Construction of high-efficiency CoS@Nb2O5 heterojunctions accelerating charge transfer for boosting photocatalytic hydrogen evolution
2022-11-05XinRnJianyouShiRuihuanDuanJunDiChaoXuXiaoLuoQingLiuMngyangXiaBoLinWuTang
Xin Rn,Jianyou Shi,Ruihuan Duan,Jun Di,Chao Xu,Xiao Luo,Qing Liu,Mngyang Xia,Bo Lin,Wu Tang
a School of Materials and Energy,University of Electronic Science and Technology of China,Chengdu 611731,China
b XJTU-Oxford International Joint Laboratory for Catalysis,School of Chemical Engineering and Technology,Xi’an Jiaotong University,Xi’an 710049,China
c Personalized Drug Therapy Key Laboratory of Sichuan Province,Sichuan Academy of Medical Science &Sichuan Provincial People’s Hospital,School of Medicine,University of Electronic Science and Technology of China,Chengdu 610072,China
d School of Materials Science and Engineering,Nanyang Technological University,639798,Singapore
e State Centre for International Cooperation on Designer Low-carbon and Environmental Materials (CDLCEM),School of Materials Science and Engineering,Zhengzhou University,Zhengzhou 450001,China
f School of Optoelectronic Science and Engineering,University of Electronic Science and Technology of China,Chengdu 611731,China
Keywords:Transition metal chalcogenides CoS cocatalyst Nb2O5 nanosheets Charge transfer Photocatalytic H2 evolution
ABSTRACT The random movement and easy recombination of photoinduced charges lead to a low conversion effi-ciency for photocatalytic hydrogen evolution.The cocatalyst design is a promising route to address such problem through introducing an appropriate cocatalyst on the semiconductor photocatalysts to construct the high-efficiency heterojunctions.Herein,novel CoS/Nb2O5 heterojunctions were constructed via in-situ loading CoS cocatalyst on the surface of Nb2O5 nanosheets.Through the femtosecond-resolved transient absorption spectroscopy,the average lifetime of charge carriers for 10 wt% CoS/Nb2O5 (159.6 ps) is drastically shortened by contrast with that of Nb2O5 (5531.9 ps),strongly suggesting the rapid charge transfer from Nb2O5 to CoS.The significantly improved charge-transfer capacity contributes to a high photocatalytic hydrogen evolution rate of 355 μmol/h,up to 17.5 times compared with pristine Nb2O5.This work would provide a new design platform in the construction of photocatalytic heterojunctions with high charge-transfer efficiency.
Photocatalytic water splitting driven by the solar energy for hydrogen generation is regarded as an efficient strategy to address the global energy crisis [1,2].The entire photocatalytic reaction can be roughly divided into 3 steps including light capture,charge separation and migration,and redox reactions at the active sites on the surface of photocatalysts [3,4].It is worth noting that the easy recombination of photogenerated carriers during the charge separation and transfer process can result in the low conversion efficiency of photocatalytic hydrogen evolution [5-7].One effective route to address such challenge is the loading of appropriate cocatalysts on the surface of photocatalysts to construct heterojunctions,which can introduce more active sites and transfer paths for charges,thus achieving high-efficiency charge separation and transfer during the photocatalytic reaction [8-10].The widely used cocatalysts for photocatalytic hydrogen evolution are noble metals such as platinum (Pt) and Aurum (Au) owing to their superior photocatalytic activity and chemical stability.However,the scarcity and high utilization cost significantly limit their practical applications [11-13].Therefore,it is of vital importance to seek the alternative cocatalysts with both excellent catalytic performance and low operating cost.
Recently,transition metal chalcogenides (shorted for TMCs)such as CdS [14],ZnS [15,16],ZnIn2S4[6,17],WS2[18],MoS2[19,20]and NiS [21],have triggered keen interest due to their suitable band gap,outstanding light harvesting,low cost,which enable TMCs to be the promising cocatalysts in photocatalytic hydrogen evolution [22-24].Among the TMCs group,CoS possesses many favorable advantages,including narrow bandgap,low toxicity and excellent conductivity,which allows it to be an ideal cocatalyst in the design of binary photocatalytic system for H2evolution [25-27].
