Corrosion protection and mechanical properties of the electroless Ni-P-MOF nanocomposite coating on AM60B magnesium alloy
2022-10-25RajaalizadehSeifzadehKhodayariShSohranezhad
Z.Rajaalizadeh ,D.Seifzadeh ,A.Khodayari ,Sh.Sohranezhad
a Applied Chemistry Department,University of Mohaghegh Ardabili,Ardabil 5619911367,Iran
bDepartment of Chemistry,Faculty of Science,University of Guilan,P.O.Box 1914,Rasht,Iran
Abstract Al-based MIL-53 MOF nanostructure was synthesized hydrothermally and then co-deposited in the electroless nickel coating on AM60B magnesium alloy using Zr pretreatment as an eco-friendly underlayer.The MIL-53(Al) nanostructure was synthesized in the form of layered semi-cube crystals with the surface area and mean pore diameter of 985.72 m2g-1 and 2.00 nm,respectively.The SEM images captured with two various zooming scales from the surface of the plain and MOF containing electroless layers showed cauliflower-like morphology with even distribution of nodule size.Also,the sub-grains of the plain coating disappeared after incorporation of the MOF.Although,both the normal and nanostructure-containing electroless layers have crystalline-amorphous structure,but the nanocomposite coating showed less crystallinity.The average surface roughness of the plain electroless coating was about 309 nm,which decreased to about 222 nm after incorporation of the MOF.The XRD patterns showed that the characteristic peak of Ni broadened after incorporation of the MOF,probably due to the decreasing of the crystallinity.For the heat-treated normal and MOF containing coatings at 200 °C no phase transition takes place,but new peaks appeared for heat-treated coatings at 400 °C due to the crystallization and second-phase precipitation.The results of the EIS tests showed an increase in the amount of the charge transfer resistance (from 19 to 29 kΩ cm2) after addition of the MOF,which means an improvement in the corrosion resistance.Also,low Jcorr of the composite coating represents its higher corrosion resistance with respect to the plain coating.The micro-hardness values of the composite coating before and after the heat treatment were higher than the plain coating.Also,the Ni-P-MOF coating has a lower wear rate both before and after the heat treatment due to an improvement in its micro-hardness.
Keywords: Electroless plating;MOF;Magnesium alloy;Wear;EIS.
1.Introduction
The use of magnesium alloys has increased significantly in recent years,mainly due to their superior properties,especially low density [1-4].These properties have attracted the interest of researchers considerably,and made these alloys desirable and even irreplaceable in several applications[5,6].AM60B is one of the most widely used Mg cast alloy especially in several automobile components like brackets,steering wheels,instrument panels,and seat frames [7].However,the use of this alloy has not yet become widespread due to high electrochemical activity and low corrosion resistance [8-10].Therefore,surface treatment is required to form a protective barrier layer and mitigate the susceptibility to the corrosion.Generally,surface treatment methods are classified into dry and wet processes,and each method has its own advantageous and disadvantageous.Due to high yield of the wet methods,they have merit and potential for large-scale production [11].Electroless deposition is one of the surface modification approaches for applying the metallic coating on the Mg alloys.Electroless nickel-based coatings are the most critical protective layers against the corrosion of substrates because these coatings have superior properties[12].However,the electroless plating of Ni on the Mg alloys faces with difficulties because of the formation of loose oxide/hydroxide magnesium layer in the aqueous electroless plating electrolyte [13].Furthermore,the applied coating on the Mg alloys should be uniform,crack/pore-free,and adhesive since the galvanic corrosion can occur between the magnesium substrate and nickel coating and deteriorates the alloy surface [14].The electrochemical inhomogeneity of the magnesium alloys is the other difficulty in the electroless plating process.Application of the pretreatments on the magnesium alloys (such as conversion coatings and etc.) is one way to overcome these problems [15-18].For this purpose,chromating is considered as a general pretreatment and also,etching the surface reduces its reaction activity.Despite the good corrosion resistance,the application of the chromium compounds was prohibited because of their carcinogenic effects.For this reason,a wide variety of researchers were focused on finding eco-friendly pretreatments prior to the electroless plating on the Mg alloy [19-23].In our recent works [15,24-29],this problem was discussed extensively and some environmentally friendly pretreatment were introduced.Also,the positive effects of using the mentioned pretreatments on the corrosion resistance of the applied electroless coating have been proven.
