MicroRNA is a potential target for therapies to improve the physiological function of skeletal muscle after trauma
2022-11-05XinYiGuBoJinZhiDanQiXiaoFengYin
Xin-Yi Gu , Bo Jin , Zhi-Dan Qi Xiao-Feng Yin
Abstract MicroRNAs can regulate the function of ion channels in many organs. Based on our previous study we propose that miR-142a-39, which is highly expressed in denervated skeletal muscle, might affect cell excitability through similar mechanisms. In this study, we overexpressed or knocked down miR-142a-3p in C2C12 cells using a lentivirus method. After 7 days of differentiation culture, whole-cell currents were recorded. The results showed that overexpression of miR-142a-3p reduced the cell membrane capacitance, increased potassium current density and decreased calcium current density. Knockdown of miR-142a-3p reduced sodium ion channel current density. The results showed that change in miR-142a-3p expression affected the ion channel currents in C2C12 cells, suggesting its possible roles in muscle cell electrophysiology. This study was approved by the Animal Ethics Committee of Peking University in July 2020 (approval No. LA2017128).
Key Words: C2C12; denervation; ion channels; microRNA; miR-142a-3p; muscle; patch clamp; potassium; sodium; whole-cell currents Chinese Library Classification No. R446; R741; R318
Introduction
Since miR-1 was first studied as a cardiac electrophysiological regulator in 2007 (Yang et al., 2007), a large number of studies have confirmed the role of microRNAs (miRNAs) in regulating ion channels in the cardiovascular system, nervous system,endocrine and cancer biology (Baroukh et al., 2007; Yang et al., 2007; Pardo and Stühmer, 2008; Saugstad, 2010). miRNAs can regulate cell electrophysiology by directly targeting mRNAs coding ion channels and transporters or indirectly regulating ion channel expression by targeting transcription factors (Pietrzykowski et al., 2008; Shi et al., 2009; Manna et al., 2021; Zeng et al., 2021). In addition, miRNA may indirectly affect ion channel function by targeting proteins that interact with channels, thereby enhancing the complexity and fine regulation of miRNA interactions. Skeletal muscle cells are excitable cells, therefore the expression changes of ion channels in muscle can lead to various diseases, affect cell excitability and electrical conduction and alter normal physiological function (Arnolds et al., 2012; Liu et al., 2015).miRNAs have powerful regulatory potential for skeletal muscle electrophysiology, but so far there have been relatively few related studies.
miR-142a-3p was one of the miRNAs with the most significant different expression in mouse skeletal muscle after denervation (Weng et al., 2018). It has been regarded as the main regulator of cell fate in the hematopoietic system(Nimmo et al., 2013) and plays a role in virus infection,inflammation and cancer (Kaduthanam et al., 2013; Kramer et al., 2015; Mandolesi et al., 2017). It also has been found that miR-142a-3p can inhibit the utilization of lipid in skeletal muscle and complements the effects of miR-27a-3p in the regulation of muscle fiber metabolism (Chemello et al., 2019).Shrestha et al. (2015) predicted and analyzed the targets of miR-142a-3p and found that miR-142a-3p may affect the regulation of the actin cytoskeleton. However, the effect of miR-142a-3p on the electrophysiology of skeletal muscle has not been explored.
Recently we confirmed that another miRNA, miR-34c-5p,is also overexpressed in denervation skeletal muscle and regulates the electrophysiology of C2C12 cells, which suggests the importance of miRNAs in regulating skeletal muscle ion channel currents (Jin et al., 2020). This further sparked our interest in miR-142a-3p. Differently expressed genes and miR-142a-3p predicted target genes were cross-labeled, and the gene oncology analysis showed that the target genes of miR-142a-3p participate highly in metal binding, cation binding,ion binding in binding gene oncology terms (Additional Figure1). miR-142a-3p was also predicted by MR-microT (www.microrna.gr/microT) to regulate gene expression (Scn4a,Scn5a,Kcna2,Kcnc1,Cacna2d1) of many ion channels (Table 1). All these findings indicate that miR-142a-3p may have an important effect on skeletal muscle ion channels.

Table 1 |Predictive targets of miR-142a-3p by MR-microT
To investigate those results further, we established C2C12 cell models of miR-142a-3p overexpression, knockdown and control groups by lentivirus. We recorded their cell membrane capacitance and various ion channel currents of each group to explore the regulatory function of miR-142a-3p on the electrophysiology of C2C12 cells.
