Clinical significance of anti-nucleocapsid-IgG sero-positivity in SARS-CoV-2 infection in hospitalized patients in North Dakota
2022-11-02BakirDzananovicMarkWilliamsonCasmiarNwaigweChittaranjanRoutray
Bakir Dzananovic,Mark Williamson, Casmiar Nwaigwe, Chittaranjan Routray
Abstract
Key Words: COVID-19; SARS-CoV-2 IgG-N; Anti-nucleocapsid IgG; Cytokines
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel virus which belongs to the family ofCoronaviridae, the causative agent for coronavirus diseases 2019 (COVID-19)[1]. SARS-CoV-2 emerged out of Wuhan in China and soon after, it spread to the entire world and thereby becoming a “Pandemic”[2-4]. After two years of rapid spread and the virus claiming over five million lives, healthcare system continues to scramble to protect patients from the atypical pneumonia-like illness caused by COVID-19. Diagnosis of SARS-CoV-2 infection is primarily dependent on reversetranscription polymerase chain reaction (RT-PCR) testing of nasopharyngeal swab samples with more recent progress into rapid antigen testing[5,6]. Rapid community spread of the infection and development of herd immunity by community exposure has been a favorite topic of discussion by epidemiologists while the scientific community have successfully raced to design several effective vaccines for COVID-19.
Taking a dive into the pathophysiology of COVID-19, a strong comprehension of the role of the humoral immunity becomes very pertinent. It is known that infection with SARS-CoV-2, elicits an adaptive immune response by producing target specific antibodies which includes IgM, IgG and IgA[7-9]. Among these antibodies, IgG has been of tremendous interest to the scientific community due to its role in long-term protection against the virus[10]. After an infection with SARS-CoV-2, it takes about 10-14 d to produce IgG antibodies which peak around the third week and continues to remain detectable for about 8-12 mo[11,12]. SARS-CoV-2 is a positive sense single stranded RNA virus comprised of four different structural proteins. Those are the nucleocapsid protein (N protein), spike protein (S-protein), matrix protein (M-protein) and the envelope protein (E-protein)[13]. Antibody against the S-protein (IgG-S) is believed to be the neutralizing antibody that is the primary target of the vaccine trials. There have been reports showing a positive correlation between higher IgG-S levels and diseases severity[14,15]. On the contrary, another study cited no association of IgG-S with patient outcomes such as need for maximal oxygen support, intensive care unit (ICU) admission, duration of hospitalization and death[16]. Alternatively, it could be argued that the non-neutralizing antibodies against the nucleocapsid protein (IgG-N) leads to robust inflammation cascade and release of cytokines thus contributing to debilitating pulmonary injury. It is believed that the cytokine storm plays a key role in the pathogenesis and COVID-19 prognosis[17,18]. A report by Batraet al[19] studied the role of IgG-N in COVID-19 and based on the findings the authors recommended using IgG-N titers as a prognostic factor for the clinical course in patients. In this study, a higher IgG-N titer was associated with extended duration of stay in the hospital and increased rate of admission into the ICU. Another study demonstrated that the stronger IgG-N seroconversion response is associated with more diseases severity compared to the weak responders[20]. There is still a paucity of data about the functionality of IgG-N in the pathophysiology of COVID-19. Given this concept of targeting various structural components of this prolific virus, study of the seroconversion and IgG-kinetics has gained a lot of importance to the researchers. Literature on the long-term kinetics of IgG antibody levels and their corresponding neutralizing effectiveness is sparse.
Practicing in a tertiary care community-based teaching hospital in North Dakota, United States, we have had experience with the pre-vaccine phase of COVID-19 pandemic. Noncompliance with public mask usage and rapid community transmission led to a sharp rise in COVID-19 illness and hospitalizations in North Dakota. In the midst of a healthcare crisis, we decided to investigate whether a qualitative IgG-N could be used as a molecular marker to determine the prognosis in the hospitalized patients. Basing our hypothesis on a theory that a rampaging community spread of SARS-CoV-2 infection led to a measurable IgG-N seroconversion of our population, thus impacting outcomes from hospitalization due to COVID-19, we retrospectively analyzed the data provided by the single-center community hospital from which we practiced.