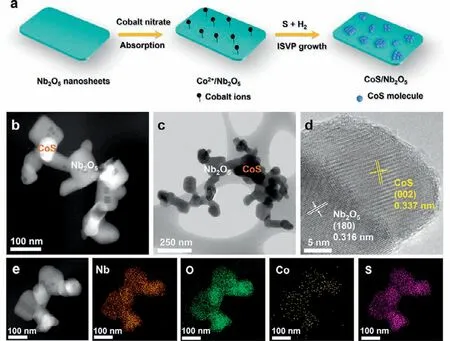
Fig.1.(a) Schematic illustration of the synthesis of CoS/Nb2O5.(b) HAADF-STEM (c)TEM and (d) HRTEM images of CoS/Nb2O5.(e) HAADF-STEM and elemental mapping images of CoS/Nb2O5.
Herein,we report an efficient CoS/Nb2O5heterostructured photocatalystviaa one-stepin-situvapor-phase growth method,where the CoS cocatalystin-situgrew on the surface of Nb2O5nanosheets with the advantages of outstanding photocatalytic H2evolution activity.Under the synergistic effects of heterojunction and CoS cocatalyst,the charge-transfer efficiency of CoS/Nb2O5is boosted significantly,thus leading to a high hydrogen evolution rate (HER)of 355 μmol/h,up to 17.5 times compared with pristine Nb2O5nanosheets.
The CoS/Nb2O5heterojunctions were synthesized using aninsituvapor-phase growth method (shorted for ISVP).As shown in Fig.1a,Nb2O5nanosheet was synthesized through a templateassisted calcination method according to the previous report [28].Then the Nb2O5nanosheet was dispersed into an aqueous solution with a certain amount of cobalt nitrate hexahydrate,where cobalt ions anchored on the surface of Nb2O5nanosheets to obtain Co2+/Nb2O5.After that,the Co2+/Nb2O5were heated to 700 °C with pure sulfur powder simultaneously in a mixed atmosphere(20% H2and 80% Ar).Under high-temperature environment,sulfur powder firstly reacted with H2to form H2S,then H2S can reduce the cobalt nitrate absorbed on the surface of Nb2O5to form the CoS nanoparticles,thus obtaining the CoS/Nb2O5heterojunctions.The morphology of CoS/Nb2O5was investigated by transmission electron microscopy (TEM) and high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM).As shown in Figs.1b and c,some well-dispersed CoS nanoparticles were loaded on the surface of Nb2O5nanosheets with an average length of 50 nm and a width of 50 nm.Furthermore,the high-resolution TEM (HRTEM) image further reveals the detail of interface structure of CoS/Nb2O5.As displayed in Fig.1d,the adjacent latticefringe spacings of 0.316 and 0.337 nm corresponded to the (180)reflection plane of Nb2O5and the (002) reflection plane of CoS respectively [29,30],which strongly supports the successful construction of the CoS/Nb2O5heterojunctions.Additionally,the elemental mapping (Fig.1e) and energy-dispersive X-ray spectroscopy (EDX)images (Fig.S1 in Supporting information) further confirm the successful construction of CoS/Nb2O5heterojunction photocatalyst.
