Profiles of interferon-gamma and interleukin-2 in patients after allogeneic hematopoietic stem cell transplantation
2022-10-31MalwinaRybickaRamosMiroslawMarkiewiczAleksandraSuszkaSwitekRyszardWiaderkiewiczSylwiaMiziaMonikaDzierzakMiettaKrzysztofBiatas
Malwina Rybicka-Ramos,Miroslaw Markiewicz, Aleksandra Suszka-Switek, Ryszard Wiaderkiewicz, SylwiaMizia,Monika Dzierzak-Mietta, Krzysztof Biatas
Abstract
Key Words: Interleukin-2; Interferon-gamma; Cytokine profiles; Acute myeloid leukemia; Allogeneic hematopoietic stem cell transplantation; Acute graft-versus-host disease
lNTRODUCTlON
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) may be related to the occurrence of complications such as graft-versus-host disease (GvHD) and infections. GvHD is a crucial and potentially fatal complication of allo-HSCT. It is observed in the case of transplantation from related or unrelated donors and occurs in two forms,i.e., acute GvHD (aGvHD) and chronic GvHD. GvHD is induced by donor T lymphocytes, which are stimulated by classic human leukocyte antigens (HLA) in the event of transplantation from not fully matched donors, or by weak human leukocyte antigens in the case of transplantation from fully matched donors[1-3]. The concept of aGvHD created by Ferraraet al[4] assumes that the pathogenesis of aGvHD involves three consecutive stages. In the first stage, pretransplantation conditioning treatment involves lesions and stimulation of host tissues with the elicitation of proinflammatory cytokines (TNF-α and interleukin [IL]-1) and induction of antigenpresenting cells. The second stage involves T lymphocytes, which recognize alloantigens on the host cells, initiate the cytokine storm, secrete interferon (IFN)-gamma and IL-2, and act on effector cells of the immune system. In the third stage, the tissues and organs are damaged by the inflammatory process activated by cytokines secreted by their cytotoxic T cells, NK cells, and macrophages. IFN-gamma and IL-2 are core cytokines inducing the graft-versus-host reaction by enhanced activation of the immune cells in response to alloantigens[5-7].
MATERlAL AND METHODS
This study included 62 subjects diagnosed with acute myeloid leukemia who underwent allo-HSCT at the Department of Hematology and Bone Marrow Transplantation of the Independent Public Clinical Hospital, Medical University of Silesia in Katowice, Poland between 2012 and 2014. The subjects included 30 (48%) males and 32 (52%) females aged 19-68 years (median age 49.5). Time from diagnosis to transplantation ranged from 4 mo to 10 years (median 11 mo). At the moment of transplantation, 54 (87%) patients were in complete remission, three (5%) in partial remission, and others did not reach remission. Conditioning treatment was based on the following regimens: TreoFluATG (n= 26, 42%), BuCyATG (n= 14, 23%), BuCy (n= 6, 10%), TreoFlu (n= 5, 8%), TBICyATG (n= 5, 8%), BuFluATG (n= 3, 5%), and in isolated cases TreoFluThymo, BuFlu, and BuCyThymo. Myeloablative conditioning was given to 42% (n= 26) of the patients, while reduced intensity conditioning (RIC) was administered to 58% (n= 36). Fifty (81%) patients underwent unrelated donor hematopoietic stem cell transplantation, while other patients (n= 12, 19%) underwent sibling hematopoietic stem cell transplantation. All patients underwent standard immunosuppressive therapy. Ninety percent (n= 59) of patients were treated with cyclosporin and methotrexate with antilymphocyte globulin in the case of unrelated donor transplantation. aGvHD was diagnosed based on clinical criteria and was assessed according to the Glucksberg scale. Infection complications were diagnosed based on clinical symptoms and the findings of bacteriological tests. Death during hospitalization was reported in four (6%) patients within +30 d after allo-HSCT. Peripheral blood samples (5 mL) were collected from each patient into clot activator tubes at the following time points: Before conditioning treatment, after its completion (day -1), and after transplantation on days +2 +4, +6, +10, +20, and +30, unless death occurred earlier. The collected blood was immediately centrifuged, and the serum was frozen at -80 °C until analysis. The levels of IFNgamma and IL-2 were determined using ELISA.