MATERIALS AND METHODS
Study population and data collection
All patients were admitted to the community hospital between December 1, 2020 and August 30, 2021. Fifty-nine patients were included in the study who were screened for IgG-N within 48 h of admission. We excluded all patients that had been admitted to the hospital with a non-COVID-19 diagnosis who incidentally tested positive for SARS-CoV-2 during screening. Patients with severe or critical COVID-19 illness as per the definition of National Institute of Health were included in the study. Those with mild and moderate illness were excluded from the study as most of them did not meet criteria for hospitalization. None of the included patients had been vaccinated against COVID-19. All the patients were confirmed positive for SARS-CoV-2 infection using RT-PCR from nasopharyngeal swab samples at admission. Both male and female patients aged 28 to96 were included in the study. All patients were checked for the presence of SARS-CoV-2-IgG-N within 48 h of admission by using Abbott SARS-CoV-2-IgG assay, that uses a two-step chemiluminescent microparticle immunoassay method with acridiniumlabeled anti-human IgG, performed at North Dakota state laboratory. Admission blood samples identified 26 patients positive for IgG-N against SARS-CoV-2 and 33 negatives. In October 2021, we started data acquisition, reviewing the electronic medical record of included patients.
Study design
As this retrospective cohort study investigated the study population from patient admission to outcome, a thorough review of the electronic medical record was performed to capture data. This data was inclusive of the following: age, gender, body mass index (BMI), duration of symptoms prior to hospitalizations (DOS), length of hospital stay measured in days (LOS), admission to ICU, need for high flow nasal cannula (HFNC), bilevel positive airway pressure ventilation (BiPAP) or mechanical ventilation (VENT) for supplemental oxygen/support, as well as the final patient outcome - discharge or death. (Table 1)
Statistical analysis
Formatting: Age, BMI and DOS were numerical variables. However, additional constructs split Age and BMI into two and three-group categories for some analyses. For example, BMI_2, patients with a BMI of < 29.9 were put in one group, and those > 30 in a second group. For BMI_3, patients with a BMI of < 25 were put into one group, those between BMI of 25-29.9 in a second group, and those with BMI > 30 in a third. For the variable labelled Age_2, patients < 75 were put in one group, and those 75+ in a second group. For Age_3, patients with an age of < 40 were put into the first group, those between 40-75 in a second, and those 75+ in a third group. It should be noted here that one patient had an extreme value for their LOS at-158 d. Models were run with both the patient included and excluded to determine the sensitivity of the models to this extreme value.
Correlation of outcomes: For each pair of outcomes (Death, ICU, BiPAP, VENT and HFNC), the phi coefficient (measure of association between binary variable, comparable to the Pearson coefficient for continuous normal variables) was calculated (Table 2).
Outcomes by IgG status alone: For each variable, the outcome was modeled as a function of IgG-N status (positive or negative) using a generalized linear distribution. For LOS, it was determined that a negative binomial distribution had a better fit than a Poisson or Gaussian distribution, as evidence by a Pearson chi-square/df value closer to 1.0. The negative binomial distribution is also less sensitive to outliers. All other outcomes utilized a binary logistic regression model.
Outcomes by IgG-N status full model: For any single models that were significant, a multiple regression model was utilized, accounting for the consequential effect (if any) of the defined confounding variables of age, sex, BMI, and duration of symptoms.
Outcomes by other factors
LOS was modeled as a function of age using a negative binomial model. From there, age (categorical), BMI (numerical), and the interaction of BMI and age were each run with and without the extreme patient LOS-value noted in the previous section. The patient outcome/death was modeled as a function of LOS using a logistic model. Then, death was modeled as a function of age (numerical), and then age (categorical). The same was done for BMI. Finally, death was modeled as a function of sex. ICU, BiPAP, VENT, and HFNC were each modeled as a function of age (numerical), BMI (numerical), and sex separately.
