pH-and H2O2-sensitive drug delivery system based on sodium xanthate: Dual-responsive supramolecular vesicles from one functional group
2022-11-05ZiyanShenNingMaFengWangJiamingRenChenxiHouShuangChaoYuxinPeiZhichaoPei
Ziyan Shen,Ning Ma,Feng Wang,Jiaming Ren,Chenxi Hou,Shuang Chao,Yuxin Pei,Zhichao Pei
Shaanxi Key Laboratory of Natural Products &Chemical Biology,College of Chemistry &Pharmacy,Northwest A&F University,Yangling 712100,China
Keywords:Pillar[5]arene Sodium xanthate Host-guest interaction pH-and H2O2-dual responsive Controllable drug delivery
ABSTRACT Nano-drug delivery systems with multiple stimulus-responsive capabilities have superior response performance and efficient drug release.Nevertheless,it is sophisticated to construct multiple stimulusresponsive systems where the two or more functional groups need to be introduced simultaneously.Xanthate,one functional group with pH and H2O2 stimulus responsiveness,has significant potential applications for building dual-responsive drug delivery system.Herein,we present a novel dual stimuliresponsive supramolecular drug delivery system by using sodium xanthate derivative (SXD) as guest molecule and quaternary ammonium capped pillar[5]arene (QAP5) as host molecule through host-guest interaction on the basis of electrostatic interaction.The amphiphile QAP5⊃SXD could self-assemble into vesicles to efficiently load the anti-cancer drug DOX.The experimental results showed that QAP5⊃SXD nanoparticles could achieve efficient drug delivery and controlled release in the tumor microenvironment.Cytotoxicity experiments proved that DOX@QAP5⊃SXD nanoparticles could significantly improve the anticancer efficiency of free DOX on cancer cells.The present study provides an efficient strategy to develop supramolecular nanocarriers with dual-responsiveness in one functional group for controlled drug release.
The tumor microenvironment (TME) is a complex integrated system,such as hypoxia,low pH,increased oxidative stress,high concentrations of glutathione (GSH) and overexpressed enzymes.Therefore,TME can supply intracellular stimuli for the construction of stimulus-responsive systems to control drug release [1,2].Stimulus-responsive systems have received extensive attention for the property that their structure can be changed by the application of a stimulus [3,4].Generally,the phenomenon of structural transformation is achieved by changes in compound hydrolysis,protonation,conformation,hydrophilic/hydrophobic ratios,and bond structure through some endogenous or exogenous stimuli [5,6].In recent years,stimulus-responsive systems have been applied extensively for gene/drug/protein delivery,fluorescent chemical sensors,smart functional nanomaterials,etc.[7-10].
The release rate of drugs in response to stimulus of TME is closely associated with anticancer efficiency [11-17].Nanodrug delivery systems with multiple stimulus-responsive capabilities have superior response performance and efficient drug release compared to single stimulus-responsive systems [18-20].Nevertheless,it is sophisticated to construct multiple stimulusresponsive systems where the two or more functional groups need to be introduced simultaneously.So far,there are no reports on the construction of multiple stimulus-responsive nano-drug delivery systems based on one functional group.Consequently,it is essential to develop a functional group that possesses multiple stimulus responsiveness.Xanthate,invented by Keller in 1920s,are widely used as trapping agents for froth flotation in mineral processing technology,precipitants in hydrometallurgy,and pharmaceutical intermediates or drying agents for agricultural crops due to its simple synthesis process,mild conditions and high yield[21,22].Many studies have shown that xanthate can be rapidly decomposed in an acidic environment or in the presence of oxidants[23-26].This phenomenon suggests that xanthate is a functional group with a dual stimuli-responsiveness of pH and H2O2.However,there are no studies on xanthate as a stimulus response group to construct dual stimulus-responsive nano-drug delivery system.
In recent years,stimulus-responsive supramolecular assemblies have been developing substantially [27-29].Pillar[n]arenes,an emerging macrocyclic molecule,have stood out among many stimulus-responsive supramolecular assemblies owing to their easy synthesis,versatile chemical modifiability,rigid electron-rich structure,tunable size of the cavity and excellent host-guest properties,which have great potential for the construction of supramolecular nano-drug delivery systems [30-35].Therefore,we envisioned sodium xanthate derivative as guest molecule and pillar[5]arene as host molecule to construct a dual-responsive nano-drug delivery system based on one functional group,which could significantly improve anticancer efficiency.