Statistical analysis
Statistical analyses were performed by a biomedical statistician. The study population was characterized by the presentation of the percentage distribution of the qualitative variable variants, while in the case of quantitative variables, the median value and range were used. The cytokine levels were initially analyzed at all checkpoints by estimating the mean, median, standard deviation, and standard error of the mean, and by defining the minimum and maximum values. Due to significant right-skewed cytokine distribution, resulting in rejecting the hypothesis of the normal distribution verified by the Shapiro-Wilk test, further analysis used the median as the measure of central tendency, and the interquartile range was used as the measure of dispersion. Moreover, non-parametric procedures were used to test statistical hypotheses. Due to multiple 0 values of the cytokines, the variables defining their levels at the test time points were categorized not only based on the cut-off point defined by the manufacturer (for IL-2, 7 pg/mL and for IFN-gamma, 5 pg/mL), but also based on the 0 value. APvalue less than 0.05 (P< 0.05) was considered statistically significant.
RESULTS
Hematological recovery after stem cell transplantation was observed in 61 (98%) patients and median time to recovery was as follows: White blood cells (> 1.0 G/L), day +15 (range 11-25 d); absolute neutrophil count (> 0.5 G/L), day +17 (11-27 d).
The manifestation of aGvHD after allo-HSCT was observed in 30 (48%) patients, and median time to manifestation was day +17 (range 8-29 d). aGvHD was diagnosed with the following grades: I, 26 (42%) patients; II, 3 (5%); III, 1 (2%); IV, not recorded. aGvHD involved the following organs: Skin, 28 (45%) patients; intestines, 3 (5%); liver, not recorded. The remaining 32 (52%) patients did not present with aGvHD symptoms. An infectious complication independent of its etiology occurred in 38 (61%) patients, and median time to the occurrence of the first incident was day +9 (range, 0-27 d). Mucositis was reported in 26 (42%) patients, and median time to the occurrence was day +1 (range, 0-20 d) (Table 1).
Twenty-one (70%) patients with aGvHD also presented with bacterial and/or fungal and/or viral infection, and median time to the first infection was day +10 (range, 1-27 d). Mucositis was reported in 15 (50%) patients, and median time to the occurrence was day +2 (0-8 d). However, it was not significantly different in comparison with the group of patients without aGvHD (Table 1).
The assessment included kinetics of IFN-gamma changes after allo-HSCT in the groups with and without aGvHD at consecutive time points, including the assessment before and after conditioning treatment (Figure 1).
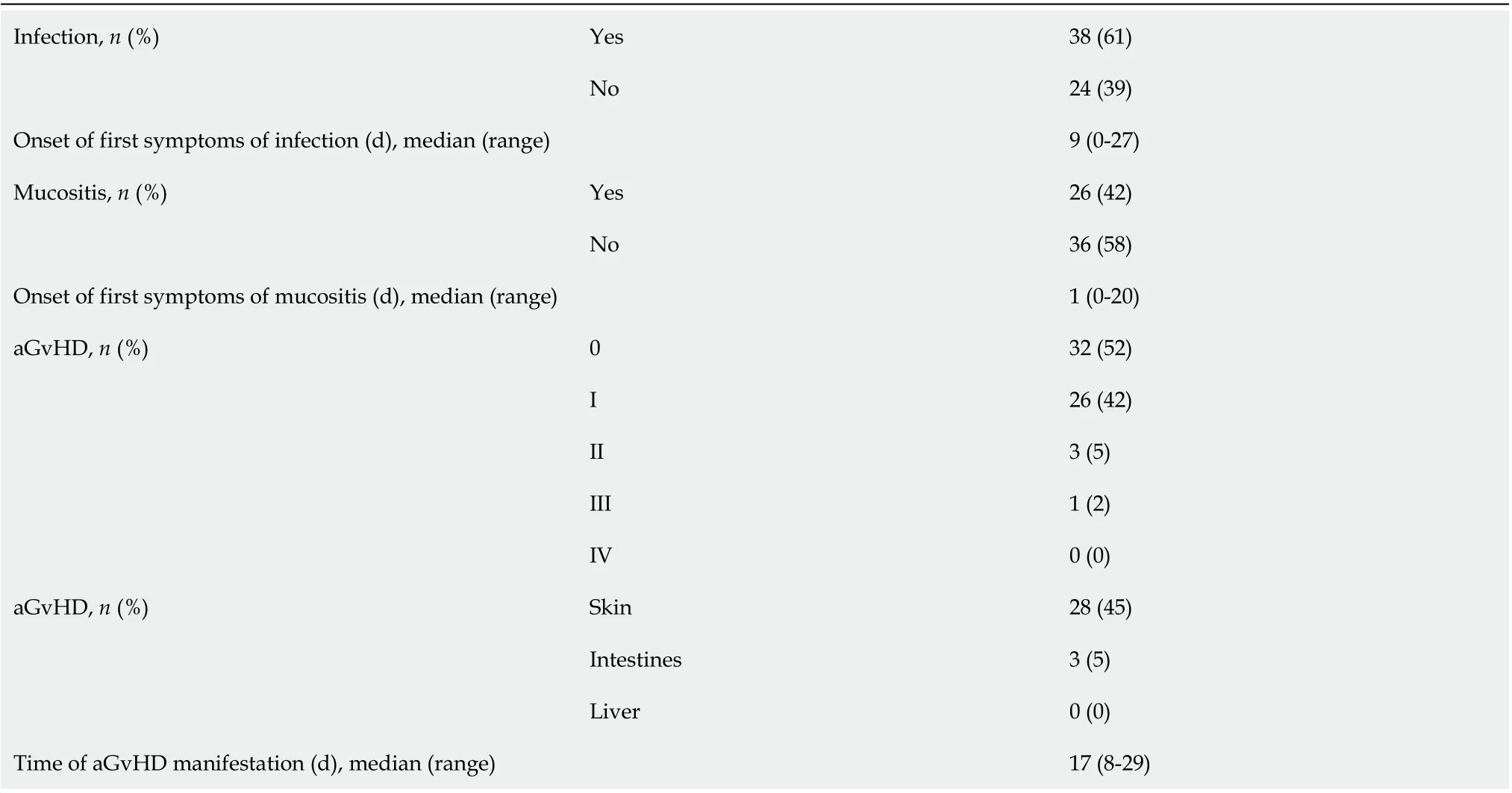
Table 1 Complications after allogeneic hematopoietic stem cell transplantation
No differences regarding the IFN levels assessed before and after conditioning treatment were observed between the subjects with and without aGvHD. In the group of patients without aGvHD, a significant increase in the cytokine level was observed on day +20. The obtained level was similar during the next measurement. In patients with aGvHD, an increase in IFN-gamma levels was observed on day +6 in relation to the levels before conditioning treatment. At this measurement point, higher IFN-gamma levels in the aGvHD group were the most pronounced in relation to the group without aGvHD. This advantage could also be observed on day +10. At the next measurement point, a shift was observed regarding IFN-gamma levels, which were higher in patients without aGvHD. The above finding may be correlated with the disease onset (median on day +17).