To explore the crystallinity of Nb2O5and CoS,the X-ray diffraction (XRD) patterns of 10 wt% CoS/Nb2O5and Nb2O5were carried out.As shown in Fig.2a,all diffraction peaks of Nb2O5well fit the orthorhombic crystalline state (JCPDS No.30-0873).For the XRD pattern of 10 wt% CoS/Nb2O5,three low-intensity peaks at 30.6°,35.3°and 46.9° are in accord with the hexagonal phase of CoS(JCPDS No.65-3418) [31],while the peaks around 25° ~28° correspond to a slight amount of cobalt sulfate (JCPDS No.15-0701) on the surface of CoS/Nb2O5sample.Additionally,all other XRD peaks are corresponded to the orthorhombic crystalline state of Nb2O5.The X-ray photoelectron spectroscopy (XPS) was carried out to further analyze the surface chemical state and element composition of Nb2O5nanosheet and 10 wt% CoS/Nb2O5.As displayed in Fig.2b and Fig.S2 (Supporting information),the XPS spectra of both samples show the similar peaks of Nb and O elements.Besides,the peaks of S and Co elements occurred in XPS spectra of 10 wt%CoS/Nb2O5,indicating the successful loading of CoS on the surface of Nb2O5.In Fig.2c,the spectrum of Co 2p region has four distinct diffraction peaks,the peaks at 782.33 and 785.95 eV are attributed to Co 2p3/2,while the peak at 798.44 and 802.83 eV corresponded to Co 2p1/2[32,33].In Fig.2d,the peaks at 207.65 and 210.39 eV are ascribed to Nb 3d5/2and Nb 3d3/2in Nb2O5nanosheets,respectively [34].As exhibited in Fig.2e,the peaks at 169.6 and 171.13 eV are attributed to S 2p3/2and S 2p1/2,which are related to the amorphous phases of CoS [35].In Fig.2f,the peak at 530.49 eV is corresponded to Nb-O bond,the other two peaks at 533.57 and 532.53 eV are associated with physically adsorbed water molecules and oxygen from the precursor of niobium [36,37].The XRD and XPS results presented above strongly demonstrated the successful construction of the CoS/Nb2O5heterostructured photocatalyst.The construction of efficient heterojunction can increase the charge separation and transfer efficiency significantly,thus boosting photocatalytic hydrogen evolution activity [38].
Time-dependent photocatalytic H2production experiments over Nb2O5and CoS/Nb2O5samples were evaluated under the simulated solar-light irradiation (λ≥300 nm) with triethanolamine(TEOA) as the hole-scavenger.As shown in Fig.3a,pristine Nb2O5exhibits a poor hydrogen evolution rate of average 20.3 μmol/h,indicating its inferior charge-transfer capacity.However,it can be observed that the CoS/Nb2O5with the optimal cocatalystloading amount (10 wt%,Fig.S3 in Supporting information)shows a high HER of 355 μmol/h,up to 17.5 times compared with pristine Nb2O5,strongly demonstrating the advantages of the CoS/Nb2O5heterostructure.The apparent quantum efficiency(AQE) of 10 wt% CoS/Nb2O5was evaluated and estimated to be 5.93% at 365 nm (Table S1 in Supporting information),exceeding most of wide-band-gap photocatalysts such as TiO2and some composite photocatalysts [18,39-42].The cycling stability of hydrogen production over CoS/Nb2O5was evaluated.As displayed in Fig.3b,after 4 cycling tests,10 wt% CoS/Nb2O5still exhibits an excellent H2evolution rate,only 9.4% decay was observed.Besides,the TEM and HAADF-STEM images of 10 wt% CoS/Nb2O5after 4 cycling tests also well support its excellent stability.As shown in Fig.S4 (Supporting information),CoS/Nb2O5still maintain a relatively intact stable heterostructure,as evidenced by the elemental mappings image (Fig.S4d).Additionally,the XRD pattern of recycled 10 wt% CoS/Nb2O5(Fig.S5 in Supporting information)shows a similar trend by contrast with fresh 10 wt% CoS/Nb2O5,further confirming its stability.
