An improved Hummers method to synthesize graphene oxide using much less concentrated sulfuric acid
2022-11-05YninZhuGngKongYulingPnLinLiuBoYngShunghongZhngDelinLiChunshnChe
Ynin Zhu,Gng Kong,Yuling Pn,Lin Liu,Bo Yng,Shunghong Zhng,Delin Li,*,Chunshn Che,*
a School of Materials Science and Engineering,South China University of Technology,Guangzhou 510641,China
b Guangzhou Special Pressure Equipment Inspection and Research Institute,Guangzhou 510663,China
Keywords:Graphene oxide Carbon materials Oxidation Low acid Hummers method
ABSTRACT The production of graphene oxide with less acid is beneficial to reduce the costs and lower the impact on the environment,but it is still a great challenge.In this work,a relatively simple,safe method for synthesizing graphene oxide with much less acid (decrease ~40%) is proposed.With assistance of the heat absorbed from environment and reaction system,the temperature of reaction system of low acid can be well controlled.More interestingly,the graphite can be completely oxidized into graphite oxide by using much less acid,with lowering the production of high-concentration aqueous waste acid (>1 mol/L,decrease ~40%).A series of characterizations show that the prepared graphene oxide has similar yield and functional groups compared with that of using the conventional method.This work provides a safe and environmentally friendly choice for the large-scale production of graphene oxide and its derivative materials.
Graphene oxide (GO),an important precursor to graphene,owns rich oxygen functional groups (i.e.,hydroxyl,epoxy,and carboxyl groups) on its carbon frame,which endues it with good chemical and physical processability.It has been studied in many fields [1],such as electrocatalysis [2],biomedical [3],separation membrane [4],sensors [5],and energy conversion and storage [6].
So far,Hummers method is the most widely adopted method to synthesize GO [7-9].The graphite is oxidized in the mixture of potassium permanganate (KMnO4) and concentrated sulfuric acid(H2SO4).KMnO4reacts with H2SO4,generating the oxidant: Mn2O7and/or MnO3[HSO4][10].Dimiev and Tour proposed three distinct independent processes of oxidation of graphite: the conversion of graphite into the stage-1 graphite intercalation compound (GIC),the conversion of stage-1 GIC into pristine graphite oxide (PGO),and finally the conversion of PGO into conventional GO after hydrolyzation [11,12].The H2SO4has a variety of roles in the oxidation process,which includes regarding as intercalation agent of the stage-1 GIC with the assistance of oxidant,stabilizing the oxidant,and acting as a solvent to transport the oxidant into the graphite interlayers.Currently,Hummers method requires at least 43 tons (~23000 L) of H2SO4to oxidize 1 ton of graphite and generates at least 207000 L of high-concentration waste dilute sulfuric acid containing metal ion (Mn2+,K+,etc.) [7].Commonly,the more concentrated sulfuric acid is used,the more waste acid will be produced.The treatment of waste acid was complicated and expensive,as reported in previous literatures [13,14].Therefore,one of the great challenges is to oxidize graphite using much less concentrated sulfuric acid.In recent years,several strategies have been used to reduce the amount of concentrated sulfuric acid,such as dry ice assisted oxidation [14],use of new oxidant [15]and cyclic utilization of acid [13,16].However,the system of insufficient acid input leads to GO with low yield or/and low oxidation degree.In addition,these methods often require special conditions and equipment.For the propose of comparison,we have summarized some literatures on the preparation of graphene oxide in recent years,as shown in Table S1 (Supporting information).To our knowledge,there are no reports on the direct use of much less concentrated sulfuric acid to completely oxidize graphite.
Herein,we present an improved Hummers method to realize high yield preparation of GO with much less acid input under relatively mild condition by only controlling the temperature of oxidation process.We investigated the oxidation behavior of natural graphite powder and compared it with the conventional Hummers method.It was found that graphite could be also completely oxidized into graphite oxide by using much less acid (GrO-LA).The reaction system of GrO-LA has a similar yield compared with that of using conventional acid amount (GrO-CA).The waste sulfuric acid generated from the GrO-LA system decreases by ~40%.A series of characterizations show that the prepared graphene oxide (GO-LA)has a similar structure and chemical composition compared with the graphene oxide (GO-CA) prepared by the conventional method.
