Effect of vibration on interfacial microstructure and mechanical properties of Mg/Al bimetal prepared by a novel compound casting
2022-10-25FengGunWenmingJingGungyuLiJunwenZhuJunlongWngGuolingJieZitinFn
Feng Gun ,Wenming Jing,∗ ,Gungyu Li ,Junwen Zhu ,Junlong Wng ,Guoling Jie ,Zitin Fn
a State Key Laboratory of Materials Processing and Die &Mould Technology,School of Materials Science and Engineering,Huazhong University of Science and Technology,Wuhan 430074,China
b State Key Laboratory for Advanced Metals and Materials,University of Science and Technology Beijing,Beijing 100083,China
Abstract In this work,a vibration was applied in the preparation of the Mg/Al bimetal by a novel compound casting in order to improve the mechanical properties of the Mg/Al bimetal,and the effect of the vibration on the interfacial microstructure and mechanical properties of the Mg/Al bimetal was investigated.The results indicated that the vibration had a significant effect on the interfacial microstructure and mechanical properties of the Mg/Al bimetal,but it did not change the phase compositions of the interface,which was composed of layer I (Al3Mg2+Mg2Si),layer II (Al12Mg17+Mg2Si) and layer III (Al12Mg17/δ-Mg).Without vibration,the Mg2Si phase with a needle-like morphology mainly aggregated in the layer II of the interface.After the application of the vibration,the SEM and EBSD analysis results showed that the Mg2Si and Al3Mg2 phases in the interface were obviously refined,and the distribution of the Mg2Si became more uniform,due to the strong forced convection of the molten metal resulting from the vibration.The TEM analysis indicated that the interface between the Al3Mg2 and Mg2Si phases was non-coherent,suggesting the Mg2Si particles cannot act as a heterogeneous nucleation base during the solidification process of the interface.Compared to the Mg/Al bimetal without vibration,the shear strength of the Mg/Al bimetal with vibration increased by about 50% from 31.7 MPa on average to 47.5 MPa,and the hardness of the layer I of the interface increased,and the hardness of the layer III decreased.The fracture surface transformed from a flat fracture morphology without vibration to an irregular zigzag fracture morphology.
Keywords: Mg/Al bimetal;Vibration;Compound casting;Interfacial microstructure;Bonding properties.
1.Introduction
The magnesium (Mg)/aluminum(Al)bimetal fabricated by welding [1,2],rolling [3],casting [4,5],and other methods[6,7]has a great application potential in automobile,aviation,and aerospace fields [8,9],resulting from the outstanding advantages of the Mg alloy and Al alloy [10-12].However,the hard and brittle intermetallic compounds (IMCs) of Al12Mg17phase and Al3Mg2phase generated in the Mg/Al interface region,sharply decreasing the mechanical properties of the Mg/Al joint [13,14].That seriously limits the practical application of the Mg/Al bimetal in the industry.Many investigations have been executed to solve this problem[15-17].The addition of alloy elements,such as Zn [18],Sn [19],Cu [20],Fe [21],Ni [22],Ti [23],Ag [24],and Ce [25],have been widely applied in the fabrication of the Mg/Al bimetal by the welding methods,to improve the bonding strength of two kinds of different substrates Al and Mg.Guo et al.[26]added Zn as an interlayer in the ZK60Mg/5083Al bimetal joint prepared by vacuum diffusion bonding.The Mg2Zn phase formed at the interface inhibits the formation of the Mg-Al IMCs,and the addition of the Zn increases the shear strength of the joint from less than 5 MPa to 38.56 MPa.Chang et al.[27] added Ni foil with the thickness of 0.5 mm in friction stir welding the AZ31Mg/AA6061-T6Al to increase the tensile strength of the joint from 95 MPa to 115 MPa.The bringing of the alloy elements can result in the formation of the other binary or ternary IMCs,and eliminate the Mg-Al IMCs within the welding joint region.Finally,the bonding strength of the Mg/Al joint was improved.
In the compound casting field,researchers have also begun to pay more attention to studying the effect of other alloy elements on the properties of the bimetal [28,29].Usually,these alloy elements are added to the bimetal interface region as the interlayers.These interlayers can be divided into two types based on the difference in the melting point.One is the interlayer with a low melting point,such as Zn and Sn.The other is the interlayer with a high melting point,and the most commonly used one is the Ni.The interlayer consisting of low melting point alloy elements melts entirely during the compound casting process,and it inhibits the formation of the Mg-Al IMCs by forming new IMCs with better mechanical properties.Therefore,the bonding properties of the Mg/Al bimetal are improved.The interlayer,which consists of a high melting point,can prevent the direct contact of the Al insert and molten Mg.So,it inhibits the formation of the Mg-Al IMCs.Zhang et al.[30] used Zn as an interlayer to prevent the reoxidation of the Al substrate and improve the bonding properties of the Mg/Al bimetal.Renata Mola et al.[31] found that adding a Zn interlayer increased the bonding strength of AZ31/6060 bimetal.When a Zn interlayer was adopted,the bonding zone was mainly composed of Mg-Al-Zn phases instead of the Mg-Al phases,and the shear strength of the AZ31/6060 bimetal increased from 5.5 to 11.3 MPa to 39.8-46.6 MPa.Liu et al.[32] investigated the effect of the Ni interlayer prepared by plasma spraying on the mechanical properties of the Mg/Al bimetal,and discovered that the addition of the Ni interlayer improved the shear strength of the Mg/Al bimetal from 17.3 MPa to 25.4 MPa.
It should be pointed out that the most significant advantage of the compounding casting is that it is suitable for preparing the bimetal with complex geometries and large dimensions[33,34].However,compared with welding or rolling methods,more complicated and severe conditions will be suffered during the compound casting process [5,35].For example,controlling the manufacturing process of the compound casting is more complicated.It has a much heavier heat input,which will lead to a large amount of melt of the solid-state substrate,and make the melt diffuse into the molten metal[36-38].If the additive amount of the alloy elements remains the same,the concentration ratio of the elements will be much smaller at the Mg/Al interface.In the case of the thickness of the interlayer is not thick enough,it cannot effectively prevent the contact between the molten metal and the solid insert.To reach the same strength improvement of the Mg/Al joint,adding a large quantity of the interlayer material is necessary.It will cause a rise in cost and make the fabricatingprocess more complicated,which is a large barrier in applying the material [39,40].In addition,the interlayer’s preparation method also significantly influences the final bonding properties of the Mg/Al bimetal [41].Preparing a suitable alloy interlayer on the surface of the Al insert will be pretty complicated and significantly increase the cost.
Although many researchers are committed to improving the properties of the Mg/Al bimetal,the knowledge on the dependence of the microstructure on the mechanical properties of the Mg/Al interface is limited.The Mg/Al bimetal prepared by the compound casting method has a much lower cooling rate,which leads to the Mg-Al IMCs at the interface zone being coarser,significantly negatively impacting the performance of the Mg/Al interface [42].Generally,the thickness of the interface is on the millimeter scale[43,44],and its mechanical properties have a lot of room for enhancement by improving its interfacial microstructure.Current researches have proved that vibration can significantly strengthen the casting and welding products [45-47].Because it can refine the grain size and reduce the porosity defects [48].Moreover,the intense convection led by the vibration can also make the microstructure become uniform [49].In addition,because there is no need to add additional alloy elements,the vibration has the advantages of low cost,simple process,and environmental protection,and it is a very potential method to improve the properties of the Mg/Al bimetal.However,the research on the effect of the vibration on the interfacial microstructure and mechanical properties of the Mg/Al bimetal is still unexplored field.