The assessment also included the kinetics of IFN-gamma changes after allo-HSCT in the groups of patients with and without infections at consecutive time points, including the assessment before and after conditioning treatment (Figure 2).

Figure 2 Serum levels of interferon-gamma in the groups with and without infection. allo-HSCT: Allogeneic hematopoietic stem cell transplantation; IFN: Interferon; Pre-C: Before conditioning treatment; Post-C: After conditioning treatment.
No significant difference in IFN-gamma levels before conditioning treatment was observed between the groups with and without infection, although a tendency to higher cytokine levels was found in the group without infection. The analysis of the effect of conditioning treatment on the cytokine level showed its decrease only in patients whose post-transplantation period was not complicated by infection, which in turn led to the reversal of proportions in both groups. A tendency to higher IFNgamma levels in the group of patients with infection after conditioning treatment was maintained during the entire post-transplantation period, and on day +6, the difference in IFN-gamma levels reached significance. In the group of patients with infection, an almost continuous increase in IFNgamma levels was observed during the entire study period, while on day +20, the concentration was significantly higher compared to baseline, and was also slightly increased at the next measurement point. In other patients, after conditioning treatment, the lowest IFN-gamma levels showed a growing tendency at subsequent measurements. However, this tendency was not as significant as that in the group of patients with infection.
Moreover, the median and maximum values of IFN-gamma levels were measured in each patient in the post-transplantation period (between days +2 and +30). These values were significantly higher in the group of subjects with infection compared with those without (median 0.058vs0 pg/mL,P= 0.043; 19.295vs2.260 pg/mL,P= 0.002, respectively) (Tables 2 and 3).
The presence of IL-2 assessed before conditioning treatment was reported only in one patient who did not develop aGvHD and had no infection due to transplantation. The levels of IL-2 decreased after conditioning treatment to the value below the cut-off level, but were maintained above 0, with no increase in the cytokine levels in other patients.
The levels of IL-2 assessed after allo-HSCT were above 0 at least at one measurement point only in five patients, including three subjects in whom the cut-off point was above 7 pg/mL. None of these patients showed manifestations of aGvHD, but three patients presented with infections independent of their etiology, and two subjects had mucositis. In all patients, the levels of IL-2 were undetectable on the day of infection onset. Figure 3 shows the kinetics of IL-2 changes in serum of patients whose level was above 0 at any time during the post-transplantation period.