To investigate the major factors for the excellent photocatalytic activity of 10 wt% CoS/Nb2O5,various properties of the synthesized samples including optics,texture and photoelectricity were studied.The UV-vis diffuse reflectance spectra (DRS) of Nb2O5and 10 wt% CoS/Nb2O5are carried out.As displayed in Fig.4a,Nb2O5has a routine light absorption in the ultraviolet region(≤400 nm),which corresponded to the calculated band-gap energy (Eg) of 3.1 eV.However,with the construction of the binary heterojunction,the light absorption capacity of 10 wt% CoS/Nb2O5was greatly enhanced whether in the ultraviolet and visible regions,which is mainly due to the effect of CoS [43].Besides,as shown in Fig.S6 (Supporting information),compared to Nb2O5sample,the color of 10 wt% CoS/Nb2O5sample changed from white to gray,indicating the variation in photo-absorption.The specific textural information of Nb2O5and 10 wt% CoS/Nb2O5is listed in Table S2 (Supporting information).As shown in Table S2,10 wt%CoS/Nb2O5possesses a higher specific surface (9.31 m2/g) and pore volume (0.04 cm3/g) compared with pristine Nb2O5(7.84 m2/g and 0.03 cm3/g,respectively),which is beneficial to the photocatalytic H2evolution by introducing more reaction active sites [44].
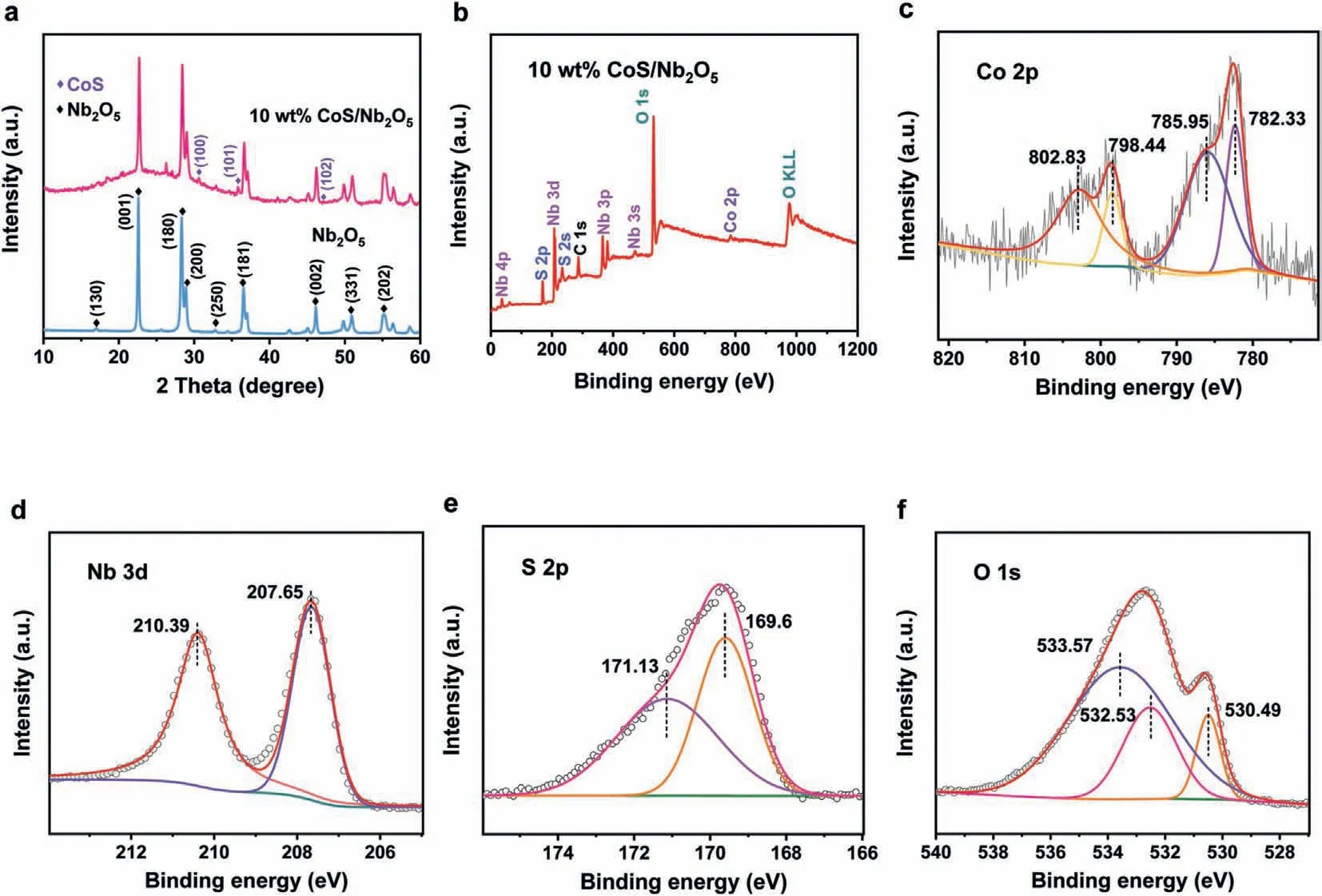
Fig.2.(a) XRD patterns of Nb2O5 and 10 wt% CoS/Nb2O5.(b) XPS patterns full spectrum of 10 wt% CoS/Nb2O5 and its corresponding regions of (c) Co 2p,(d) Nb 3d,(e) S 2p,(f) O 1s.