The present study used a novel lost foam compound casting(LFCC)process to manufacture the AZ91D/A356 bimetal,and a mechanical vibration was applied in the compound casting process to improve the mechanical properties of the AZ91D/A356 bimetal.The effect of the vibration on the interfacial microstructure and mechanical properties of the AZ91D/A356 bimetal was investigated,and the influence mechanism of the vibration on the microstructure and bonding properties of the AZ91D/A356 bimetal was analyzed.
2.Experimental
2.1.Experimental materials
In this study,commercial A356 Al and AZ91D Mg alloys were chosen to prepare the Mg/Al bimetal.Table 1 lists their detailed chemical compositions.The expanded polystyrene(EPS) material with a density of 0.02 g/cm3was used to manufacture a foam pattern.Cylindrical rods with a diameterof 10 mm and a height of 110 mm were obtained from the A356 ingot by wire-electrode cutting method.The A356 rods were polished with silicon carbide sandpaper of 240 mesh,500 mesh,1000 mesh,and 2000 mesh in sequence.After that,these A356 rods were washed with acetone in the ultrasonic cleaners for 10 min,and they were then pickled with mixed acid to remove the oxide film on the surface.In addition,a thin layer of Zn was deposited by chemical deposition to avoid reoxidation of the surface of the A356 rods.The chemical formulation and specific parameters are listed in Table 2.Finally,the A356 rods were washed with absolute ethanol and then dried.
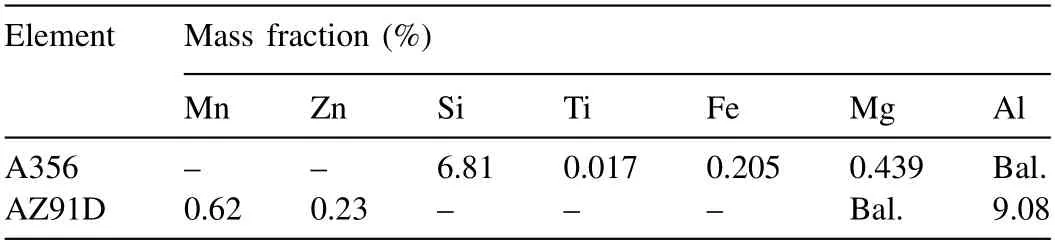
Table 1 Chemical compositions of A356 and AZ91D alloys.
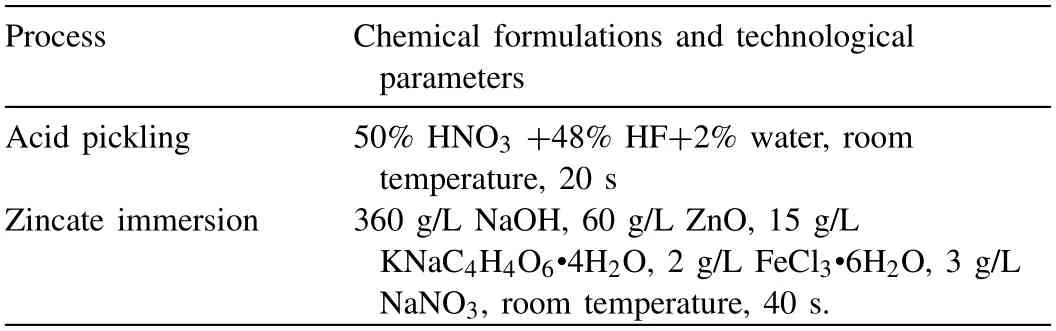
Table 2 Chemical formulations and technological parameters in the surface treatment process of the A356 inserts.

Table 3 Phase transition time during the solidification processes with and without vibration.
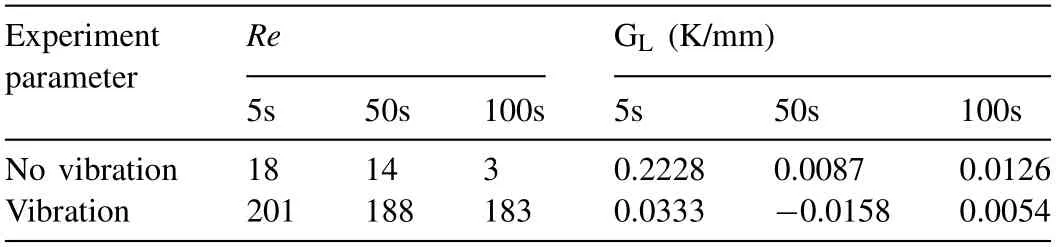
Table 4The Renold number (Re) of the flow field and the horizontal temperature gradient (GL) near the insert surfaces with and without vibration.

Fig.1.Schematic diagram of the experiment.
2.2.Compound casting process
The LFCC method was utilized to fabricate the Mg/Al bimetal.The A356 rods were first assembled with the EPS foam patterns.The foam patterns assembled with the Al inserts were immersed into a water-based refractory slurry,and then dried in an oven.Subsequently,it was placed within a sand flask which was later filled with unbonded loose sand.The loose sand was compacted using a vibration table and finally covered with a plastic film waiting for the pouring process.The schematic illustration of the experimental equipment in this work is illustrated in Fig.1.
The AZ91D Mg alloy was melted at 750 °C in an electric resistance furnace under a CO2-0.5% SF6protective gas mixture.The pouring temperature and vacuum degree were 730 °C and 0.03 MPa in the present work,respectively.The mechanical vibration with a frequency of 20 Hz and an amplitude of 0.17 mm was applied in the LFCC process through a vibrating table.The vibration was maintained for about 260 s after the completion of the pouring.Based on the current tests,these vibration parameters have produced the best results.This work mainly compared the results under these parameters with those without vibration.The same pouring temperature and vacuum degree parameters were used for casting under the condition without vibration,which was used as the control group in this experiment.
2.3.Microstructure and bonding properties analyses
The metallographic specimens were cut from the crosssection of the Mg/Al bimetal,as shown in Fig.2(a).Different areas of the same cross section were used for microstructure and mechanical properties analyses to evaluate the uniform effect of the vibration operation in the bimetal.The thermocouple was arranged on the surface of the Al insert in the middle of the Mg/Al bimetal,and the samples for the performance testing and observation were selected from the adjacent area above the temperature measuring point.The specimens were ground and polished before etching using a 4%nital solution.The macro surface morphology of the Mg/Al bimetallic interface was observed by an optical microscope(OM).A Quanta 200 scanning electron microscope (SEM)equipped with energy-dispersive X-ray spectroscopy (EDS)was used to analyze the interfacial microstructure and chemical component of the Mg/Al bimetal.Further investigation of the constitutive phases in the interface was performed using transmission electron microscopy (TEM;JEOL2100) and X-ray diffractometry (XRD) analyses.The specimens for the TEM analysis were prepared by the focused ion beam (FIB)technique.Electron backscatter diffraction(EBSD)scans were performed,and the analysis of the EBSD data was accomplished using Channel 5 software.For the EBSD testing,the specimens were polished with diamond suspension,and a final step of broad ion beam milling was used with the equipment of PECS II 685 to obtain a strain-free surface.In addition,the simulation software was used to simulate the flow and temperature fields of the solidification process of the Mg/Al bimetals with and without vibration.The Renold number (Re) of the flow field and the temperature gradient (GL)near the insert surface were calculated to assist the analysis of the microstructure of the bimetals.