In addition to the crucial role of the pretreatment layer on the corrosion protection,the tribological properties (wear resistance and micro-hardness) of the final deposit on the magnesium alloys should be considered.These properties could be improved by the incorporation of the additional metal elements (poly-alloy coating) and/or hard particles (composite coating) into the nickel coating [30].The selection of the codeposited nanoparticles is mostly dependent on the expected final property of the nickel electroless coating.Wang et al.[31] found that the microstructural and mechanical characteristics of the electroless Ni coating on AZ31 alloy improved by incorporation of Si3N4nanowires.According to the discussions,the result of adding Si3N4nanowires (1.5 g L-1) was the formation of a regular nodular structure.However,as the concentration increases,the porosity and roughness of the coating increase due to the agglomeration of the nanowires.Additionally,the micro-hardness of the coating was increased to 790 HV by incorporation of Si3N4nanowires.Also,the friction coefficient and wear rate of the Ni-P-Si3N4nanocomposite was about 1/6 of the plain deposit.Hu et al.[32] showed the improved anti-corrosion and microhardness of Ni-P-Al2O3composite versus Ni-P layer.This improvement was explained by the suitable crystal plane spacing and grain size of the applied composite coatings.Song et al.[33] examined Ni-P-ZrO2deposits on AZ91D alloy.They found that the applied composite coating reaches to the maximum hardness value at 350 °C.Moreover,the compact structure of the composite coating led to the higher corrosion resistance with respect to the Ni-P coated alloy.In the other study by Ghavidel et al.[34],the electroless Ni-P-SiC layer was coated on AZ31 alloy.The addition of SiC nanoparticles showed promising results in improving the corrosion resistance,hardness,and wear resistance.
Metal-Organic Frameworks (MOFs) are new classes of the porous and crystalline materials that comprises of metal ions linked with organic molecules in a broad framework.The main characteristics of MOFs such as high porosity,low density,and high surface area make them promising compounds for various applications [35,36].MIL-53(Al) is a carboxylate based MOF and has some advantages such as high thermal stability,cheap,and available raw materials compared with other MOFs.The other properties of MIL-53(Al) are moisture-resistance and relatively high surface area [37-39].This type of nanostructure has some advantages such as easy preparation method,high stability,low toxicity,and cheapness compared to the conventionally-used nanostructures in the plating.Moreover,the inherent porosity of these nanostructures makes them as suitable nano-carriers to apply the smart electroless coatings containing corrosion inhibitors.For this purpose,the first prerequisite is to study the impact of the presence of these nanostructures in the coating itself,which is discussed in this work.The intelligent electroless coating could be considered in the next works.
In this work,Al-based MIL-53 MOF was synthesized and then co-deposited in the electroless Ni-P coating on AM60B magnesium alloy using an eco-friendly Zr pretreatment.The SEM (Scanning Electron Microscopy),FTIR (Fourier-Transform Infrared Spectroscopy),TGA (Thermo Gravimetric Analysis),and BET (Brunauer-Emmett-Teller) methods were used to identify the synthesized nanostructures.The FESEM (Field Emission Scanning Electron Microscopy),EDS(Energy-Dispersive X-ray Spectroscopy),and AFM (Atomic Force Microscopy) methods were employed to characterize the plain and Ni-P-MOF coatings from the morphological,elemental composition,and surface roughness aspects,respectively.The XRD (X-Ray Diffraction) analysis was applied to examine the microstructure of the coatings.In addition,EIS(Electrochemical Impedance Spectroscopy) and PDP (Potentiodynamic Polarization) tests were performed to determine the corrosion resistance of the Ni-P and Ni-P-MOF coatings.Finally,the effects of the MOF incorporation and heat treatment on the mechanical properties of the coatings were investigated.