Materials and Methods
Cell culture
C2C12 cell line (RRID: CVCL_0188) was purchased from the American Type Culture Collection (Atlanta, MD, USA) and identified by STR. The cells were cultured at 37°C, 5% CO2,and the growth medium (GM) was changed every three days.The GM consists of Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum (Gibco, New York, NY, USA) and 1%penicillin/streptomycin (Gibco).
C2C12 myotube differentiation
After the cells were cultured at equal density, the GM was replaced by the differentiation medium. The differentiation medium consists of Dulbecco’s modified Eagle’s medium with 2% horse serum (Biological Іndustries, Beit Haemek, Іsrael)and 1% penicillin/streptomycin. The differentiation medium was changed every 3 days, and the cells were collected on the 7thday for the subsequent experiments (Kubo, 1991).
Plasmid construction and cell transfection
In this experiment, miR-142a-3p overexpression, knockdown and control groups were constructed using the plasmid hU6-MCS-Ubiquitin-EGFP-IRES-puromycin (Genechem, Shanghai,China). Lentivirus (Genechem) was used to package the plasmid, and finally used to transfect cells. The method is as follows: the vector and the target fragment were each digested with the same restriction enzymes (Beyotime,Shanghai, China) and, after agarose electrophoresis, the gel was cut to recover the product. The recovered vector and the target fragment were ligated overnight at 16°C; the ligation product was used to transform Escherichia coli. The Plasmid Midi Preparation Kit (Beyotime) was used to extract the plasmids.
The cells were planted in a 24-well plate at a density of 30–50%, and GM, lentiviral infection enhancing reagent(Genechem) and 1.5 × 107TU/mL lentivirus were added sequentially. The total volume was 500 µL. After 72 hours of culture, the culture medium was replaced with GM and the transfection efficiency was observed under a fluorescence microscope (MZ75, Leica, Bensheim, Germany). Cells expressing green fluorescent protein were deemed to be successfully transfected. The cell transfection rate was calculated as the number of green fluorescent cells/total cells.
Muscle sampling
To exclude the interference of the physiological cycle and hormone secretion in female animals, nine healthy male C57BL/6 mice (specific pathogen free, 6–8 weeks, 22–25 g;purchased by Charles River, Boston, MA, USA) were used in this study. The study was approved by the Animal Ethics Committee of Peking University in July 2020 (approval No.LA2017128).
Each experimental animal was anesthetized with 1.5%isoflurane (Huazhong Haiwei (Beijing) Gene Technology Co., Ltd., Beijing, China). After anesthesia, the right lower limb of the experimental animal was shaved, and the skin was disinfected with iodophor (Shandong Likang Medical Equipment Technology Co., Ltd., Linyi, China). An incision was made and the gastrocnemius muscle of the right lower limb of the mouse was completely separated from the Achilles tendon to the knee joint for measurement and comparison.The gastrocnemius muscle of the right lower extremity was harvested at 0, 1 or 2 weeks after surgery.
Quantitative real-time polymerase chain reaction
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA from the gastrocnemius muscle according to the manufacturer’s instructions. 5X All-Іn-One RT MasterMix Kit (Abm, Vancouver, Canada) was used to reverse transcribe RNA into DNA. The Bio-Rad iQTM5 system (Bio-Rad,Mississauga, Canada) was used to perform quantitative realtime reverse transcription-polymerase chain reaction (qRTPCR) and the qRT-PCR data was analyzed by 2–∆∆Ctmethod (Livak Method). Mouse glyceraldehyde-3-phosphate dehydrogenase(GAPDH) was the reference gene in this experiment, and the gene primer sequences are listed inTable 2.
Electrophysiology
Electrode preparation and experiment recording
The whole-cell patch clamp solution formulae for sodium current (INa), potassium current (IKd) and L-type calcium current(ICa,L) were in accordance with a study by Nakada et al. (2018),as shown inTable 3.