The microhardness of the Mg/Al bimetal was measured using a TWVS-1 hardness tester with 300 g load and 15 s holding time.The bonding properties of the Mg/Al bimetal were tested by a push-out experiment in the ZwickZ100 universal testing machine,and the compression rate was 0.5 mm/min.The schematic diagram of the push-out test for shear strength testing is shown in Fig.2(b).The bonding properties were evaluated according to Eq.(1).

Where F is the maximum force loaded during the compression process,d is the diameter of the solid Al insert,and h is the height of the test specimen.The fracture behavior of the Mg/Al bimetal was analyzed using the OM and SEM methods.

Fig.2.(a) Schematic diagram of sampling position;(b) schematic diagram of the push-out test for the shear strength testing.
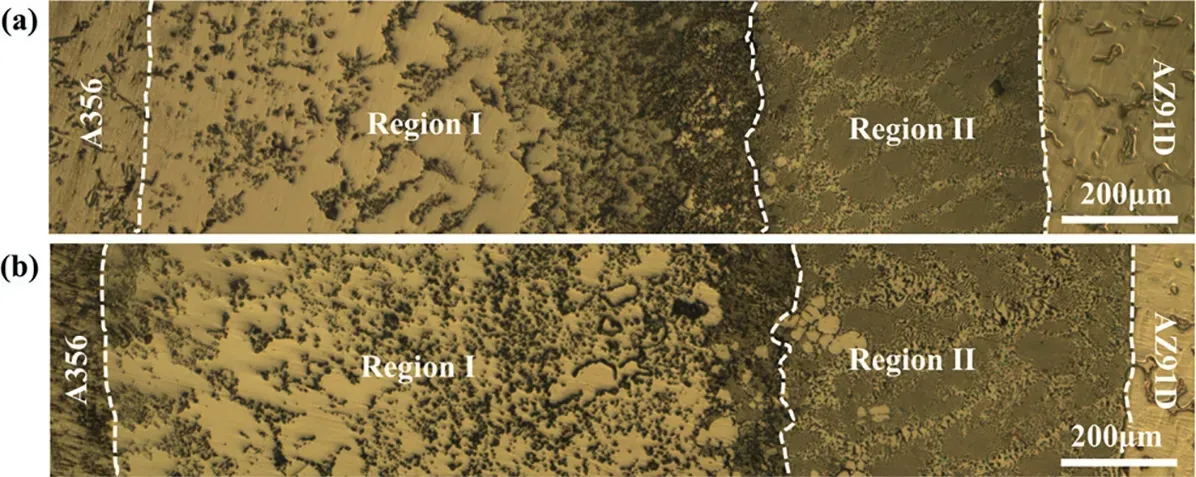
Fig.3.Optical morphologies of the Mg/Al bimetallic interfaces: (a) without vibration;(b) with vibration.
3.Results
3.1.The effect of vibration on the interfacial microstructure of the Mg/Al bimetal
The optical morphologies of the Mg/Al interface of the samples fabricated with and without vibration are shown in Fig.3.As can be seen,the interface can be divided into two regions according to its morphology.The region where the black phase is distributed is named region I.The region where there are few black phases is called region II.In the region II,the microstructure is composed of eutectic microstructure and primary dendrites.It is found that the distribution of the black phases in the region I is not uniform under no vibration condition,and it mainly gathers at the side near the region II.While the vibration was applied,the distribution of the black precipitates becomes more uniform,as shown in Fig.3(b).
Fig.4 shows the SEM image and the corresponding EDS mappings of the Mg/Al interface.The composition distributions of the Si,Al,and Mg elements are observed at the interface zone,showing that the rule of the distributions of the Al and Mg elements is uniform under the conditions with or without vibration.But,under the condition without vibration,the Si aggregates locally in the interface.For comparison,the distribution of the Si element becomes more uniform after applying the vibration,as shown in Fig.4(b).Therefore,it suggests that the assistance of the vibration can reduce the component segregation at the Mg/Al interface region,which may positively affect the microstructure and bonding properties of the Mg/Al bimetal.
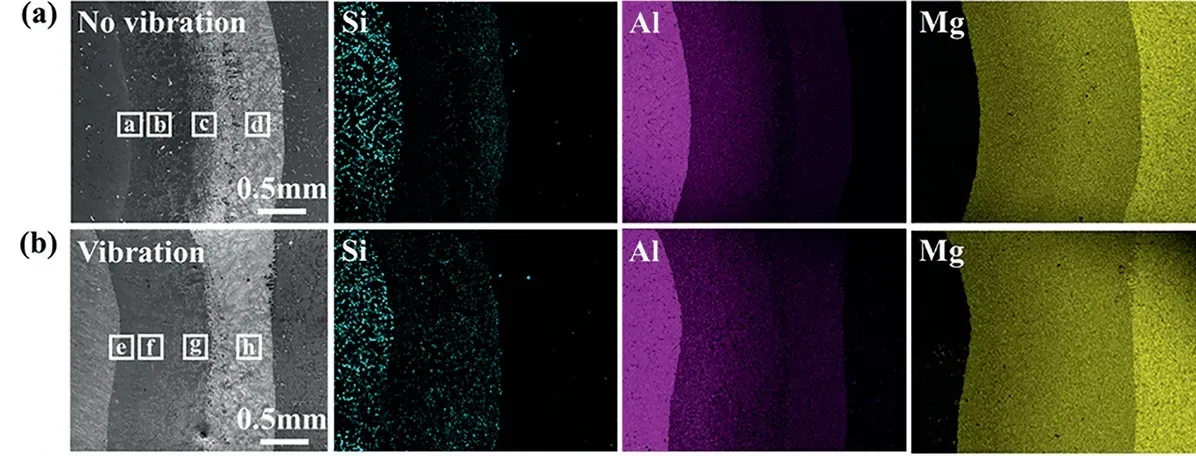
Fig.4.EDS mapping analysis results of the Mg/Al bimetallic interfaces: (a)without vibration;(b) with vibration.
In addition,four regions,marked in Fig.4(a) and (b),were chosen for more detailed observation and compositional analysis from the Mg/Al interface region.Observation images of the corresponding areas are shown in Fig.5,marked by the corresponding letters.According to the EDS point analysis results shown in Fig.5,as well as the Al-Mg and Mg-Si binary phase diagrams [43,50],the interfaces with and without vibration are mainly composed ofδ-Mg phase,and IMCs including Al3Mg2and Al12Mg17phases,and Mg2Si phase dispersed at the region adjacent to the A356 substrate.The XRD testing result,shown in Fig.7,also confirms that the Mg/Al bimetallic interface is mainly composed of the Al3Mg2,Al12Mg17,and Mg2Si phases.The result is consistent with the XRD analysis results in our previous study[51].The research of Emami also confirmed the existence of the Al3Mg2and Al12Mg17phases in the Mg/Al bimetallic interface [43].Thus,the interface region can be divided into three layers: layer I (Al3Mg2+Mg2Si phase) and layer II(Al12Mg17+Mg2Si phase),and layer III (Al12Mg17/δ-Mg eutectic).Fig.5(a)shows that the Mg2Si phase is mainly needlelike or worm-like in the layer I under no vibration condition.But,the morphology of the Mg2Si phase becomes much more round under the vibration,as shown in Fig.5(e).Fig.5(c)and(g) indicate that the layer II is mainly composed of the gray Al12Mg17matrix and dispersive granular dark Mg2Si phase whose morphology shows little difference.As for the eutectic tissue shown in Fig.5(d) and (h),no significant change is observed,regardless of whether the vibration was applied.Moreover,the morphology and size of the Mg2Si phase in the layer I were quantitatively analyzed by three parameters of the length,width and aspect ratio,as shown in Fig.6.It can be found that the length and the aspect ratio of the Mg2Si phase under the vibration significantly decrease compared to the no vibration condition,indicating that the size and morphology of the Mg2Si phase in the layer I are remarkably improved by the vibration.