In this study,we report a pH-and H2O2-sensitive drug delivery system based on quaternary ammonium capped pillar[5]arene(QAP5) and sodium xanthate derivative (SXD) with dual stimulus responsiveness through host-guest interaction on the basis of electrostatic interaction.As shown in Scheme 1,the supramolecular drug delivery system (QAP5⊃SXD) can be successfully obtained by self-assembly of QAP5 and SXD in aqueous solution.Doxorubicin (DOX) as a model anticancer drug was loaded to prepare DOX@QAP5⊃SXD nanoparticles (NPs).At low pH and high concentrations of H2O2,SXD can be quickly decomposed,which leads to the disassembly of DOX@QAP5⊃SXD NPs,resulting in the release of the loaded drug DOX.Finally,the massive accumulation of DOX in a brief period of time caused damage to DNA and ultimately led to the death of cancer cells.
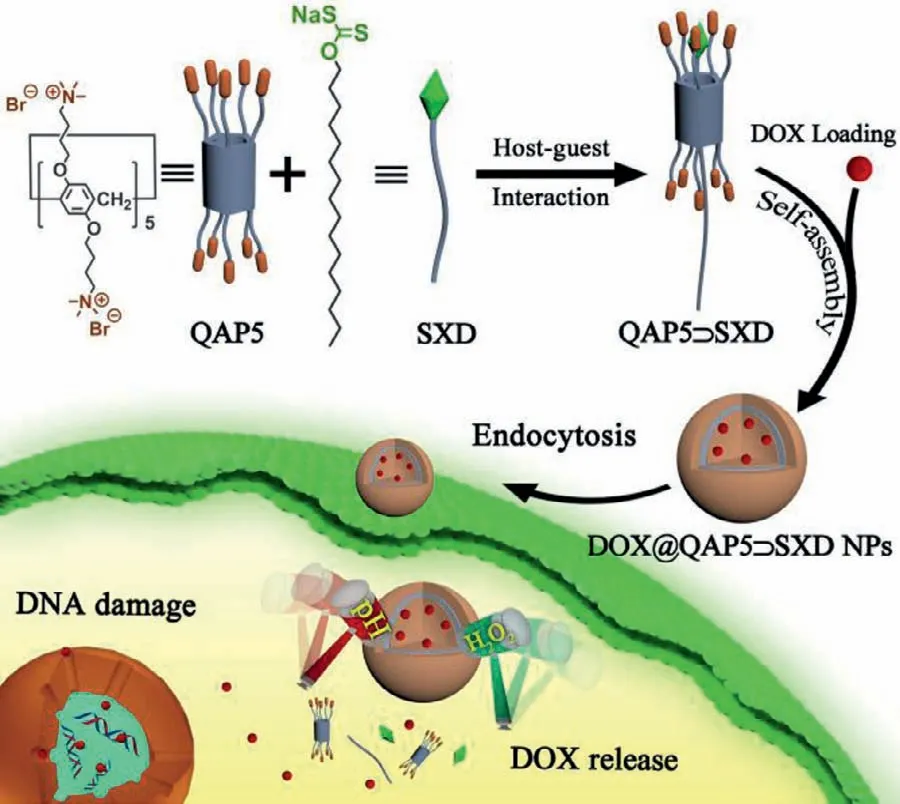
Scheme 1.Schematic illustration of the construction of the supramolecular system based on quaternary ammonium capped pillar[5]arene (QAP5) and sodium xanthate derivative (SXD) and its application in controllable drug delivery.
The synthetic routes QAP5 and SXD were shown in Schemes S1 and S2 (Supporting information).The chemical structures of QAP5 and SXD were characterizedvia1H NMR and13C NMR (Figs.S1-S5 in Supporting information).Meanwhile,a water-soluble sodium xanthate derivative (W-SXD) was synthesized (Scheme S3 and Fig.S6 in Supporting information) to replace the hydrophobic SXD for studying the decomposition of sodium xanthate in aqueous solutions with an acidic environment,H2O2conditions,or both.Sodium xanthate decomposition in an acidic environment or under H2O2conditions is shown in Fig.1a [23-26].As exhibited in Figs.1b and c,the W-SXD was barely decomposed under both H2O2-free and neutral conditions.However,when the solution pH was adjusted to 4.5,the decomposition rate of W-SXD reached about 89% (Fig.1b).Meanwhile,it was shown that the decomposition amount of W-SXD gradually enhanced from 43.8% to 78.8% with the increase of H2O2concentration from 25 μmol/L to 100 μmol/L(Fig.1c).Furthermore,when W-SXD was in an acidic solution containing H2O2,the decomposition rate of W-SXD was significantly accelerated where the cumulative decomposition amount was also increased (Fig.1d).The results revealed that xanthate is a group with dual responsiveness of pH and H2O2which could be introduced into the drug delivery system to control drug release.