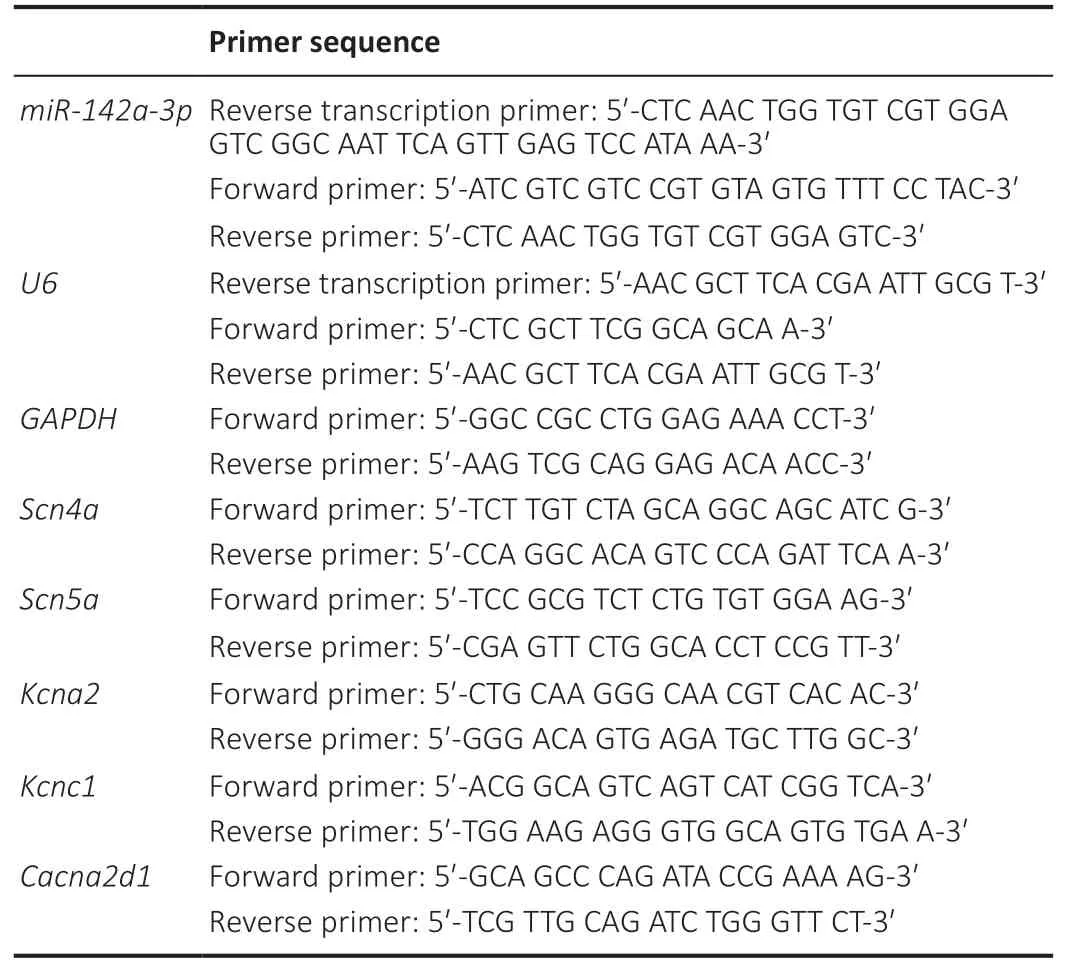
Table 2 |Primer sequence

Table 3 |Whole-cell patch clamp solution formulae
Cacna2d1: Calcium voltage-gated channel auxiliary subunit alpha2delta 1;GAPDH: glyceraldehyde-3-phosphate dehydrogenase; Kcna2: potassium voltage-gated channel subfamily A member 2; Kcnc1: potassium voltage-gated channel subfamily C member 1; Scn4a: sodium voltage-gated channel alpha subunit 4.