Fig.5.SEM images of the microstructures of the interface layers with and without vibration: (a-d) SEM microstructures without vibration corresponding to the region a-d marked in Fig.4,respectively;(e-h) SEM microstructures with vibration corresponding to the region e-h marked in Fig.4,respectively.
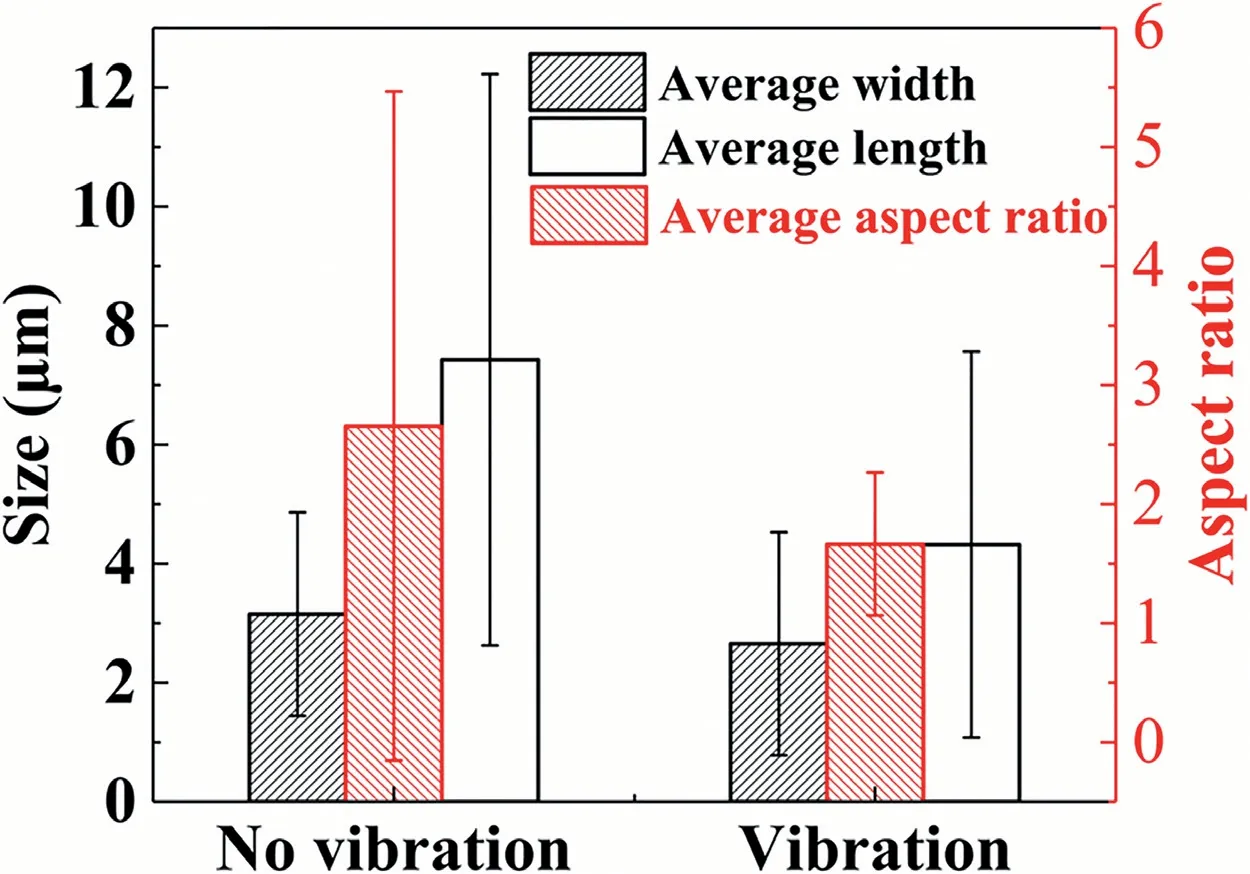
Fig.6.Quantitative analysis of the Mg2Si phase in the layer I of the interfaces with and without vibration.

Fig.7.XRD analysis result of the Mg/Al bimetallic interface with vibration.

Fig.8.TEM analysis images of the Mg/Al interfaces with and without vibration: (a) and (b) TEM bright-field images of the layer I without vibration;(c),(f),and (h) SAED patterns of the Mg2Si,Al3Mg2, and Al18Mg3Mn2 phases in (b);(d),(e) and (g) high-resolution TEM images of the region marked with letters A,B,and C in (b);(i) TEM bright-field image of the layer I with vibration.
To further confirm the phase composition of the Mg/Al bimetallic interface,the Mg/Al interface was observed by the transmission electron microscopy (TEM).The results are shown in Fig.8.Fig.8(a) is a TEM bright-field image in the layer I without vibration.It can be seen that the Mg2Si particles aggregate into clusters,and obvious black dislocation defects are observed in the Mg2Si particles.After the vibration was applied,the sizes of the Mg2Si particles and clusters are reduced,as shown in Fig.8(i).The areas marked in Fig.8(b) were then selected to analyze the composition of the phase and the orientation relationship among different phases in the layer I.The analysis of the diffraction spots of the selected area electron diffraction shown in Fig.8(c),(f),and(h) shows that the layer I is composed of the Al3Mg2,Mg2Si and Al18Mg3Mn2phases.According to the high-resolution transmission electron microscope (HRTEM) images shown in Fig.8(c) and (h),it is found that there is an orientation relationship ofoccurs between the Mg2Si and Al18Mg3Mn2particles.Correspondingly,it can be observed from Fig.8(b) that a straight interface is formed between the Al18Mg3Mn2and Mg2Si particles.The orientation relationships of the Mg2Si-Mg2Si grain boundary and the Mg2Si-Al3Mg2phase boundary were also studied.Fig.8(d)is an HRTEM image of the grain boundary formed by the contact of the Mg2Si particles.The grain boundary is parallel to theplane of the Mg2Si phase.Atoms tend to overlap with crystal planes with high binding energy.Among the {100},{110},{111} crystal planes of the Mg2Si crystal,the {100} crystal plane has the lowest surface density,and the {111} crystal plane is the dense row plane.Considering the attachment energy for new atoms at faces in the Mg2Si crystal,the crystal face with high binding energy is the plane atoms prefer to pack on [52].As a result,the growth rate of the crystal planes in the Mg2Si crystal is {100},{110},{111} crystal planes from fast to slow.Since the {100} crystal plane has the fastest growth rate,during the growth of the Mg2Si particles,it will first come into contact with other particles and eventually form a grain boundary parallel to the{100} crystal plane.Fig.8(e) and (g) are HRTEM images of the grain boundary between the Mg2Si and Al3Mg2substrate.In region B,shown in Fig.8(g),the lattice mismatchδbetween thecrystal planes is about 85%.In region C,shown in Fig.8(e),it is about 39% between.These results indicate that the lattice mismatchδbetween the Al3Mg2and Mg2Si phases is far more larger than 25%,so the interface between the two phases is non-coherent.It also means that the Mg2Si particles cannot act as a heterogeneous nucleation base during the solidification process of the interface.Consequently,it is difficult to achieve the effect of refining the interfacial microstructure through the heterogeneous nucleation way.