In the final analysis, the patients were divided into four groups based on the occurrence of aGvHD and infection: Group I, patients (n= 15, 24%) who presented with no signs of acute GvHD or infection; group II, patients (n= 17, 27%) who showed complications in the form of infection after allo-HSCT without acute GvHD; group III, patients (n= 9, 15%) who showed acute GvHD with no infection; group IV, patients (n= 21, 34%) who showed both acute GvHD and infection. The analysis of IFN-gamma levels measured at subsequent measurement points showed the occurrence of differences between the groups on days +20 and +30 after allo-HSCT (Table 4).
Significantly higher levels of this cytokine were observed in group II on days +20 (P= 0.014) and +30 (P= 0.008) in comparison with other groups of patients. Post-hoc tests revealed significantly lower IFNgamma levels on day +30 in groups I (P= 0.039) and IV (P= 0.017) in comparison with group II. The levels of IL-2 were undetectable in most patients at all checkpoints. Figure 4 shows mean IFN-gamma levels before and after allo-HSCT in the four groups of patients.
DlSCUSSlON
This paper shows the profiles of IL-2 and IFN-gamma levels depending on the occurrence of aGvHDand infection complications after allo-HSCT. The cytokine levels was assessed in the early period after allo-HSCT (within the first 30 d after transplantation). Before, during, and after blood sampling for cytokine determination, cytokine-producing cells were not stimulated with mitogenic substances such as lipopolysaccharide (LPS) or phytohemagglutinin (PHA). Therefore, the obtained cytokine levels and their profiles may be regarded as those which reflect real values in patients who underwent allo-HSCT. Low levels of IFN-gamma and IL-2 before and after allo-HSCT may be caused by severe pancytopenia