The borosilicate capillary glass was placed in a PC-10 Puller(Narishige, Tokyo, Japan) and stretched through a twostep process. After filling the inner liquid, the resistance of borosilicate capillary glass was 3–5 MΩ. Vacuum suction was applied to complete the Giga-ohm seal, and then negative pressure was released to rupture the cell membrane. The series resistance was between 4–7 MΩ, and the compensation was 70–90%. Digidata 1440A and MultiClamp 700B hardware were used for the experimental recordings. Clampex 10.6 and MultiClamp 700B command software were used for data acquisition. The above hardware and software were provided by Molecular Device-Axon Instruments (San Jose, CA, USA).Stimulus waveform and membrane capacitance recordingRecordings were made from C2C12 myotubes in each experimental group.INawere elicited by a series of 20 ms lasting depolarizations from a –120 mV holding potential to voltage ranging from a program of –80 to +20 mV in 5 mV steps.IKdwere induced by the following protocol: a holding potential at –80 mV for 10 ms, then changing to a series of depolarizing steps from –100 to +80 mV in 20 mV increments and then returning to –60 mV, were used to obtain theIKdcurrent density curve. TheICa,Lstimulation square wave was as follows: the initial voltage was maintained at –80 mV,then rose to –40 mV for 1 second, after that, rose to –60 to+70 mV (step 10 mV) for 2 seconds, and finally held at –80 mV for 7 seconds (Beam et al., 1986). The capacitance was compensated and recorded, and the compensation mode was 100% compensation. The cell membrane capacitances of each group was then counted.
Statistical analysis
Clampfit 10.6 (Molecular Devices, San Jose, CA, USA) and OriginPro 2019 (OriginLab, Northampton, MA, USA) were used. Significant differences were determined using a twotailed Student’st-test and data are expressed as mean ±standard error of mean (SEM). A probability value ofP< 0.05 was considered statistically significant.
Results
miR-142a-3p expression and transfection efficiency
The expression of miR-142a-3p was detected by qRT-PCR at 0, 1 and 2 weeks after sciatic nerve excision. The miR-142a-3p expression had significantly increased by the 2ndweek after injury (P< 0.01,vs.0 week,Figure 1A). The transfected cells were screened with puromycin, then qRT-PCR was used to verify the expression of miR-142a-3p in each group of cells (Figure 1B). At each time, the transfection efficiency was recorded under a microscope. The cells with green fluorescence were regarded as successful transfection cells.The transfection efficiency in each group was ~0.9 (Figure 1CandD).
Effects of miR-142a-3p on the voltage-gated sodium,potassium and calcium channel currents in C2C12 myotubes
After 7 days of differentiation, the membrane capacitance of each group was calculated. Compared with the negative control (NC) group, the membrane capacitance in the miR-142a-3p overexpression group was down-regulated (P< 0.01),and the membrane capacitance of miR-142a-3p knockdown group was up-regulated (P< 0.01) (Figure 2).
In the miR-142a-3p overexpression group, the peak currents density of theINadid not change significantly (P= 0.53,vs. NC group), whereas, in the miR-142a-3p knockdown group, the peak currents density of theINawas lower than in the control group (P< 0.05,vs. NC group) (Figure 3).
In the miR-142a-3p overexpression group, the peak currents density of theIKdwas higher than in the control group (P< 0.05,vs. NC group), however, there was no significant change in the peak currents density of theIKdin the miR-142a-3p knockdown group(P= 0.34,vs. NC group) (Figure 4).
In the miR-142a-3p overexpression group, the peak currents density of theICa,Ldecreased relative to the control group (P<0.05,vs. NC group), whereas in the miR-142a-3p knockdown group, the peak currents density of theICa,Ldid not change significantly (P= 0.12,vs. NC group) (Figure 5).
Prediction of ion channel-related targets of miR-142a-3p
We predicted the ion channel-related targets of miR-142a-3p by MR-microT, and found its potential regulatory targetsScn4a,Scn5a,Kcna2,Kcnc1, andCacna2d1(Table 1). The cells in each group were differentiated for 7 days. qRT-PCR results showed thatScn4a,Scn5a,Kcna2, andCacna2d1were significantly down-regulated in the miR-142a-3p overexpression group (P< 0.05,vs. NC group), and upregulated in the miR-142a-3p knockdown group (P< 0.05,vs.NC group) compared with the control group (Figure 6).
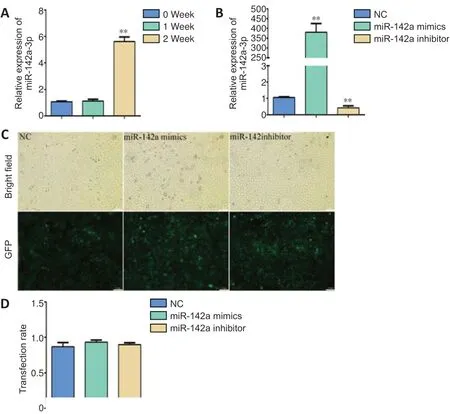
Figure 1|Detection of miR-142a-3p in denervated muscle and establishment of cell models.