Fig.9(a) and (c) show the orientation distributions of the Mg/Al interfaces with and without vibration using the EBSD method.It can be found that the growth of the microstructure in the interface region is significantly directional.In the layers I and II without vibration,coarse grains are observed in the interface,exhibiting an apparent characteristic of directional solidification,as shown in Fig.9(a).The reason is that the chilling effect of the Al insert leads to the generation of a temperature gradient perpendicular to its surface.Under the vibration condition,the microstructure of the layer I changes significantly,as shown in Fig.9(c).The grain size of the Al3Mg2phase is decreased,and it still has the characteristic of columnar crystals.But the growth direction has changed from horizontal growth to upward growth.In addition,the morphology of the crystal grains of the layer I becomes more irregular,and the tip of the crystal grain appears an obvious bifurcation.
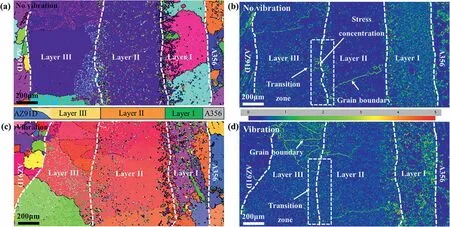
Fig.9.Orientation distributions and stress maps of the Mg/Al bimetallic interfaces with and without vibration obtained by EBSD test: (a,b) orientation distribution and stress map without vibration,respectively;(c,d) orientation distribution and stress map with vibration,respectively.
Fig.9(b) and (d) show the stress distribution in the Mg/Al interface area.Except for the observed stress concentration at the grain boundary position in the interface,as shown in Fig.9(b) and (d),the results also show a slight stress concentration at the transition zone between the layer II and layer III without vibration,as shown in Fig.9(b).After applying vibration,the stress concentration at the transition zone disappears,as shown in Fig.9(d).Since the thermal expansion coefficient of the Mg2Si is smaller than that of the substrate,considerable residual stress is generated in the interface region,which induces a large amount of misfit dislocation.In the absence of the vibration,the Mg2Si phase accumulates in this area,resulting in the stress concentration.After the application of vibration,the Mg2Si phase accumulated in the transition zone is evenly dispersed in the layer II and layer III.Therefore,the stress concentration at the transition zone disappears.
3.2.The effect of vibration on the mechanical properties of the Mg/Al bimetal
The Vickers-hardnesses of the Mg/Al bimetallic interfaces obtained with and without vibration are shown in Fig.10(a).The results show that under the vibration,the hardness of the A356 and AZ91D substrate does not change significantly.However,the vibration has a significant effect on the hardness of the interface.The hardness of the layer I obviously increases under the vibration,due to the refinement and uniform distribution of the Mg2Si phase in the IMCs layer.Furthermore,the vibration can also promote the diffusion in the molten metal by the vigorous convection [53] and make the solid solution of the Al inδ-Mg decrease,as shown in EDS results in Fig.5,resulting in a reduction of the hardness of the layer III.The results of the shear testing are shown in Fig.10(b).As can be seen,the shear strength of the Mg/Al bimetal increases significantly from 31.7 MPa to 47.5 MPa,with an increase of about 50% when the vibration was applied.
The morphologies of the shear fractures of the Mg/Al bimetals with and without vibration were observed,as shown in Fig.11.Fig.11(a) shows the morphology of the shear fracture on the side of the Mg substrate under no vibration condition.It is found that the crack first penetrates the entire IMCs layers and then expands along with the interface between the layers II and III,and the morphology of the fracture is relatively flat.While the vibration was applied,the morphology of the shear fracture changes significantly,as shown in Fig.11(b).Under the vibration condition,when the fracture occurs,the crack only expands in the IMCs layers,and the direction of the crack propagation is deflected during this process.The fracture surface exhibits an irregular zigzag morphology.In addition,many coarse and fine cracks can be observed in the IMCs layers.It means that the application of the vibration can significantly increase the length of the crack propagation path during the fracture process,which will absorb and consume more energy.
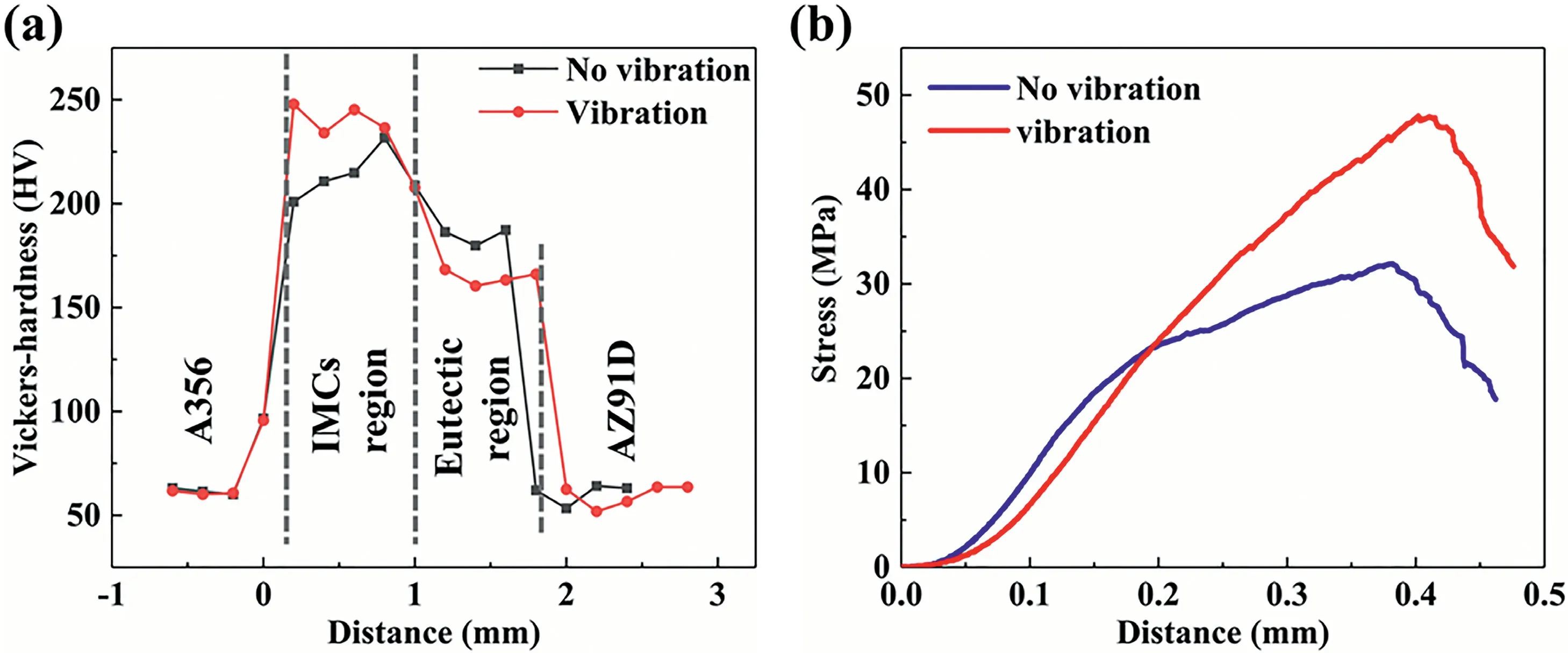
Fig.10.The mechanical properties of the Mg/Al bimetals with and without vibration: (a) Vickers-hardnesses of the Mg/Al bimetallic interfaces;(b) bonding properties of the Mg/Al bimetals.