Statistical analysis used SAS Studio V.3.8 (Cary, North Carolina, United States). The statistical review of the study was performed by a biomedical statistician.
RESULTS
We conducted a retrospective cohort study among fifty-nine adults aged between 28-96, admitted to the hospital with severe or critical COVID-19 illness between December 2020 and August 2021.
Correlation of outcomes
Unsurprisingly, most outcomes were strongly correlated. VENT and ICU rates were very strongly correlated (Phi Coeff = 0.94). All but one patient who went on mechanical ventilation was also admitted to the ICU. In contrast, VENT and HFNC rates were only moderately correlated (Phi Coeff = 0.43).
Outcomes by IgG status alone
Patient outcome, ICU admissions, HFNC, BiPAP and VENT rates were not significantly different across IgG-N status (Figure 1A-E). However, LOS by IgG-N status was found to be significant (tvalue = 2.16,Pvalue = 0.0349) when including the extreme value (LOS > 150 d). IgG-N negative patients had higher average LOS than IgG-N positive patients (15.12vs9.35 d). However, when removing the extreme value (LOS of 150 d), IgG-N negative patients still had slightly higher average LOS (10.66vs9.35 d), but the relationship was no longer significant (Figure 1F). Furthermore, median LOS was lower in IgG-N negative patients (6.5vs7.5 d).
Outcomes by IgG-N status full model
Because LOS-days and IgG-N status was significant, at least when not removing the extreme value, the full model was considered which included age, BMI, and sex, and duration of symptoms. However, in the full model, IgG-N was not significant when controlling for the other variables. This remained true when using a model without the extreme value.

Table 1 Summary statistics by IgG-N status
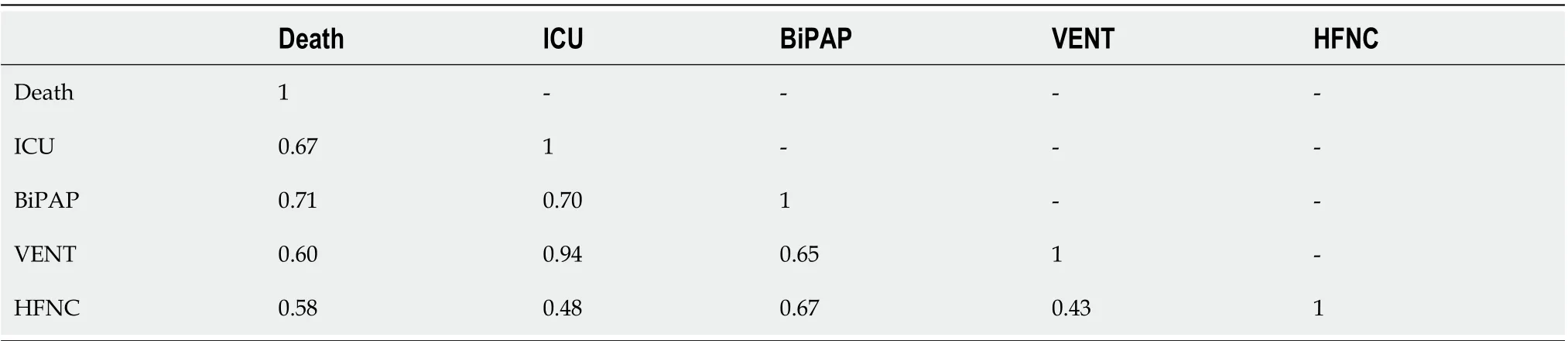
Table 2 Matrix of Phi coefficient for binary outcomes
Outcomes by other factors
For death, only age (numerical) was a significant predictor (Fvalue = 5.07,Pvalue = 0.0283). As age increased, the probability of having an endpoint of one (Death) increased (Figure 2). No other variables for any of the outcomes were significant.