Considering the limited water solubility of SXD,water-soluble model compound W-SXD was used to investigate the host-guest interaction between QAP5 and the sodium xanthate group by1H NMR spectroscopy.As shown in Fig.S7 (Supporting information),with the addition of QAP5 to W-SXD solution,H1-H7 on W-SXD shifted upfield,indicating that part of W-SXD group approached to the ammonium salt groups on QAP5.This would suggest that QAP5 forms a complex with W-SXD due to host-guest assembly driven by electrostatic interactions.
The QAP5⊃SXD complex was obtained using the hydrophobic sodium xanthate derivative (SXD) as the guest molecule and the hydrophilic QAP5 as the host molecular by host-guest interaction on the basis of electrostatic interaction.Stable QAP5⊃SXD NPs can be formed by self-assembly of amphiphilic QAP5⊃SXD in aqueous solution.As shown in Fig.2a,with the measurement of the optical transmittance of the nanoparticle solution,the calculated critical aggregation concentration (CAC) of QAP5⊃SXD NPs was 47.68 μmol/L.The clear Tyndall effect evidenced the formation of QAP5⊃SXD NPs (Fig.2b).Moreover,the QAP5⊃SXD NPs were characterized by DLS,SEM and TEM for their assembled morphology and size,respectively.As shown in Fig.2c,the DLS results showed that the average diameter of the QAP5⊃SXD NPs wasca.180 nm.The SEM observed spherical NPs with an average diameter ofca.200 nm (Fig.2d).QAP5⊃SXD NPs could be identified as vesicles by TEM (Fig.2e).
According to the dual-responsive character of sodium xanthate,we selected the hydrophobic anti-cancer drug DOX as the model drug to study the drug loading efficiency and drug release behavior of the QAP5⊃SXD NPs.We further measured the DOX loading capability of QAP5⊃SXD NPs,which was calculated to be 29.8%.Furthermore,the size of the DOX@QAP5⊃SXD NPs is smaller than that of the blank QAP5⊃SXD NPs as shown by the DLS results (Fig.S8 in Supporting information).Next,the dual-responsive properties of DOX@QAP5⊃SXD NPs were investigated.As shown in Fig.2f,the experimental result showed that DOX was rapidly released from the DOX@QAP5⊃SXD NPs under the co-triggered conditions.The cumulative release of DOX was up to about 80% after 5 h.No obvious spherical nanoparticle could be observed by SEM under low pH and high H2O2concentration (Fig.S9 in Supporting information),which indicated the disassembly of DOX@QAP5⊃SXD NPs.The phenomenon is similar with the decomposition behavior of xanthate,which implies that the xanthate moiety with dual responsiveness of pH and H2O2is a very helpful feature in drug delivery.
The cellular uptake of DOX@QAP5⊃SXD NPs was evaluated by the confocal laser scanning microscopy (CLSM) and flow cytometry assay toward HepG2 cells.As show in Fig.3,the fluorescence intensity of DOX@QAP5⊃SXD NPs group was much higher than that of the free DOX group.The red fluorescence of DOX appeared in cells after incubation for 1 h demonstrated that DOX@QAP5⊃SXD NPs were successfully internalized by HepG2 cells.Meanwhile,the stronger red fluorescence in quantitative experiments performed by flow fluorescence of DOX@QAP5⊃SXD group was observed than free DOX group after incubation for 6 h.The enhanced fluorescence of DOX@QAP5⊃SXD group was mainly due to enhanced intracellular accumulation of nanoparticles through endocytosis,where free DOX group was mainly internalised by the cell through passive diffusion [36].Subsequently,it was further demonstrated in quantitative experiments performed by flow cytometry that the cellular uptake activity of DOX@QAP5⊃SXD NPs was much higher than that of free DOX (Fig.S10 in Supporting information).The above results suggested that QAP5⊃SXD NPs could effectively transport anticancer drugs into cancer cells.As a drug carrier,good cytocompatibility is essential for the use of QAP5⊃SXD NPs in biomedical applications.The MTT method was used to evaluate the cytocompatibility of QAP5⊃SXD NPs and guest molecule SXD with different concentrations towards HL7702 cells (normal hepatocytes).The results displayed the effect on the survival rate and proliferation rate of HL7702 cells was minimal,where HL7702 cells incubated with SXD and QAP5⊃SXD at concentrations ranging from 10 μmol/L to 60 μmol/L for 24 h.The cell viability was still above 80% when the concentration of SXD and QAP5⊃SXD were increased to 60 μmol/L,which indicated the good biocompatibility of QAP5⊃SXD (Fig.S11 in Supporting information).Next,the anticancer efficiency of the DOX@QAP5⊃SXD NPs was further evaluated.As shown in Fig.4,DOX@QAP5⊃SXD group was more effective than DOX group after incubation of HepG2 cells with DOX@QAP5⊃SXD NPs and DOX for 24 h and 48 h,respectively.This result indicated that QAP5⊃SXD NPs have excellent drug delivery capabilities.