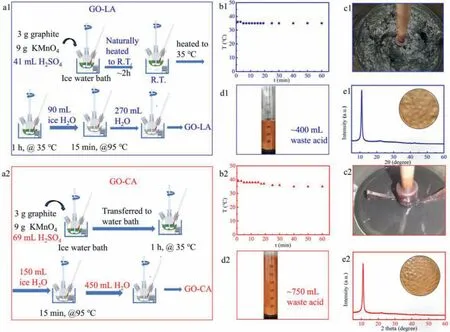
Fig.1.(a1,a2) Schematic procedure of preparation of GrO-LA and GrO-CA;(b1,b2) Reaction temperature change of GrO-LA and GrO-CA within 60 min (at 35 °C) when the graphite flakes were oxidized by KMnO4 in H2SO4;(c1,c2) Images of the GrO-LA and GrO-CA after the graphite flakes have oxidized 60 min by KMnO4 in H2SO4;(d1,d2)Images of the suspension of as-prepared GrO-LA and GrO-CA after the addition of H2O2;(e1,e2) XRD patterns of freeze-dried GrO-LA and GrO-CA after purification.Insets in Figs.e1 and e2 show the pictures of filter cakes from the corresponding suspensions.
Firstly,we selected 10-30 μm of natural graphite flakes (Fig.S1 in the Supporting information) for oxidation,because small graphite flakes are easier to oxidize,as reported by the literature[17].The amount of oxidant we used is the same as the original Hummers method [12],i.e.,graphite:KMnO4=3 g:9 g.The amount of sulfuric acid is a critical factor.The low acid system with Mn2O7generation was prone to explosion even when the system was cooled in an ice bath,as reported by the literature [14].In order to ensure the safety of the oxidation as much as possible,the insoluble Mn2O7should be avoid to produce on the surface of reaction system.Therefore,through quantitative experimental observations,we used a slightly excess amount of concentrated sulfuric acid for the exploratory experiments in this work (graphite:KMnO4: H2SO4=3 g:9 g:41 mL),as shown in Fig.S2 (Supporting information).
To initiate GO synthesis,KMnO4was added to the mixture of graphite and concentrated H2SO4.When the reaction system was directly transferred from ice water bath to a 35 °C water bath,a violent reaction with a sharp temperature rise occurred,as shown in Fig.S3 (Supporting information).It indicates that the low acid system exhibits highly oxidative reactivity.
For our improved Hummers method,as shown in Fig.1a1,in order to control the reactivity of the high-concentration oxidant system,the ice-water bath was naturally heated to room temperature by absorbing the heat from the environment and reaction system,to decrease the concentration of oxidant and pre-oxidize the graphite.In general,the oxidation of graphite is accompanied by a decrease of the concentration of oxidant.The change of oxidant concentration can be roughly reflected by the color of the reaction system after the natural heating process.The color of the reaction system changes from dark green to grey green,as shown in Figs.S4a and b (Supporting information),indicating the consumption of oxidant.It should be mentioned that the oxidant in concentrated sulfuric acid is easily decomposed after being diluted by water,so it is difficult to directly determine the concentration of the oxidant,as reported by previous studies [18].The oxidation of graphite can be reflected by the XRD pattern of the reaction product after the natural heating process.As shown in Fig.S4c (Supporting information),the peak located at 9.6° is attributed to GO (001)and another broad peak (located at around 25°) contains graphite peak (~26.6°),implying the partial oxidation of graphite.Indeed,our method can be proceeded at 35 °C very mildly,as shown in Fig.1b1.This shows that the improved Hummers method is safe.The reaction product after oxidation is also sticky (Fig.1c1).After quenching the reaction system with ice and H2O2,the graphite oxide (GrO-LA) and its filter cake are bright yellow (Figs.1d1 and e1)),indicating the high oxidation degree.This assumption was demonstrated by the XRD data (Fig.1e1) of freeze-dried reaction product after purification.The XRD pattern of GrO-LA shows only one peak located at 2θ=10.97°,which is attributed to the GrO(001).No apparent graphite peak (2θ=26.6°) can be detected.
The graphite oxide was also prepared using the conventional method (GrO-CA),as shown in Fig.1a2.As seen in Figs.1b2,d2 and e2),the information of colors (bright yellow) and XRD of GrOCA (2θ=10.97°) is very similar to the GrO-LA.The reaction product after oxidation is not very sticky (Fig.1c2).Furthermore,in order to compare the structure information of GrO-LA and GrO-CA with different ratio of KMnO4to graphite,their XRD patterns were detected,as shown in Fig.S5 (Supporting information).The structure information of GO-LA samples and GO-CA samples are also approximate.The colors of corresponding samples were also recorded,as shown in Fig.S6 (Supporting information).