DISCUSSION
SARS-CoV-2 infection causes an atypical pneumonia like respiratory illness known as COVID-19 characterized by fever, dyspnea, anosmia and a worsening hypoxia[21,22]. Among those hospitalized with COVID-19, patients often required supplemental oxygen using HFNC, BiPAP and an increased admission into the ICU requiring mechanical ventilation depending on the severity of the respiratory failure and lung parenchymal involvement. The pathophysiology of COVID-19 is primarily an immunemediated process with a variety of antibody signatures among which the IgG signatures were of interest to our study. A robust immune-mediated inflammatory cascade guides the pathophysiology of the COVID-19 illness[22-24]. Different proinflammatory cytokines such as IL-6 and TNF-α have been correlated with diseases severity[25].

Figure 1 Clinical outcomes across Covid IgG status (0 = negative, 1 = positive). A: Mortality rates; B: High flow nasal cannula rates; C: Bilevel positive airway pressure ventilation rates; D: Intensive care unit admission rates; E: Mechanical ventilation rates; F: Mean length of stay. COVID: Coronavirus disease.
There is some data available to understand the humoral response to SARS-CoV-2 infection and role of various IgG subtypes in the body’s line of defense. Much of it was inherited from the studies of SARSCoV-1[26]. IgG antibodies directed towards the spike protein (IgG-S) and that of the nucleocapsid protein (IgG-N) are the two important components of humoral immunity against SARS-CoV-2 infection. SARS-CoV-2 uses the spike protein to bind to the target cell through its receptor-binding domain and therefore is the target site for neutralizing antibody, IgG-S[27]. The role of IgG-S in early viral clearance is crucial for favorable clinical course and survival[28-30]. IgG-S is considered the neutralizing antibody which may elicit a protection against SARS-CoV-2 by interfering with virion binding to host cell receptors, blocking cellular uptake and preventing endosomal processing of viral genome[13,27]. However, the kinetics of the antibody response becomes more complex to understand with current available literature, which is conflicting. In one interesting study, there is a link between the IgG-S response and COVID-19 severity, but the antibody response has to develop in a specific time window to improve viral clearance and disease outcomes. A faster antibody response was associated with better survival (within the first 14 d of infection) and deceased patients showed a slower antibody response although they reached higher IgG titers later in the disease trajectory[31]. Other studies have shown that, severely ill patients exhibit higher peak, faster and stronger antibody response compared to mildly symptomatic patients[13,32]. Severely ill COVID-19 patients have been found to produce a unique serologic signature with increased IgG-S with afucosylated Fc glycans. The Fc modification of IgG-S triggers activation of natural killers cells and enhances production of IL-6 and TNF-α by primary monocytes that results in more severe disease[33].
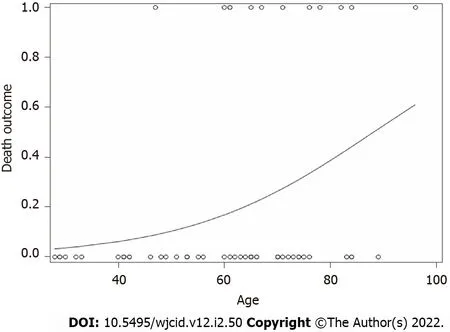
Figure 2 Logistic regression results of death outcome by age (Death = 1, Recovery = 0) by age (numerical). As age increased, the probability of mortality increased (F value = 5.07, P value = 0.0283).
The role of IgG-N in the pathogenesis and clinical course of COVID-19 remains largely unknown. As to our current knowledge, severe COVID-19 is characterized by a series of inflammatory signatures including a cytokine storm, inflammatory alveolar infiltrates and formation of vascular microthrombi[33]. During the peak of the pandemic, clinicians took their chance to use different inflammatory markers such as C-reactive protein, platelet count, D-dimer and Ferritin, to name a few to monitor diseases progression and crisis planning. However, data to support the specificity of these inflammatory signatures as reliable prognostic markers for COVID-19 is limited[34,35]. As per one report by Batraet al[19], showed that titers of IgG-N at the time of admission can be a prognostic factor in the clinical course of the diseases and was associated with increased incidence of hypoxemia, admission into the ICU and extended length of stay in the hospital. In our study, we hypothesized that the presence of IgG-N at the time of admission into the hospital could be used as a marker of impending diseases severity and determine hospital course. We pursued a qualitative measurement of IgG-N on all our patients. Some key parameters such as the degree of hypoxemia, mean length of hospitalization, ICU admission, need for mechanical ventilation and patient outcome as in-hospital datasets were examined in our study group. We enrolled a total of 59 patients who were admitted with hypoxia secondary to COVID-19, out of which 26 (44%) patients had IgG-N antibody at the time of admission into the hospital. Our goal was to investigate the role of IgG-N as a marker to anticipate the clinical course in hospitalized patients. Based on our results, we concluded that IgG-N might not be a reliable predictor of COVID-19 diseases severity.