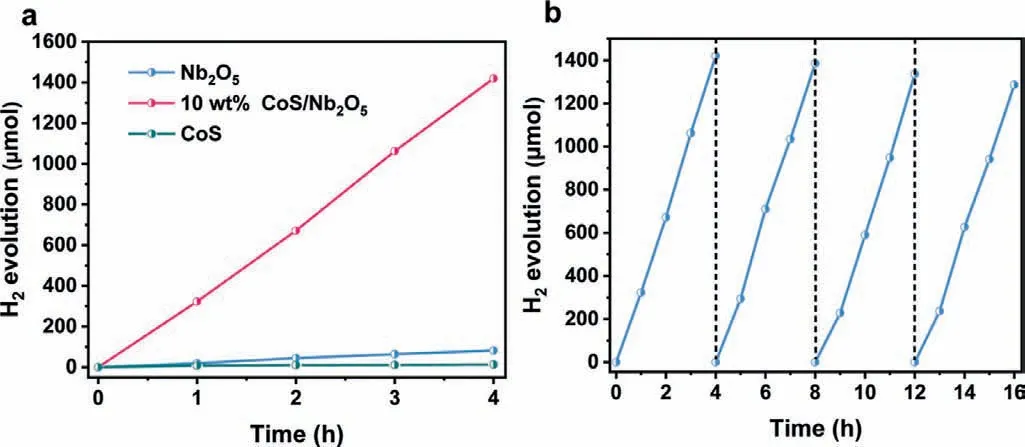
Fig.3.(a) Time-dependent photocatalytic H2 evolution of Nb2O5 and 10 wt%CoS/Nb2O5 under solar-light irradiation (λ ≥300 nm).(b) Cycling H2 evolution tests of 10 wt% CoS/Nb2O5 under solar-light irradiation (λ ≥300 nm).
To investigate the charge separation and transfer capacity of Nb2O5and 10 wt% CoS/Nb2O5,transient photocurrent responses,electrochemical impedance spectroscopy (EIS) and photoluminescence (PL) spectra were carried out.As exhibited in Fig.4b,10 wt%CoS/Nb2O5displays a higher transient photocurrent density than Nb2O5,indicating the superior charge transfer efficiency [45].Besides,the EIS spectra (Fig.4c) show that 10 wt% CoS/Nb2O5displays a smaller radius of Nyquist circle by contrast with pristine Nb2O5,which strongly prove the minimum charge transfer obstruction of 10 wt% CoS/Nb2O5.Photoluminescence spectrum (PL)is an important characterization to study the separation and recombination of photogenerated charges,which is closely connected to the photocatalytic process.As the PL spectra shown in Fig.4d,a lower emission peak intensity was observed of 10 wt% CoS/Nb2O5by contrast with pristine Nb2O5.This result indicates that the suppressive recombination of charges leads to the reduced energy released in the form of PL.Therefore,more charges in CoS/Nb2O5system can participate in the photocatalytic reaction,thus achieving a high hydrogen evolution rate of 355 μmol/h [46-49].