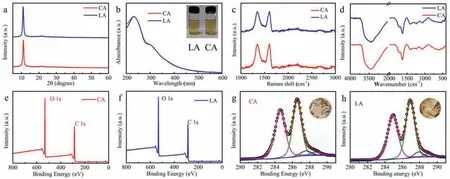
Fig.2.(a) XRD spectra,(b) UV-vis absorbance spectra,(c) Raman spectra,(d) FT-IR spectra,(e,f) Full XPS spectra and (g,h) High-resolution C 1s spectra of the of GO samples;Inset in (b) is the dilute GO aqueous dispersion (0.1 mg/mL);Insets in (g,h) are the corresponding photographs of the corresponding freeze-dried GO aerogels with the same concentration (8.5 mg/mL).
Notably,by using water with the same ratio of water to concentrated sulfuric acid to quench the reaction product,the system of GO-LA decreases ~40% of waste acid (~1.8 mol/L waste sulfuric acid containing metal ion) because of lower acid input,as shown in Figs.1d1 and d2.The purification of both GO samples show no apparent differences.Therefore,it is beneficial to reduce the costs of production of GO and lower the impact on the environment.
After purification and centrifugation,the yields of graphene oxide-LA (GO-LA) and graphene oxide-CA (GO-CA) were calculated to 164% ± 5% and 160% ± 3%,respectively.XRD was used to reflect the structure of the GO,as displayed in Fig.2a.Both of two GO samples have a weak and broad diffraction peak located at 11.0o(d spacing=8.1 ˚A).UV-vis spectra were used to identify GO,as shown in Fig.2b.The two spectra have two classic features of GO: A main peak at ~231 nm,corresponding to theπ-π*transitions of aromatic C=C bonds,and a shoulder at ~300 nm,which is due to the n-π*transitions of C=O bonds [19].Fig.2c shows the Raman spectra of the samples.The intensity ratio of D peak to G peak (GO-LA,ID/IG=0.98;GO-CA,ID/IG=0.98) indicates the high defect degree of the both GO samples because of the chemical oxidation.
FTIR were used to study the functional groups of the samples,as seen in Fig.2d.The two spectra present three peaks at 3435 cm-1,1722 cm-1and 1224 cm-1,which can be assigned to the stretching vibrations of-OH (intercalated water),C=O (carboxyl and carbonyl),C-O-C (epoxy groups) bonds,respectively [9].The peak at 1410 cm-1is ascribed to the O-H deformation and the peak of 1048 cm-1is the stretching vibration of C-O (alkoxy).In addition,the peak at 1620 cm-1can be recognized to vibration of intercalated water [20].Thermalgravimetric analysis (TGA) curves of GO-CA and GO-LA (Fig.S7 in Supporting information) exhibit similar characteristics: A weight loss before 100 °C is attributed to the releasing of adsorbed water between GO sheets;the sharp weight loss between 180 °C and 225 °C is due to the removal of less stable oxygenated groups on GO sheets;a gradual mass loss in the range of 225-800 °C is related to the decomposition of more stable functional groups [21,22].The content of the oxygen containing group of GO-LA is almost the same as that of GO-CA.XPS was further used to confirm the chemical composition of GO samples.Figs.2e and f show two high intensity peaks,i.e.,C 1s (284.6 eV) and O 1s (532.9 eV).Figs.2g and h show that the C 1s of GO can be divided into four peaks at 284.6 eV (C=C),286.6 eV (C-O),287.8 eV (>C=O),and 289.0 eV (O-C=O),severally.The C/O ratio of the GO-CA and GO-LA are 1.84 and 1.78,respectively,according to the C 1s spectra [9,23].The contents of different oxygenated groups of GO-LA and GO-CA are basically similar,as shown in Fig.S8 (Supporting information).