Figure 2|Cell membrane capacitance in miR-142a-3p overexpression and knockdown C2C12 cells.
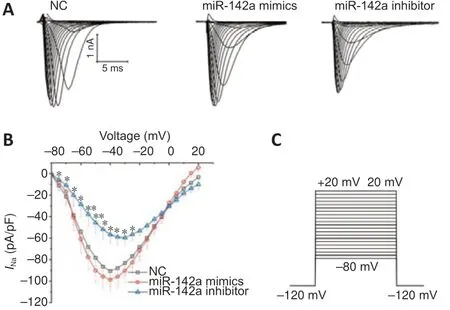
Figure 3|Knockdown of miR-142a-3p decreases the whole-cell voltagegated sodium channel currents (INa) of C2C12 cells.

Figure 4|Overexpression of miR-142a-3p decreases the potassium ion channel currents (IKd) of C2C12 cells.
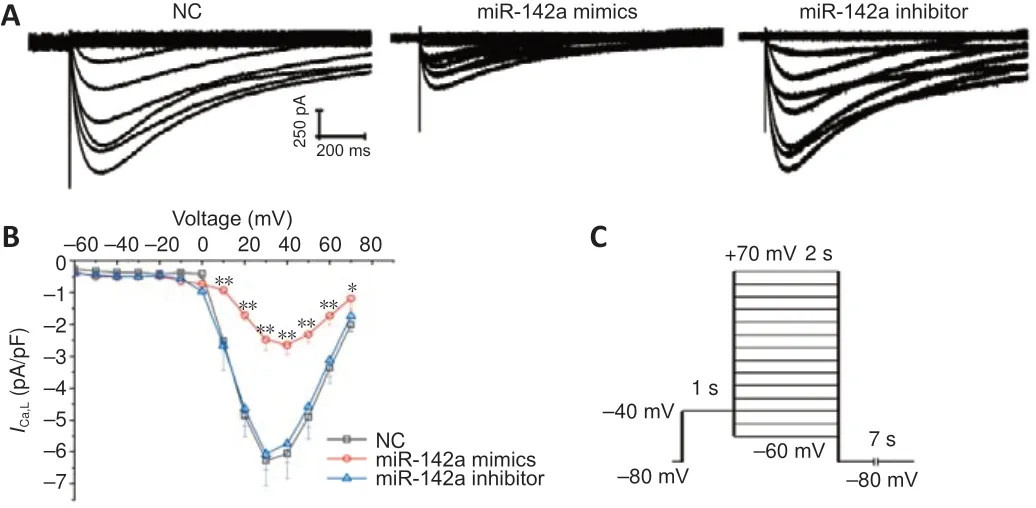
Figure 5|Knockdown of miR-142a-3p decreases the L-type calcium channel (ICa,L) current density of C2C12 cells.
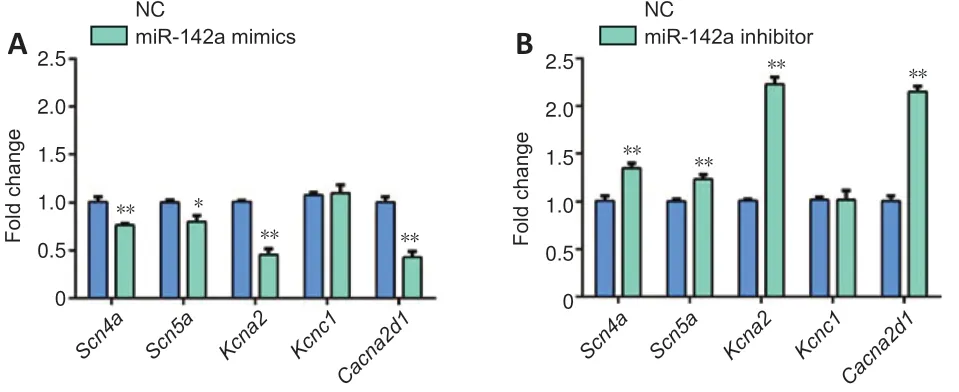
Figure 6|MiR-142a-3p affects the expression of ion channel-related genes in C2C12 cells.