Our data indicate that age was a single independent predictor of death following hospitalization, which is in support of reports published earlier[36,37]. As age increased, the probability of death increased (Figure 2). Mortality rate was not significantly different in IgG-N positive groupvsnegative (Figure 1A). We did not find any statistical difference with the need to use HFNC between the two groups (Figure 1B). Many of our patient population had clinically progressive diseases with worsening respiratory failure requiring BiPAP or transfer to ICU to be intubated and placed on mechanical ventilation. After following the patient pool until discharge, we did not find any significant difference with the need to use BiPAP between the two groups with and without IgG-N at the time of admission (Figure 1C). The admission rate into the ICU and need for mechanical ventilation was not statistically significant either (Figures 1D and E). Although we saw an extended LOS among the IgG-N negative group, but after adjusting for the extreme outlier, the findings were no longer significant (Figure 1F). Furthermore, median LOS was actually lower in the IgG-N negative group, showing that the extreme value was skewing the LOS average.
Our study had several limitations. We did not measure the IgG-N antibody titers in our study and so we cannot imply if highvslow antibody titers have any direct impact on the disease severity and mortality in COVID-19. Since every individual patient was enrolled into the study only when they were symptomatic enough to meet criteria for hospitalization, especially hypoxic with oxygen saturation < 90%, it could be argued that they may be at different stage of the diseases course and different phase of the seroconversion. This could have confounded our findings since seroconversion and viral kinetics are time dependent phenomena. We did not standardize our patients based on their underlying comorbidities, which further could have influenced our results. More investigation using a larger sample size and different IgM/IgG subtypes is warranted to put more light in this area.
CONCLUSION
We have analyzed the presence or absence of IgG-N in patients admitted to the hospital with severe or critical COVID-19 illness and evaluated the effects of presence of IgG-N on clinical severity and outcome. Age happens to be the single independent risk factor for a worse outcome. Our analysis revealed no significant correlation between IgG-N status and degree of respiratory failure or mortality. The degree of respiratory failure was characterized by the utilization of high flow nasal canula, bilevel positive pressure ventilation and intubation with mechanical ventilation. IgG-N seroconversion had no significant effect on mean length of stay in the hospital. Further studies with large cohorts and riskadjusted comorbidities are needed to demonstrate the more accurate role of IgG-N seroconversion on clinical outcome.
ARTICLE HIGHLIGHTS

ACKNOWLEDGEMENTS
We would like to acknowledge Ms. Shannon Yarbrough, for her contribution with medical library services.
FOOTNOTES
Author contributions:Routray C was the principal investigator and designed the study; Nwaigwe C was the coinvestigator, participating in study design and revision of manuscript for intellectual content; Dzananovic B helped with data acquisition, analysis and initial manuscript writing; Williamson M performed the biostatistical analysis and interpretation of the data.
Supported bythe National Institute of General Medical Sciences of the National Institutes of Health under Award, No. U54GM128729.
Institutional review board statement:Institutional review board statement:This study was reviewed and approved by the Trinity Hospital, Institutional Review Board Committee.
Informed consent statement:Obtaining informed consent was waived by the IRB committee since this was a retrospective cohort study.
Conflict-of-interest statement:There are no conflict of interest to report.
Data sharing statement:No additional data are available.
STROBE statement:The authors have read the STROBE statement- checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Chittaranjan Routray 0000-0001-7004-0372.
S-Editor:Liu JH
L-Editor:A
P-Editor:Liu JH