after conditioning treatment and the use of antithymocyte globulin (ATG), which results in a deficiency of function and the production of cytokines. Low IFN-gamma and IL-2 levels may indicate successful immunosuppressive treatment to some extent. However, beginning with day 20 after allo-HSCT, the cytokine levels gradually increased with progressive reconstitution of the hematopoietic cells.
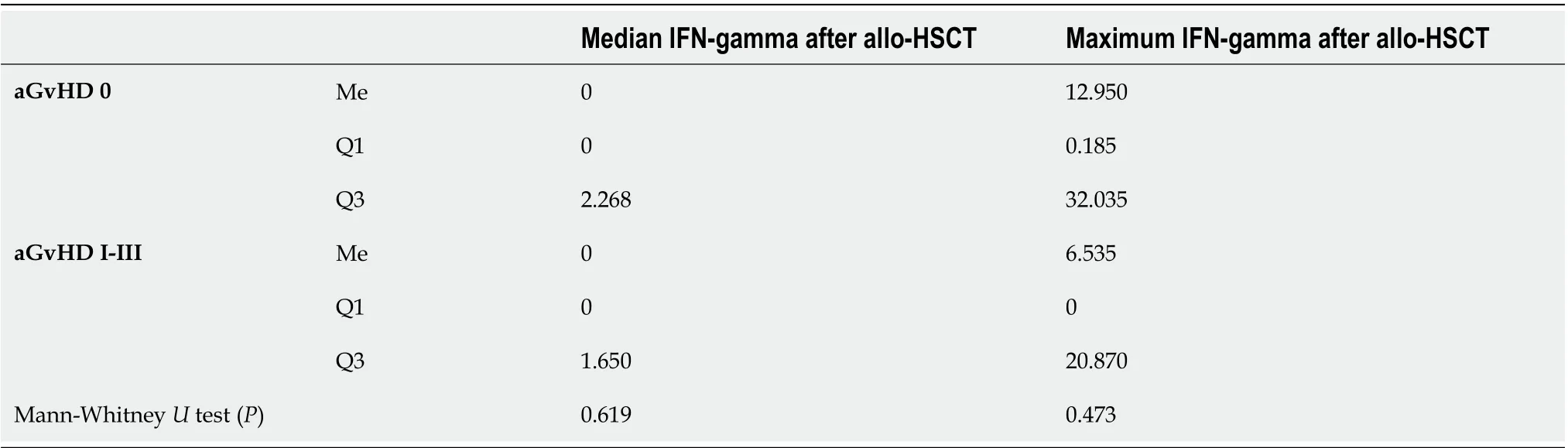
Table 2 Median and maximum values of serum levels of interferon-gamma (pg/mL) in patients with and without acute graft-versus-host disease in the post-transplantation period (between days +2 and +30)
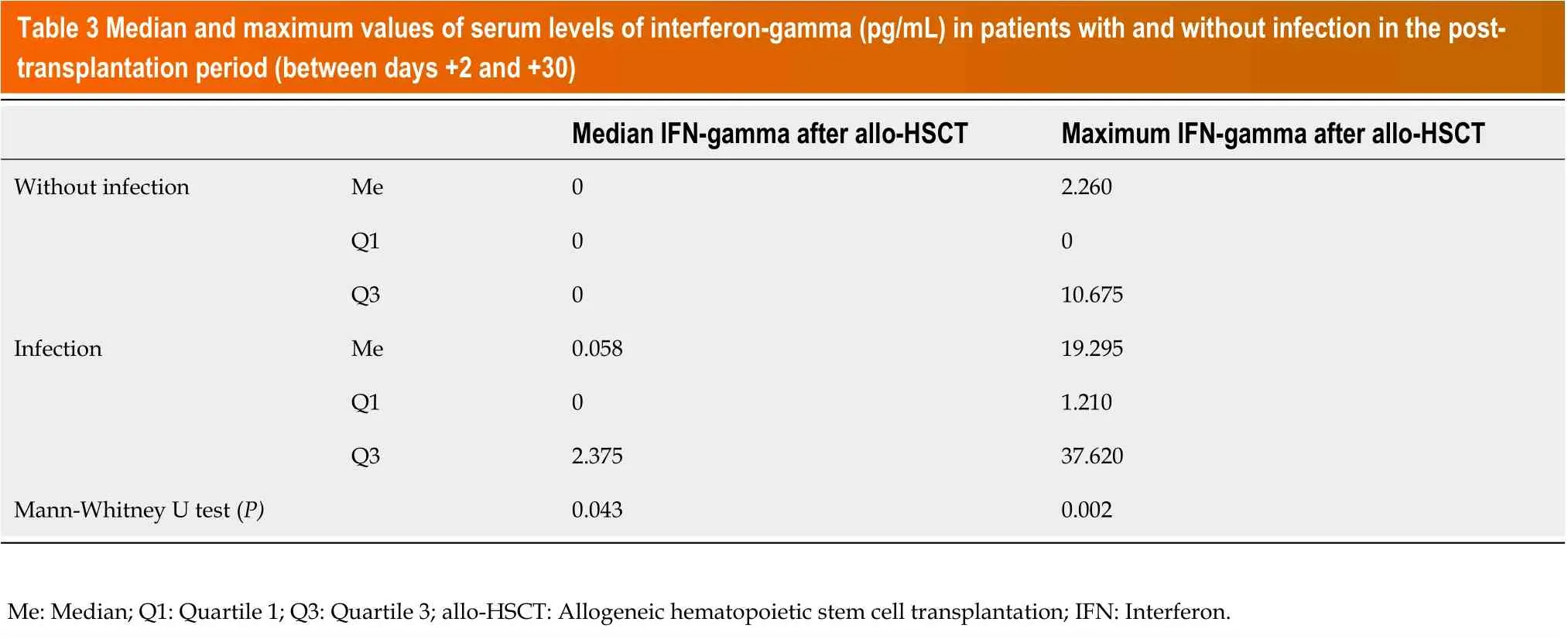
Table 3 Median and maximum values of serum levels of interferon-gamma (pg/mL) in patients with and without infection in the posttransplantation period (between days +2 and +30)Median lFN-gamma after allo-HSCT Maximum lFN-gamma after allo-HSCT Without infection Me 0 2.260 Q1 0 0 Q3 0 10.675 Infection Me 0.058 19.295 Q1 0 1.210 Q3 2.375 37.620 Mann-Whitney U test (P)0.043 0.002 Me: Median; Q1: Quartile 1; Q3: Quartile 3; allo-HSCT: Allogeneic hematopoietic stem cell transplantation; IFN: Interferon.

Table 4 Serum levels of interferon-gamma (pg/mL) in patients divided into groups based on the occurrence of acute graft-versus-host disease and infection
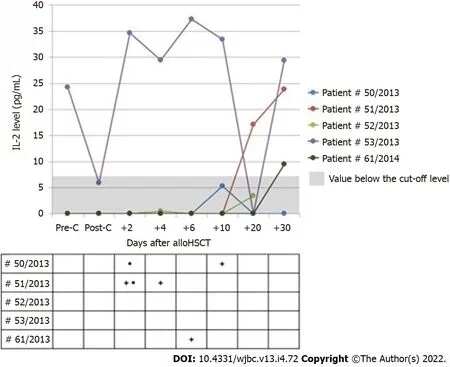
Figure 3 Serum levels of interleukin-2 in patients* with the value above 0 at least once in the post-transplantation period. *None of these patients presented with aGvHD. “+”: Day of the occurrence of infection independent of its etiology; “·”: Day of the occurrence of mucositis. Allo-HSCT: Allogeneic hematopoietic stem cell transplantation; IL-2: Interleukin-2; Pre-C: Before conditioning treatment; Post-C: After conditioning treatment.