Discussion
Іon channels are critical to the function of excitable cells and they have been found to be regulated by miRNAs in many organs, including skeletal muscles. miR-142a-3p and many ion channel coding genes have been found to be highly differently expressed in skeletal muscle after denervation (AdditionalTable 1) (Weng et al., 2018). The predicted target genes of miR-142a-3p showed a high degree of participation in the binding of metals, cations and ions (Additional Figure 1).Thus, we propose that miR-142a-3p may regulate skeletal muscle electrophysiology.
In this study, whole-cell currents were recorded after 7-day differentiation. Our results showed that in the miR-142a-3p overexpression group cells had smaller membrane capacitance, higher potassium current density and lower L-calcium current density; in contrast, the miR-142a-3p knockdown group exhibited greater membrane capacitance and lower sodium ion channel current density.
Our experiments showed that miR-142a affected cell fusion, and the miR-142a overexpression group had more mature, differentiated cells. The differing development level of myotubes between the different groups could be associated with differences in the expression of ion channels(Moody and Bosma, 2005). In order to analyze the possible mechanism of miR-142a-3p in regulating ion channel current,we predicted the target genes of miR-142a-3p. We showed that miR-142a-3p may targetScn4a,Scn5a,Kcnc1,Kcna2,andCacna2d1. Scn5a was one of the few ion channel coding genes with increased expression. There was also evidence that expression of the voltage-gated sodium channel, Nav1.5,appeared together with the fibrillation potentials in the early stage of denervation atrophy, and that inhibition of Nav1.5 could weaken fibrillation potentials (Sekiguchi et al., 2012).There is no direct evidence that miR-142a targets Scn5a, but some studies have found that ectopic expression of miR-142-3p inhibited the expression of Tbx5, and Tbx5 drives Scn5a expression to influence the functioning of the cardiac conduction system (Arnolds et al., 2012; Chen et al., 2017).We also searched the gene expression files of nine human muscle diseases. Compared with normal human skeletal muscle,Scn4a,Kcna1,Cacna1aandCacna1bare generally upregulated, andKcna2,Kcna3andCacna1care down-regulated in a variety of diseases (National Institutes of Health, 2005)(Additional Table 2). miR-142a-3p targets ion channel genes and inhibits their expression, which may be of significance in the development of skeletal muscle diseases.
We found that knocking down miR-142a-3p can reduce sodium currents density. The action potentials of muscles, as in nerves, are initiated with the opening of sodium channels.Sodium currents cause rapid depolarization of the membrane and the propagation of potential and electrical input from negative to slightly positive passes along the fiber (Hodgkin and Huxley, 1952; Adrian et al., 1970; Hudson et al., 1995).Jurkat-Rott et al. (2000) studied periodic paralysis and found that decreased inward sodium currents in skeletal muscle can lead to low excitability of the fibrous membrane and cause muscle weakness. Therefore, knocking down miR-142a-3p can reduce sodium current density, which may further affect cell excitability. Іn addition, changes in ion channel expression may have important effects on the electrophysiological properties of a cell. The mouse model showed that decreased expression of Scn5a in the heart would slow down ventricular conduction(van Veen et al., 2005), whereas overexpression of Scn5a would enhance AV conduction, which mimics the human syndrome of enhanced atrioventricular nodal conduction (Liu et al., 2015). The possible regulatory effects of miR-142a-3p onScn4aandScn5amay also affect cell excitability and electrical conduction.
Up-regulation of miR-142a-3p increased the current density ofIKd, suggesting that the cells are more easily repolarized,resulting in a shorter time course of their action potentials.SinceIKdare responsible for the repolarization phase of the action potential, the increase in delayed outward current helps to establish the excitement cycle and shorten the action potential (Moody and Bosma, 2005). Therefore, the elevated potassium current in C2C12 may hasten their return to a resting state, maintain the stability of the cell state and resist external stimuli.