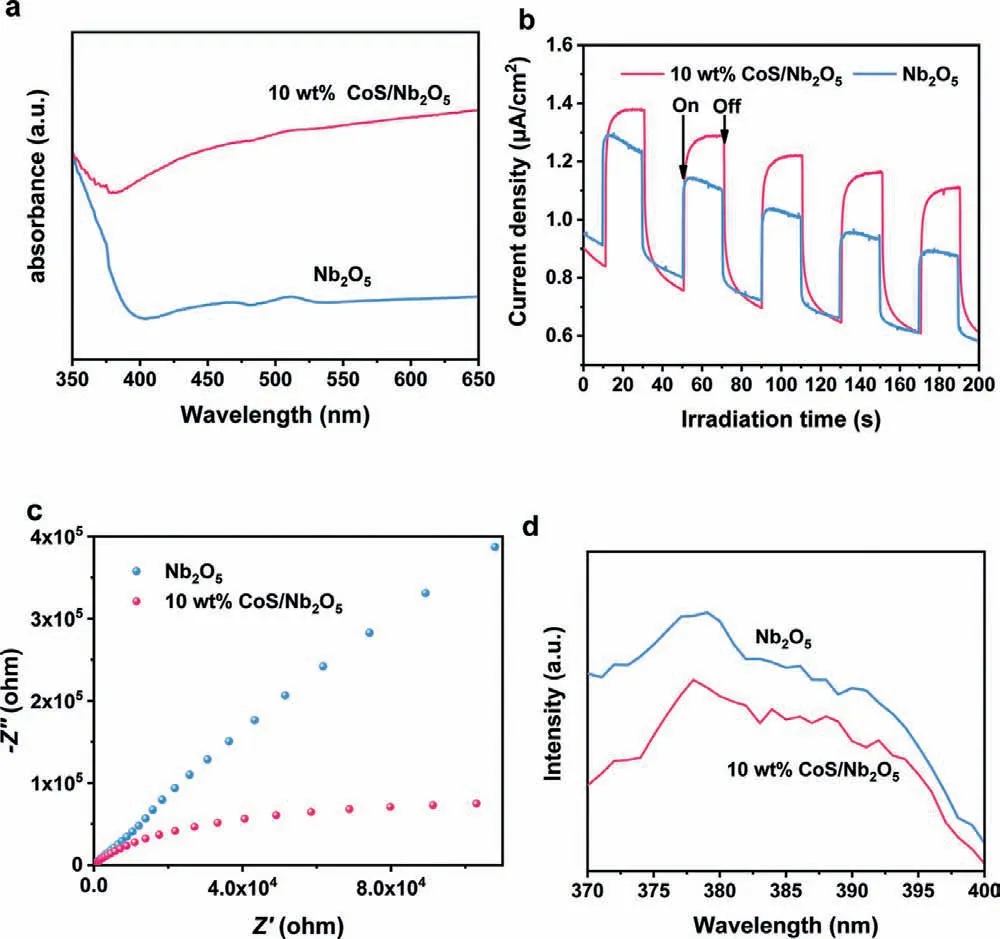
Fig.4.(a) UV-vis diffuse reflectance spectra of Nb2O5 and 10 wt% CoS/Nb2O5.(b)Transient photocurrent responses of Nb2O5 and 10 wt% CoS/Nb2O5.(c) Electrochemical impedance spectroscopy Nyquist plots of Nb2O5 and 10 wt% CoS/Nb2O5.(d)Photoluminescence spectra of Nb2O5 and 10 wt% CoS/Nb2O5.
Furthermore,femtosecond-resolved transient absorption spectroscopy (TAS) is powerful technique for investigating the separation and transfer of charges.As displayed in Figs.5a and b,the specific-time-points TAS spectra from 1 ps to 1000 ps of Nb2O5and 10 wt% CoS/Nb2O5are marked with different colors.Both Nb2O5and 10 wt% CoS/Nb2O5exhibit a broad negative induced absorption,which are attributed to the effect of overlapping electron and hole absorption in Nb2O5[50-52].The kinetics of TAS from 360 nm to 420 nm (Fig.5c) of Nb2O5and 10 wt% CoS/Nb2O5is investigated to reveal the lifetime of charge carriers.As shown in Table S3 (Supporting information),by fitting the kinetics curve with two-exponential decay functions,the average lifetime of charge carriers for 10 wt% CoS/Nb2O5is 159.6 ps,which is far lower than that of Nb2O5(5531.9 ps).The considerably shorted lifetime strongly indicates the rapid charge transfer from Nb2O5to CoS,which is in accord with the time-resolved fluorescence decay spectra results (Fig.5d and Table S4 in Supporting information) [50,53].