In summary,we have successfully constructed a novel dual stimulus-responsive supramolecular drug delivery system by using sodium xanthate derivatives (SXD) with pH and H2O2stimulus responsiveness in one functional group as guest molecule and quaternary ammonium capped pillar[5]arene (QAP5) as host molecule through host-guest interactions on the basis of electrostatic interaction.QAP5⊃SXD complex can self-assemble to supramolecular vesicles in water,which could effectively encapsulate hydrophobic anticancer drug DOX.Furthermore,the loaded anticancer drug DOX could be released rapidly at the acidic microenvironment with high concentration of H2O2.Further cellular uptake and intracellular localization experiments have demonstrated that these supramolecular nanocarriers are introduced into cancer cells mainly through endocytosis,which could lead to the accumulation of large amounts of drugs in HepG2 cancer cells.Cytotoxicity experiments prove that DOX@QAP5⊃SXD NPs significantly improve the anticancer effi-ciency of free DOX on cancer cells.The present study provides an efficient strategy to develop supramolecular nanocarriers with dual-responsiveness in one functional group for controlled drug release.
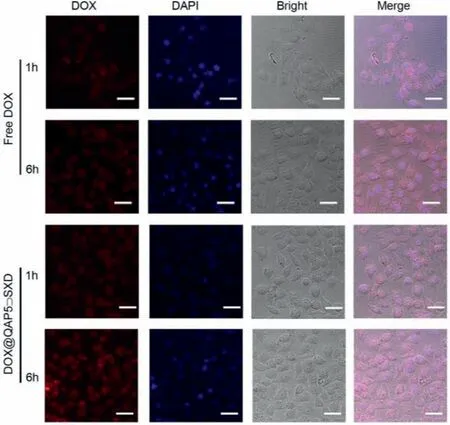
Fig.3.CLSM of HepG2 cells incubated with free DOX and DOX@QAP5⊃SXD NPs for 1 h and 6 h.The scale bar is 20 μm.[DOX]=5 μmol/L.
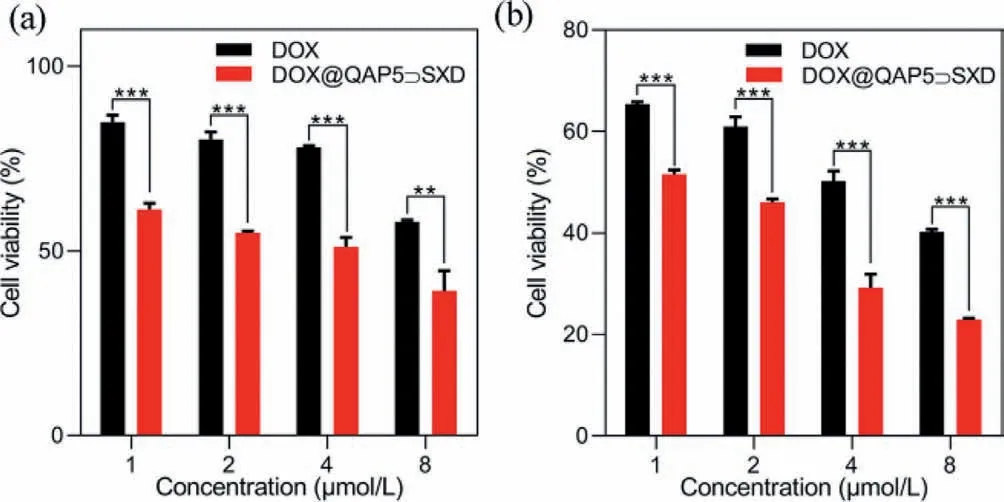
Fig.4.In vitro cytotoxicity results of free DOX and DOX@ QAP5⊃SXD NPs on HepG2 cells for 24 h (a) and 48 h (b) (**P<0.01,***P<0.001).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research work was supported by the National Natural Science Foundation of China (Nos.21877088,22171230) and China Postdoctoral Science Foundation (No.2016M602861).The authors thank Life Science Research Core Services (LSRCS),Northwest A&F University for helping with characterizations including SEM,TEM,CLSM and flow cytometer.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.01.069.
杂志排行
Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