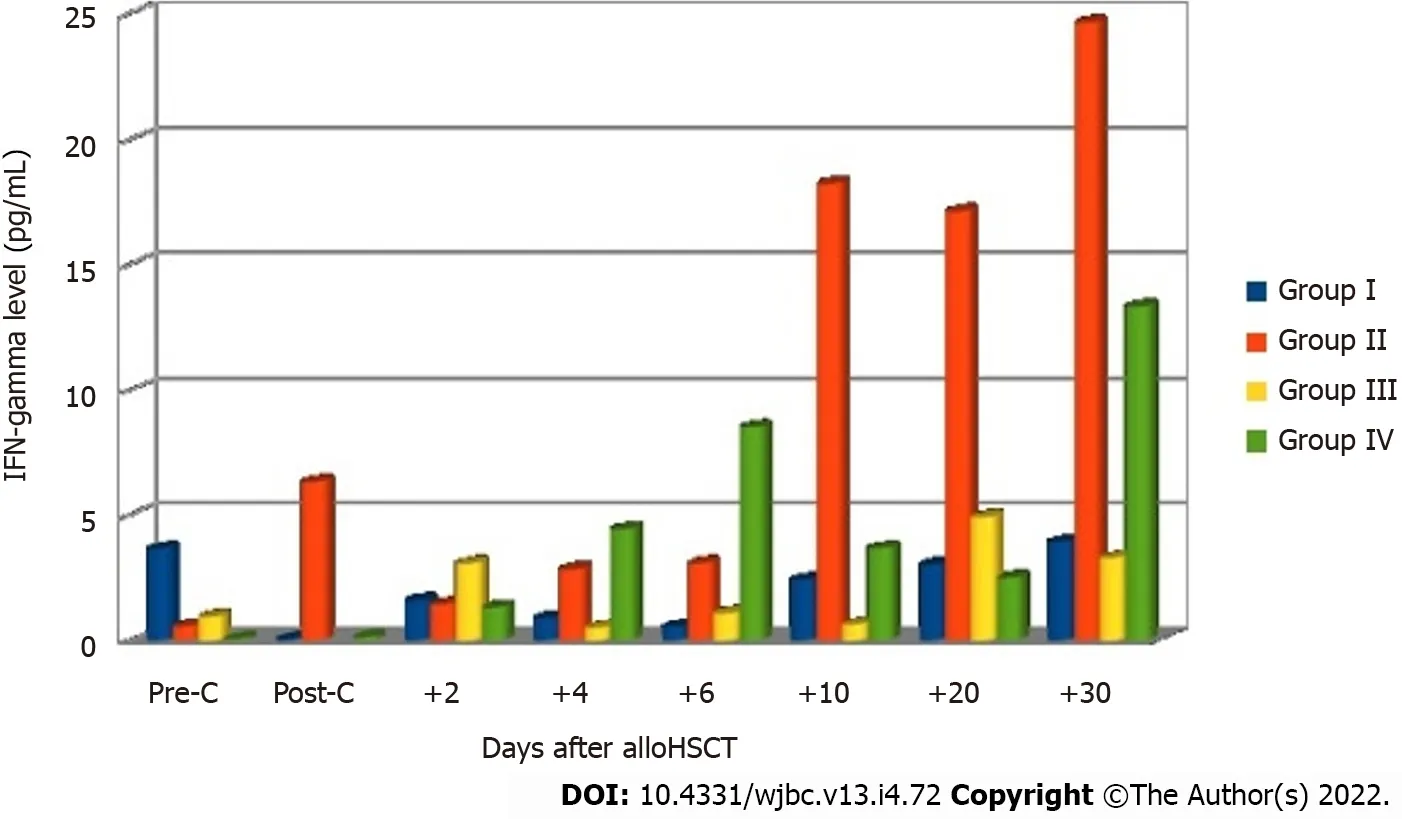
Figure 4 Mean levels of interferon-gamma before and after allogeneic hematopoietic stem cell transplantation in the four groups of patients. Allo-HSCT: Allogeneic hematopoietic stem cell transplantation; IFN: Interferon; Pre-C: Before conditioning treatment; Post-C: After conditioning treatment.