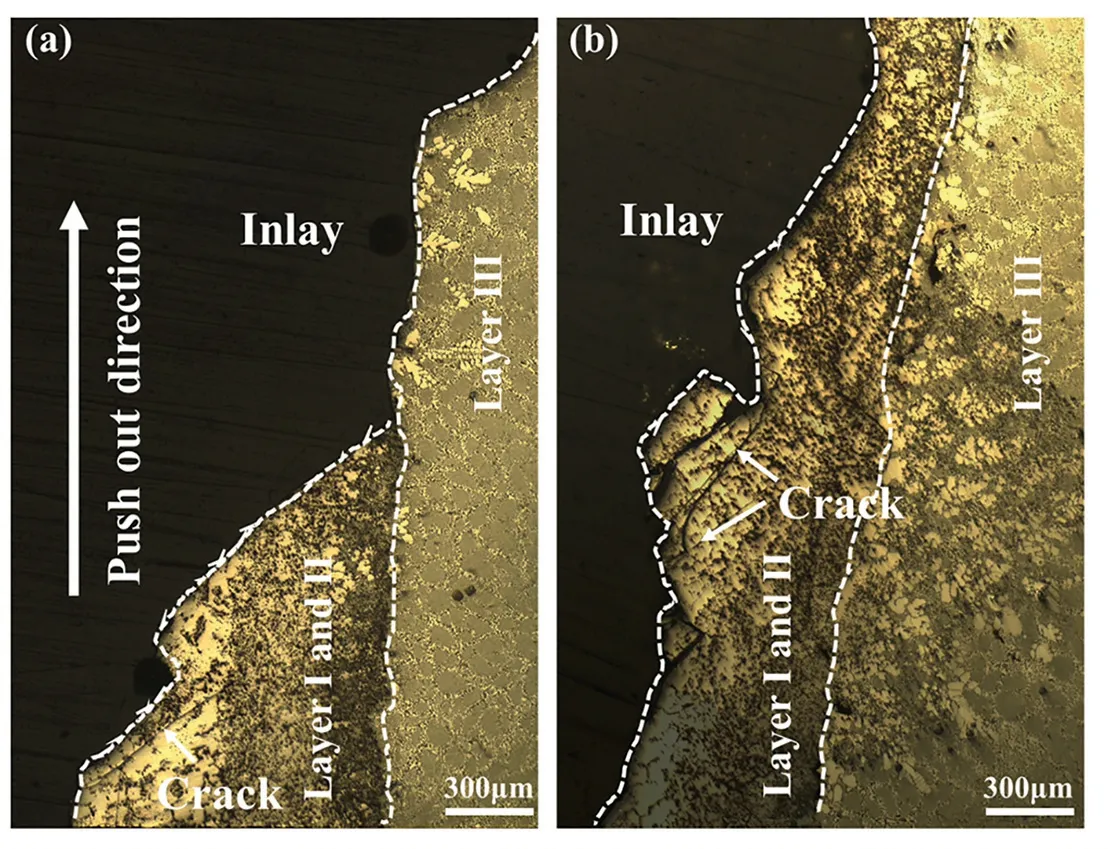
Fig.11.The morphologies of the shear fractures on the Mg substrate sides of the Mg/Al bimetals with and without vibration: (a) without vibration;(b)with vibration.
Furthermore,the micrographs of the fracture surfaces were also observed by the SEM,as shown in Fig.12.According to the EDS point analysis,the component of the phases that exist in the fracture surface are marked in Fig.12.No matter whether or not the vibration was applied,the plentiful cleavage planes and river patterns are observed on the fracture surfaces of the Mg/Al bimetals,displaying a typical brittle fracture characteristic.Under the condition of no vibration,an obvious step between the upper and lower parts of the fracture surface is shown in Fig.12(a).It is consistent with the phenomenon observed in Fig.11(a) that the cracks penetrate rapidly through the layer I and layer II,and then expand along with the interface between the layer II and layer III during the fracture process.Fig.12(b) and (d) show that theδ-Mg and Al12Mg17are detected on the fracture surface of the Al and Mg sides without vibration,respectively.Theδ-Mg exists in the eutectic tissue,according to the interfacial microstructure of the Mg/Al bimetal.So theδ-Mg and Al12Mg17marked in Fig.12(b) and (d) may come from the eutectic tissue in the transition zone between layers II and III,indicating that the Mg/Al bimetal without vibration may crack along the transition zone.Fig.12(f) and (h) show that the Al3Mg2and Al12Mg17are observed on the fracture surface of the Al and Mg sides with vibration,respectively.In the Mg/Al bimetallic interface,the Al3Mg2and Al12Mg17can be observed at the entire layer II and layer III.It indicates that both the layer II and layer III crack during the fracture process.These results further prove that the crack propagation paths are changed with the application of the vibration.
4.Discussion
The solidification curve of the Mg/Al bimetal was measured by the thermocouple on the surface of the Al insert,which can be divided into three stages,named stage I,II,and III,as shown in Fig.13(a).The stage I refers to a heating stage,and the Al insert begins to come into contact with the molten metal.The solidification curves show a rapid increase at this stage.In the stage II,the interface region is in a relatively higher temperature range,and it is named the high-temperature stage.The stage III is the stage where the solidification reaction occurs intensively.

Fig.12.SEM micrographs of the fracture surfaces of the Mg/Al bimetals with and without vibration after the push-out testing: (a) and (c) macro morphologies of the shear fracture surface on the Al substrate and Mg substrate sides without vibration,respectively;(e) and (g) macro morphologies of the shear fracture surface on the Al substrate and Mg substrate sides with vibration,respectively;(b),(d),(f),and (h) are the high magnification micrographs of the samples corresponding to the samples observed in (a),(c),(e),(g),respectively.
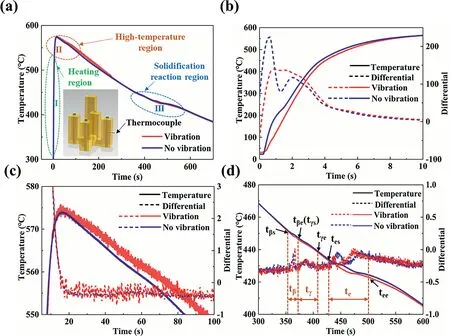
Fig.13.(a) Results of thermocouple measurement;(b-d) temperature curves and first-order differential curve in the region I,II,III in (a),respectively.