Calcium current density was reduced by overexpression of miR-142a-3p, which may be related to the excitationcontraction coupling. Two types of voltage-gated calcium channel (VDCC) are found in skeletal muscle: 1) L-type VDCC generated by the CaV1.1/α1s subunit, which plays a key role in excitatory contraction (Tanabe et al., 1993) and 2)t-type VDCC, generated by the CaV3.2/α1H subunit, which is important in muscle development (Cognard et al., 1986).This experiment recorded L-type VDCC currents, and the L-type Ca2+channel, 4-1-dihydropyridine receptor complex was used as an excitation-contraction coupling voltage sensor(Tanabe et al., 1988). During membrane depolarization, the conformational change of dihydropyridine receptor is coupled with ryanodine receptor 1, allowing the calcium ions to enter the sarcoplasm via ryanodine receptor 1 to initiate contraction(Endo, 1977; Lee et al., 2006). Therefore,ICa,Lcurrents are very important for skeletal muscle excitation-contraction coupling,and reducedICa,Lcurrents in C2C12 may have similar effects to skeletal muscle. Cacna2d1 encodes the α2 and δ1 subunits of theICa,L(Arikkath and Campbell, 2003) and Schug et al.(2013) found that miR-142a-3p targets Cacna2d1 in mice liver cells by high-throughput sequencing with crosslinkingimmunoprecipitation. However, it is not clear that miR-142a-3p regulatesICa,Lby targetingCacna2din muscle cells.These miRNA/ion channel mechanisms are also valuable for explaining the adaptive changes of muscle cell excitationcontraction coupling after denervation.
Comparing our previous work on miR-34c-5p with this study(Jin et al., 2020), we found that both miR-34c-5p and miR-142a-3p are significantly up-regulated in denervated skeletal muscle, whereas their effects on specific currents are totally different. It is interesting that one side-regulation on ion channels was observed with each miRNA but to different effects. For sodium channels, there is no significant change inINawhen miR-34c-5p is overexpressed or knocked down,whereas knockdown of miR-142a-3p can reduceINa. For the delayed rectified potassium current, knockdown of miR-34c-5p and overexpression of miR-142a-3p can increaseIKd. For the L-type calcium current, overexpression of miR-34c-5p can increaseICa,Lcurrent, while overexpression of miR-142a-3p can reduce theICa,Lcurrent. Similar phenomena also appear in other tissues. For example, overexpression of miR-370-3p reduces the hyperpolarization-activated channel current, but the knockdown group was not different from the control group(Yanni et al., 2020). The possible reason for this phenomenon is that different miRNAs have different regulatory targets and thus perform different functions.
In summary, we have studied the regulatory effect of miR-142a-3p on C2C12 ion channels. We used the C2C12 cell line, whose physiological characteristics may be slightly different from true skeletal muscle cells, but the results still suggest that miRNAs function as regulators of ion channels.The specific channel subtypes and regulatory mechanisms of miR-142a-3p in regulating whole-cell sodium, potassium and calcium channels need further investigation and this is the direction of our follow-up experiments.
Author contributions:Study conceptualization, methodology, and data analysis: XFY; data curation, andoriginal draft preparation: XYG;visualization, and investigation: BJ; supervision: ZDQ. All authorsapproved the final version of the manuscript.
Conflicts of interest:The authors declare that there is no conflict of interests.
Financial support:This study was supported by the National Natural Science Foundation of China, Nos. 82072162, 81971177; and Beijing Municipal Natural Science Foundation of China, No. 7192215 (all to XFY).The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:The study was approved by the Animal Ethics Committee of Peking University in July 2020 (approval No.LA2017128).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Yao Tong, The Scripps Research Institute, USA.
Additional files:
Additional Table 1: Differentially expressed ion channel coding genes in skeletal muscle after denervation.
Additional Table 2: Differential expression of ion channel genes in multiple human muscle diseases.
Additional Figure 1: Gene Ontology (GO) analysis of miR-142a-3p predictive target mRNAs at each time point.
Additional file 1: Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Lycium barbarum polysaccharides and ferroptosis: jumping into the era of novel regulated cell death
- Corrigendum
- Therapeutic potential of prophylactic exercise for intracerebral hemorrhage
- Neuronal and endothelial transglutaminase-2 expression in experimental autoimmune encephalomyelitis and multiple sclerosis
- Loss of smell in COVID-19: reasons for variable recovery patterns from anosmia
- Phosphoinositide-3-kinase regulatory subunit 4 participates in the occurrence and development of amyotrophic lateral sclerosis by regulating autophagy