In the present study, the levels of the cytokines were low. In a small number of patients, they exceeded the value of the cut-off point, which was considered a positive result. This may be related to lymphocyte dysfunction caused by intensive acute myeloid leukemia (AML) treatment. In AML patients, T lymphocytes show genetic and phenotypic disorders, as well as impaired function and reduced cell count[8,9]. It was demonstrated that this could be related to an impaired function of the T lymphocyte receptor (TCR) (especially subunit ζ), whose damage causes impaired immunity in leukemia. In addition, T lymphocytes, especially with TCR Vβ, are not fully recovered after induction treatment in AML patients[10,11]. Moreover, long-term antigen stimulation of T lymphocytes leads to the “exhaustion” of these cells, which means that T lymphocytes lose their ability to secrete cytokines such as IL-2, TNF-α, and IFN-gamma, proliferate, and induce cytotoxic reactions[12-14].
Sadeghiet al[15] showed a murine model of aGvHD based on conditioning with high-dose chemotherapy. The animals underwent conditioning, and were divided into two groups. Group I comprised animals that underwent allogeneic transplantation, while group II included animals with syngeneic transplantation. The authors did not use immunosuppressive treatment in the posttransplantation period. The period of bone marrow cell regeneration was varied depending on the type of transplantation; in mice after syngeneic transplantation, it was more rapid with a shorter duration (onset of recovery on day +1, peak on day +5, and the end on day +21, while in mice after allogeneic transplantation, no complete recovery was reported by day +21). Analyzing the collected blood samples, the authors examined reconstitution of the immune cells of mice and the kinetics of IFNgamma, IL-2, and TNF-α in the early period after transplantation. The study showed that in mice after allogeneic transplantation, the proliferation and maturation of dendritic cells and CD8+ T lymphocytes of the donor were more rapid in comparison with the mice after syngeneic transplantation: Day +3 and from day +5 after transplantation, respectively. The study found a regular increase in all cytokine levels that corresponded with the rate of bone marrow cell recovery and the presence of dendritic cells and T lymphocytes in the circulation. The increase in the cytokine levels was higher in mice after allogeneic transplantation with aGvHD. While gradually increasing from low levels on the day of transplantation, the levels of the cytokines reached their peak on day +5 in mice that underwent allogeneic stem cell transplantation which developed aGvHD, followed by a decrease on subsequent days. No such phenomenon was observed in the animals after syngeneic transplantation.
In contrast to the present study, the authors of the above study based it on a murine model and did not include immunosuppressive treatment. Therefore, the kinetics of the cytokine level changes and reconstitution of bone marrow cells were deprived of the blocking effect of immunosuppressive agents on the immune cells and cytokine secretion. This is reflected by rapid recovery of bone marrow cells with a simultaneous increase in the cytokine levels. The study of Sadeghiet al[15] did not include the period before conditioning treatment, and the follow-up period was shorter (21 d after transplantation).
Juet al[16] analyzed the cytokine expression at a molecular level and the protein expression in 30 patients after allogeneic peripheral blood stem cell transplantation (allo-PBSCT). The group of patients was not homogeneous with regard to the disease being an indication for transplantation. A myeloablative regimen of Cy+VP16+TBI was used as conditioning treatment, while ciclosporin A + and methotrexate were used as aGvHD prevention. Blood samples were collected before allo-PBSCT, during the occurrence of the first aGvHD symptoms and after pharmacological control of the disease symptoms. The samples were incubated and a solution of lipopolysaccharide and PHA was added, and the levels of IFN-gamma, IL-2, IL-4, IL-10, IL-12, and IL-18 were measured using ELISA. Of 30 patients, 16 did not develop aGvHD, 7 presented with grade I aGvHD symptoms, and 7 presented with grades IIIV aGvHD. The expression of the cytokines, especially of IL-2 and IFN-gamma, was significantly higher in patients with aGvHD at the mRNA and protein levels. The expansion of cytokines was observed with increased severity of aGvHD, and a significant decrease was reported when aGvHD symptoms were controlled. In the above study, a correlation between the protein levels of the above cytokines and aGvHD symptoms was stronger than between their mRNA expression and disease symptoms.
Unlike the authors of this paper, the authors of the above report measured the cytokine levels after stimulation with LPS and PHA, which increase cytokine secretion and result in high concentrations. Moreover, despite standard immunosuppressive treatment, high IL-2 levels (> 100 pg/mL) were observed both in the group of patients with aGvHD and in the group of patients without aGvHD symptoms. In the present study, IL-2 levels were below the detection limit in almost all patients, regardless of the presence of aGvHD symptoms, and IFN-gamma levels were higher in the group without aGvHD symptoms. Despite using standard immunosuppression, high IL-2 levels in both groups of patients with or without aGvHD indicated a possible decisive effect of LPS and PHA stimulation of the immune cells on achieving high cytokine levels. For this reason, the real picture of the kinetics of IL-2 and IFN-gamma level changes in the post-transplantation period in patients developing aGvHD is unclear in the study of Juet al[16].