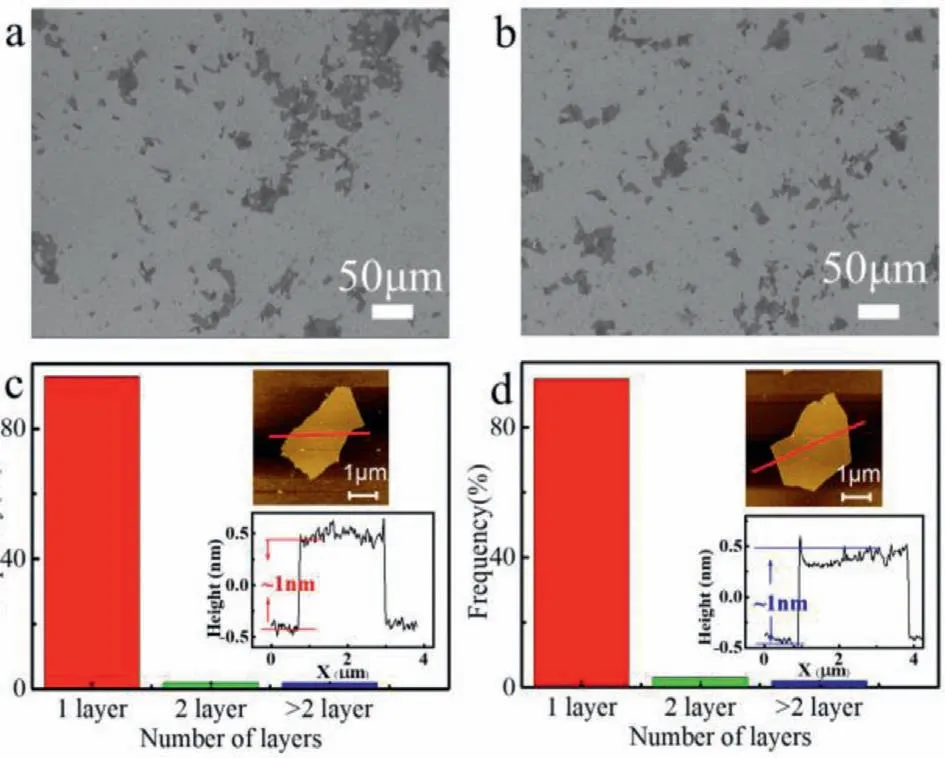
Fig.3.(a,b) SEM images of GO-CA and GO-LA.(c,d) The statistic results of number of layers of GO-CA and GO-LA,respectively.Insets in (c,d) are a typical AFM image and its height profile.
The GO-CA and GO-LA sheets have similar size distributions,as shown in Figs.3a and b.Their lateral dimensions are in the range of several micrometers to tens of micrometers.As shown in Figs.3c and d,the thickness of GO-CA and GO-LA sheets are approximately 1 nm,which is typical thickness for a single layer of GO sheet deposited on the mica substrate [9,24].
The GO films were prepared by the GO dispersions with the same concentration.After reduction by HI,the silvery grey reduced graphene oxide (RGO) films were prepared (insets of Figs.4d and e).The Raman spectroscopy was applied to study the structures of RGO-CA and RGO-LA films,as shown in Fig.4a.TheID/IGratio can be applied to access the mean defect degree.TheID/IGratio of the former is 1.58 and that of the latter is 1.57,respectively [25].The structure of RGO-LA and RGO-CA films were characterized by XRD.As shown in Fig.4b,the XRD pattern of RGOLA exhibits single characteristic reflection peak located at 24.43°(d-spacing=0.372 nm),and that of RGO-CA shows a similar peak located at 24.48° (d-spacing=0.372 nm).Comparing with the dspacing of graphite (2θ=26.6°,d-spacing=0.339 nm),the corresponding d-spacing of both films are slightly larger,which can be attributed to the residue of oxygenated functional groups between RGO layers [17].The C/O ratio of RGO-CA and RGO-LA films were 9.19 and 8.49,indicating the similarity in their chemical compositions (Fig.4c).According to Figs.4d and e,and Fig.S9 (Supporting information),the peak intensity percentages of intact carbon atoms (C=C) of RGO-LA and RGO-CA films in the C 1s spectra reached 73.02% and 72.26%,respectively.The RGO-LA and RGO-CA films have conductivity of 156±5 S/cm,167±7 S/cm,respectively.The above results demonstrate that the structures of RGO derived from two GO samples are nearly the same.The improved Hummers method is also a good choice to be used as starting material for RGO applications,considering the much less acid input and the waste acid output of GO-LA production.
In conclusion,we report a simple,low-cost improved Hummers method of producing GO with much less concentrated sulfuric acid.By assistance of the heat absorbed from environment and reaction system to control the reaction temperature,the problem of safety risks caused by insufficient acid in the synthesis process can be solved.The graphite (10~30 μm) can be fully oxidized by using the much less acid.Much less waste sulfuric acid (decrease of 40%) will be generated by the GO-LA system.Compared with GO-CA system,the GO-LA system has similar yield and oxidation degree.Our method could provide a reference for other Hummers oxidation systems of insufficient acid.It can be anticipated that this method could be suitable to achieve low-cost synthesis of GO on a large scale.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors thank the Guangzhou Municipal Science and Technology Bureau (Nos.202002030368,202102080408) for financial support.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.01.060.
杂志排行
Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