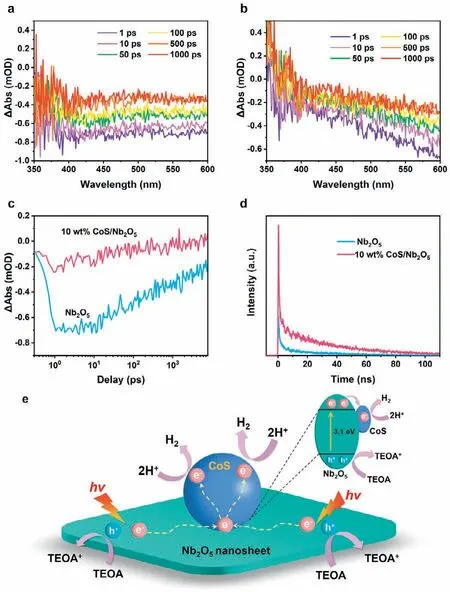
Fig.5.Femtosecond-resolved TAS spectra of (a) Nb2O5 and (b) 10 wt% CoS/Nb2O5.(c) TAS kinetics probed at 360 to 420 nm for Nb2O5 and 10 wt% CoS/Nb2O5.(d)Time-resolved fluorescence decay spectra of Nb2O5 and 10 wt% CoS/Nb2O5.(e) The schematic of charge transfer in the CoS/Nb2O5 system for photocatalytic H2 evolution.
According to all the characterization and analytical results presented above,a probable mechanism for the charge transfer in photocatalytic hydrogen evolution of CoS/Nb2O5photocatalyst has been proposed.As illustrated in Fig.5e,Nb2O5nanosheets in the CoS/Nb2O5system can be excited to generate numerous photoinduced electron-hole pairs under the simulated solar-light irradiation (λ≥300 nm).The photoinduced electrons are rapidly injected into its conduction band (CB),while the photoinduced holes that remained in the valence band (VB) are captured by the hole-scavenger of triethanolamine (TEOA).Owing to the construction of the binary CoS/Nb2O5system,the photoinduced electrons on the Nb2O5nanosheets can rapidly transfer to CoS with abundant active sitesviathe heterojunction interfaces.Then the electrons reduce hydrogen ions in aqueous solution into H2at the active sites on the surface of CoS cocatalyst [54-56].The significantly accelerated charge transfer contributes to the excellent photocatalytic H2evolution activity of 10 wt% CoS/Nb2O5.
In summary,the high-efficiency CoS/Nb2O5heterojunctions were successfully synthesized through a one-step vapor growth method.Taking advantage of the advanced femtosecond-resolved ultrafast TAS spectra,we reveal that the average lifetime of charge carriers for 10 wt% CoS/Nb2O5(159.6 ps) is drastically shortened by contrast with that of Nb2O5(5531.9 ps),strongly suggesting the rapid charge transfer from Nb2O5to CoS.The improved charge transfer efficiency leads to a high photocatalytic H2evolution of 355 μmol/h,up to 17.5 times by contrast with pure Nb2O5.
Declaration of competing interest
The authors declare no conflict of competing interest.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No.22002014),Applied Basic Research Program of Sichuan Province (No.2020YJ0068),“Young Talent Support Plan”of Xi’an Jiaotong University,National Key Research and Development Program of China (No.2020YFC2005500),Key Research and Development Program of Science and Technology Department of Sichuan Province (No.2019YFS0514).Dr.Chao Xue acknowledges financial support from the National Natural Science Foundation of China (No.22102152).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.12.076.
杂志排行
Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