As the stage I shown in Fig.13(b),due to the chilling effect of the Al insert,a downward peak appears on the differential curve without vibration.However,only a slight decrease occurs on the differential curve with vibration,which means that the vibration may significantly improve the heating transfer process at the solid-liquid contact interface and weaken the chilling effect of the insert.In addition,previous research has shown that under the vibration,the disturbance and convection of the molten metal promote the heat exchange at the solid-liquid interface [54],which makes the temperature of the Mg/Al interface become unstable,as shown in Fig.13(c).In this case,the strong forced convection of the molten metal resulting from the vibration will cause the fragmentation of the first precipitated Mg2Si phase.Consequently,the size of the Mg2Si phase is refined,and the needle-like Mg2Si phase is broken and its distribution becomes more uniform.According to the results of Haq et al.[55],an increase in the derivate curve means the formation of a new phase,and the derivative decreases when the phase transformation ends.As the temperature decreases,the Al3Mg2,Al12Mg17phases,and the Al12Mg17/δ-Mg eutectic begin to form at the Mg/Al interface,which may create a series of ascending peaks on the differential curve,as shown in Fig.13(d).According to the Al-Mg binary phase diagram and the microstructure of the Mg/Al bimetallic interface,the times for the formation of the layer I,layer II and layer III may respectively respond to tβ,tγ,te.The phase transition times of these reactions are measured and summarized in the Table 3.The result shows that the reaction time decreases significantly after applying the vibration,and it suggests that the solidification rate of the bimetallic interface increases under the vibration.
Fig.14 shows the temperature and flow fields of the Mg/Al bimetals with and without vibration during the solidification process at 5 s,50 s,and 100 s obtained by the simulation method.The Renold number (Re) of the flow field and the horizontal temperature gradient (GL) near the insert surface were also calculated,and the results are shown in Table 4.
The Reynold numbers of the natural convection flow field can be calculated with the Eq.(2):

Wherelis half the distance between the insert and liquid surface,vis the maximum velocity of the molten metal near the surface of the insert,ρis the density of the molten metal,andηis the dynamic viscosity of the molten metal.
Fig.14(a)indicates that a large horizontal temperature gradient (0.2228 K/mm near the insert surface) is formed in the melt without vibration due to the chilling effect of the solid insert at 5 s,resulting in a strong natural convection with theReof 18,5 s after the cooling process begins,as shown in Fig.14(d).With the homogenization of the temperature field in the horizontal direction at 50 s and 100 s,as shown in Fig.14(b) and (c),the flow rate of the natural convection slows down rapidly,as shown in Fig.14(e) and (f),with theReof the flow field decreasing from 18 to 14 and 3.
When the vibration was applied,it can be found that the temperature gradient in the bimetal decreases by measuring the difference between the maximum and minimum values in the temperature field,as shown in Fig.14 (g-i).It points out that the temperature field of the Mg/Al bimetal becomes more uniform.Moreover,when it comes to 100 s,Fig.14(c) and(i) show a similar geometric shape for the temperature fields with and without vibration,while their temperature distributions are quite different before that time.The results suggest that the temperature field in the bimetal may converge to a certain stable state under the vibration parameters used in the experiment.At this time,the horizontal temperature gradient near the surface of the insert is 0.0054 K/mm,which is lower than 0.0126 K/mm without vibration.
The flow field illustrates that the flow velocity of the molten metal increases significantly under the vibration condition,as shown in Fig.14 (j-l).Here,theReis also calculated with Eq.(2),and the results are shown in the Table 4.But in this case,vis the average velocity of the flow field,andlis the distance between the solid surface and the liquid surface.The result shows that theReof the flow field can be maintained in a relatively stable range of about 180,supporting that the intensity of the convection in the molten metal is enhanced and sustained long after applying the vibration.As a result,the solidification of the Mg/Al bimetal may be significantly influenced by the changing of constitutional undercooling conditions caused by the vibration.
When the mechanical vibration has been applied in the solidification process,the refinement of the Al3Mg2phase can be explained by the following mechanisms.First,the forced convection induced by the vibration inserts external forces on the dendrite arms in flow direction[56].As a result,the initial solidified fine dendrites forming on surface of the A356 insert can be broken off by the vibrating forces from the vibration.Meanwhile,the dendrite arms can be carried by the forced convection into the melt,acting as new nuclei,resulting in the refinement of the microstructure.
On the other hand,the microstructure is also influenced by the growth condition.The constitutional undercooling has a significant influence on the growth process of the solid-liquid interface.The temperature gradient (GL) and the solid-liquid interface growth rate (R) are two main factors affecting the interface growth.Here,two assumptions are made to simplify the analysis.(i) The diffusion in the solid is negligible;(ii) The convection in the solid-liquid interface is negligible.It means that the diffusion is the principal solute transport mechanism because the laminar boundary layer in the liquid close to the solid-liquid interface may be the same order of magnitude as the diffusion layer surrounding the growing grain [57].So,the conditions for the occurrence of the constitutional undercooling can be expressed by the Eq.(3).

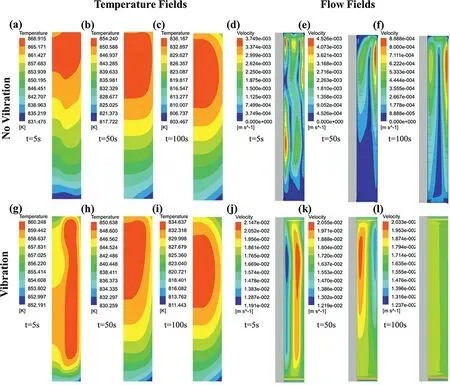
Fig.14.Simulation results of the temperature and flow fields of the Mg/Al bimetals with and without vibration during the solidification process at 5 s,50 s,and 100 s: (a-c) temperature field without vibration at 5 s,50 s,100 s,respectively;(d-f) flow field without vibration at 5 s,50 s,100 s,respectively;(g-i)temperature field with vibration at 5 s,50 s,100 s,respectively;(j-l) flow field with vibration at 5 s,50 s,100 s,respectively.
WheremLis the slope of the liquidus,C0is the concentration of the solute in the liquid,kis the distribution coefficient,andDLis the diffusion coefficients in the liquid.
It can be seen from Table 3 that the phase transition time of the Al3Mg2(tβ) decreases significantly after applying the vibration.It suggests that the growth rate of the layer I increases under the vibration.The result of Table 4 also indicates that the horizontal temperature gradient near the surface of the insert decreases under the stable state,after the application of vibration.With the decreasing of the GLand the increasing of the R,the constitutional undercooling is enhanced after applying the vibration,which will reduce the grain size of the layer I.As a result,the microstructure of the layer I is refined under the combined influence of the vibrating forces that can break initial solidified fine dendrites and the constitutional undercooling,thereby improving the performance of the Mg/Al bimetal.
Fig.15(a) and (b) show the orientation distribution of the layer I obtained by the EBSD.As can be seen,the irregular Mg2Si phase distributed on the interface is composed of fine Mg2Si particles exhibiting aggregation morphology.The research indicated that the morphology of primary Si will have an important influence on the Mg2Si,formed during the solid-liquid compound casting process [42].
