein expressions of Bax and NF-κB were significantly decreased and the expression of Bcl-2 was significantly increased in the BDNF, TrkB and CREB overexpression groups, indicating that regulating the expression of BDNF, TrkB and CREB may inhibit the apoptosis of nerve cells by regulating the expression of apoptotic proteins and improve the brain tissue injury in the model young rats.
In conclusion, the expression of BDNF, TrkB and CREB is abnormal in the HIBI model young mice. Overexpression of BDNF,TrkB and CREB can improve the learning and memory ability of the young mice, repair the brain tissue damage and inhibit the apoptosis of hippocampal cells. Therefore, the mechanism of HIBI may be related to the BDNF, TrkB and CREB pathways.
Author's contribution
Lu Tian-tian: experimental design, experimental operation, index detection, writing a paper; Zhang Yao and Liang Bin: assist in experimental modeling and index detection; Liu Min and Chen Xiu-ling: collect data and make statistical analysis; Jia Yan-ping:experimental guidance, paper revision and review.
All authors declare that they have no conflict of interest.
杂志排行
Journal of Hainan Medical College的其它文章
- Research progress on inflammatory mechanism and traditional Chinese medicine in the treatment of diabetic retinopathy
- Research progress on the mechanism of TCM treatment of depression based on NLRP3 pathway
- Effect of Shengsui Jiangu Capsule on bone conversion in rats with alcoholic osteoporosis
- Effectiveness and Safety of Chinese Medicine in the Treatment of PostTACE Syndrome of Primary Hepatocellular Carcinoma: A Systematic Review
- Real world analysis on renal impairment of elemene emulsion injection based on propensity score matching
- Clinical observation of kidney tonifying and collateral tonifying acupuncture combined with thermosensitive moxibustion in the treatment of knee osteoarthritis and its effect on TRACP and CTX-I