Visentaineret al[17] included 13 patients after allo-HSCT from fully matched donors in whom serum cytokine levels were determined within 15 wk after allo-HSCT using ELISA. Only the levels of IL-10 and the soluble receptor for IL-2 were significantly higher in the group of patients with aGvHD symptoms in comparison with those without. Moreover, the increase in the level of the soluble receptor for IL-2 had a direct correlation with implantation of bone marrow cells and onset of aGvHD symptoms.
In terms of the correlation between IFN-gamma and infection complications in patients after allo-HSCT, several available papers have shown contradictory results. In a study by Gayosoet al[18], a group of 26 patients were assessed for a correlation between the IFN-gamma level and the occurrence of cytomegalovirus (CMV) infection 6 mo after allo-HSCT. The prophylaxis of aGvHD included the use of cyclosporin and methotrexate in patients after myeloablative conditioning, and the use of cyclosporin with mycophenolate mofetil after RIC. If CMV reactivation was observed, patients were treated with valganciclovir. Blood samples were collected 6 mo after allo-HSCT. They were centrifuged and IFNgamma levels were determined in the supernatant using ELISA. The authors proved that in patients with CMV reactivation, IFN-gamma levels were higher (> 0.2 IU/mL) than those in the group where no reactivation was observed.
Penget al[19] analyzed the correlation between proinflammatory cytokines and the occurrence of invasive fungal infection in patients after allo-HSCT. Their analysis included 47 patients who underwent allo-HSCT due to various hematological diseases, and a control group (40 healthy volunteers). All the subjects were given myeloablative conditioning treatment, including 17 patients with ATG, and 30 patients without ATG. Immunosuppressive treatment for preventing aGvHD included tacrolimus with methotrexate and mycophenolate mofetil in 30 patients, and cyclosporin with methotrexate and mycophenolate mofetil in 17 patients. The levels of IL-6, IL-10, IFN-gamma, and TGFβ cytokines were determined using ELISA at 1, 2, and 3 mo after allo-HSCT. A comparison of the results in patients after allo-HSCT and the control group showed that IL-6 levels gradually increased and reached the peak value at 2 mo after allo-HSCT, and then decreased. At 3 mo, however, they were significantly higher in patients after allo-HSCT. Similarly, IL-10 levels increased in the posttransplantation period, while TGF-β levels gradually decreased. For IFN-gamma levels, no significant differences were observed between the groups. Moreover, there was no correlation between invasive fungal infection and changes in the concentrations of IFN-gamma and other cytokines.
In the above papers, their authors focused on showing a correlation between one selected infection complication and the concentration of specific proinflammatory cytokines. The study groups were not homogeneous with regard to the disease being an indication for allo-HSCT. Cytokine levels were determined in the late post-transplantation period with no regard to the effect of GvHD and other complications in the form of (bacterial) infections on the results. Contrary to our study, the above papers reflected the kinetics of cytokine level changes in a later period after allo-HSCT in relation to selected infection complications, without considering the early post-transplantation period. The present study showed a potential correlation between IFN-gamma levels and the occurrence of infection complications, while the findings reported in the above papers were inconsistent in this respect.
CONCLUSlON
Higher IFN-gamma levels are more related to infection complications than the occurrence of aGvHD. IFN-gamma levels in the group of patients with acute GvHD, in the group with acute GvHD and infection, and in the group without GvHD and infection are significantly lower than those in the group of patients with infection only. Within the first 30 d after allo-HSCT, serum IL-2 levels in patients with AML are very low and diagnostically insignificant.
ARTlCLE HlGHLlGHTS

Research conclusions
Higher IFN-gamma levels are more related to infectious complications than the occurrence of aGvHD. Within the first 30 d after allo-HSCT, serum levels of IL-2 in patients with AML are very low and diagnostically insignificant.
Research perspectives
More studies are warranted to create a more precise cytokine profile.
FOOTNOTES
Author contributions:Rybicka-Ramos M contributed to conceptualization, methodology, investigation, data curation and analysis, original draft preparation, project administration, and fund acquisition; Markiewicz M contributed to methodology, investigation, manuscript review and editing, supervision, and fund acquisition; Dzierzak-Mietla M and Bialas K contributed to investigation; Suszka-Świtek A, Wiaderkiewicz R, and Mizia S contributed to data curation and analysis.
lnstitutional review board statement:The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Medical University of Silesia in Katowice (No. KNW/0022/KB1/71/I/12).
Conflict-of-interest statement:All the authors declare no conflict of interest for this article.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Poland
ORClD number:Malwina Rybicka-Ramos 0000-0002-0159-8126.
S-Editor:Liu JH
L-Editor:Wang TQ
P-Editor:Liu JH