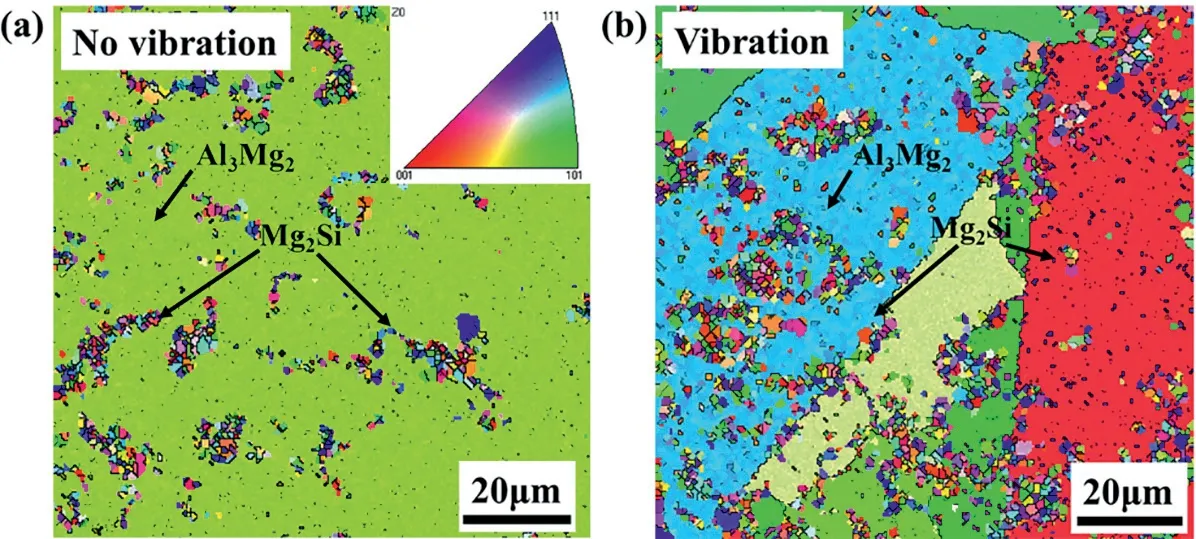
Fig.15.Orientation distributions of the layer I with and without vibration obtained by the EBSD.
Fig.16 shows the effect mechanism of the morphology of the Mg2Si phase under vibration.The molten metal first decomposes the foam,and then fills its position during the casting process.Under a high temperature condition,the mutual diffusion of the Al and Mg elements occurs between the solid Al insert and the molten Mg alloy,which will change the composition of the insert surface and decrease the melting point of the insert surface [58].The insert melts to form a molten pool on the surface of the solid insert,as shown in Fig.16(a).A diffusion zone then occurs near the insert surface,and the unmelted silicon on the A356 substrate may enter the molten metal,as shown in Fig.16(b).Subsequently,the Si element will diffusion into the melt and react with the Mg element to form the Mg2Si phase,as shown in Fig.16(c).As a result,many fine Mg2Si particles can be observed,as shown in Fig.5(a) and (b),under the condition of no vibration.When the vibration was applied,the flow velocity and shear interaction between the fluid and solid in the molten metal are enhanced.Thus,the Mg2Si is not easy to agglomerate in the interface layer,but tends to be uniformly distributed in small and dispersed particles,as shown in Fig.12(d) and(e).It was beneficial to strengthen the performance of the interface layer because the refined Mg2Si phase may produce more minor splitting and stress concentration in the nearby substrate.Thus,the shear strength of the Mg/Al bimetal is enhanced.

Fig.16.Effect mechanism of the morphology of the Mg2Si phase under vibration: (a) filling process;(b) needle-like Si frees into the diffusion layer;(c) Si element diffuse into the melt and react with the Mg element to form Mg2Si;(d) the agglomerated Mg2Si particle is dispersed and broken;(e) the distribution of Mg2Si becomes homogeneous;(f) interface layer forms.
The Vickers-hardness was tested,and the indentations were observed to further investigate the reason for the improvement of the shear strength of the Mg/Al bimetallic interface.The crack growth near the indentation was analyzed,and the microstructures near the indentations were observed,as shown in Fig.17.The region shown in Fig.17 is divided into three areas,the layer I,the layer II,and the transition zone between them.According to the result of the sample without vibration,the transverse propagation length of the crack at the middle two indentations is significantly longer than that of the longitudinal,as shown in Fig.17(a).The reason may be that the IMCs in the layer I and layer II grow preferentially along the temperature gradient direction,which causes a significant anisotropy of the performance of the interface.That may explain why the cracks are quickly deflected to the side of the Mg matrix.In addition,there is apparent stress concentration in the area between the layer II and III without vibration,and it makes this area relatively weak.Then,the crack propagates along the stress concentration area when it achieves there,leading to a decrease of the shear strength of the Mg/Al bimetal.
Compared with Fig.17(a) and (b),it shows that the cracking situation is improved under the vibration,and the length of the crack is remarkably reduced.Further observation of the crack,as shown in the high magnification pictures in the lower-left corner of Fig.17,indicates that the Mg2Si makes the crack growth path deflect and bend,increasing the energy consume in the process of the cracking and reducing the length of the cracks.After applying the vibration,the grain size of the Al3Mg2phase in the layer I is refined,and the size of the Mg2Si phase is also refined,and the needle-like Mg2Si phase is broken.The distributions of them become more uniform.Thus,the anisotropy of the performance is weakened.Finally,the fine dispersed Mg2Si phase and refined Al3Mg2phase improve the interface performance by making the crack deflect and bend.
5.Conclusion
In the current investigation,the effect of the mechanical vibration on the interfacial microstructure and mechanical properties of the Mg/Al bimetal fabricated by the LFCC process was investigated.The obtained results can be summarized as follows.
(1) With the application of the vibration during the solidification of the Mg/Al bimetal using the LFCC process,the Mg2Si and Al3Mg2phases in the Mg/Al bimetallic interface were significantly refined,and the morphology of the Mg2Si particle was changed from needle-like to granular,exhibiting an uniform distribution in the whole interface.
(2) The hardness of the layer I in the Mg/Al bimetallic interface increased due to the uniform distribution of the Mg2Si phase under the vibration condition.The hardness of the layer III decreased,resulting from the decrease of the solid solution of the Al in theδ-Mg.The shear strength of the Mg/Al bimetal with vibration increased significantly from 31.7 MPa to 47.5 MPa,with an increase of about 50%.
(3) When the vibration was applied,the molten metal was subjected to a violent impact,which caused the forced convection in the molten metal.The initial solidified fine dendrites formed on the surface of the Al insert was broken by the vibrating forces from the vibration.The strong forced convection promoted the decrease of the temperature gradient and increased the solidification rate of the bimetal,resulting in the increase of the constitutional undercooling.Under the combined influence of the vibrating forces and constitutional undercooling,the interfacial microstructure of the Mg/Al bimetal was improved.
(4) The crack first penetrated the entire IMCs layers,and then expanded along with the interface between the layers II and III under no vibration condition,showing a flat fracture morphology.When the vibration was applied,the crack developed in the IMCs layers.The fine dispersed Mg2Si phase and the refined Al3Mg2improved the interface performance by making the crack deflect and bend.Thus,the direction of the crack propagation was deflected,exhibiting an irregular zigzag fracture morphology.
Acknowledgements
The authors gratefully acknowledge the supports provided by the National Natural Science Foundation of China (No.52075198),the National Key Research and Development Program of China (Nos.2020YFB2008300,2020YFB2008304),the State Key Lab of Advanced Metals and Materials (No.2021-ZD07),and the Analytical and Testing Center,HUST.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Improving the Young’s modulus of Mg via alloying and compositing -A short review
- Surface modifciation of magnesium alloys using thermal and solid-state cold spray processes: Challenges and latest progresses
- Preparation,structure and properties of Mg/Al laminated metal composites fabricated by roll-bonding,a review
- A review on recent advancements in biodegradable Mg-Ca alloys
- Analysis of element loss,densification,and defects in laser-based powder-bed fusion of magnesium alloy WE43
- Pore structure of porous Mg-1Mn-xZn alloy fabricated by metal-gas eutectic unidirectional solidification✩