2.Experimental
2.1.Materials and chemicals
The AM60B magnesium alloy substrate containing Al(6.33%),Zn (0.68%),Mn (0.24%) in wt.% and Mg balance was purchased from Nanjing Welbow Metals Co.,Ltd.Also,the name,brand,and purity of the used chemical materials are as follows:
Al(NO3)3·9H2O (Merck,98.5%),C8H6O4(Merck,98%),Acetone (Atlas shimi,99%),HNO3(Royalex,69-71%),H2ZrF6(Aldrich,50 wt.% in H2O),NaOH (Loba Chemie,98%),NiSO4·6H2O (Merck,99%),NaH2PO2.H2O (Loba Chemie,99%),HF (Loba Chemie,40% v/v),NaC2H3O2(Merck,>99.5%),NH4HF2(Loba Chemie,98%),Thiourea(Merck,extra pure),CuSO4·5H2O (Merck,extra pure),and NH3(Rankem,30%).
2.2.Preparation of the MOF
First of all,0.025 mol of Al(NO3)3.9H2O was dissolved in deionized water (36 mL) and the solution was magnetically agitated for 10 min after addition of 0.013 mol C8H6O4.Also,the pH of the solution was adjusted to 1-2 by addition of HNO3.Then,the obtained solution was transferred into a Teflon-lined stainless steel autoclave and heated for three days at 220 °C under an Ar atmosphere.The byproducts were removed by washing the obtained white precipitate using the deionized water several times.The obtained solid product was dried under vacuum condition for 1 h.Removing of the encapsulated/unreacted terephthalic acid and water molecules through the pores of the synthesized nanostructure was done by drying of the obtained solid product under vacuum condition for 1 h.Afterward,the calcination was operated for 24 h at 400 °C under air atmosphere,and finally,MIL-53(Al) nanostructures with the general formula of Al(OH)[O2CC6H4CO2] were obtained [40-42].
2.3.Ni-P and Ni-P-MOF coatings
2.3.1.Zr pretreatment layer
The substrate was cut into a rectangular shape parts with dimension of 10 × 30 × 2.0 mm3.Then,the alloy samples were abraded with silicon carbide abrasive paper (grits size of 400 to 2000).Next they were etched in HNO3(50 mL L-1)for about 10 s after ultrasonically cleaning for 15 min in acetone at 40 °C in the ultrasonic bath (BANDELIN SONOREX DIGITEC),Then,the cleaned specimens were exposed to Zr pretreatment solution (1.5 g L-1of H2ZrF6with pH~4.5 which was adjusted by NaOH) for 6 min at 30 °C.
2.3.2.Electroless plating
To apply the Ni-P coating,the Zr-pretreated alloy was inserted in the electroless Ni-P bath containing 15 g L-1NiSO4·6H2O,14 g L-1NaH2PO2·H2O,13 g L-1NaC2H3O2,8 g L-1NH4HF2,12 mL L-1HF,0.2 g L-1CuSO4·5H2O,and 1 ppm Thiourea.NH3was used for pH adjusting to 6.4 and the plating was done for 3 h at 65 °C.For each sample,200 mL of the plating solution was used [43].Composite Ni-P-MOF plating was performed in two stages.First,the Zr-pretreated sample was inserted in 150 mL of the electroless plating solution which its chemical composition was expressed above,and the plating process was performed for 1 h In the second step,50 mL of the plating solution containing 0.3 g L-1of the MOF nanostructures was added to the first plating solution and the process was continued for another 2 h It should be noted that the solution containing MOF was subjected to the ultrasound irradiation for 1 h before being added to the plating solution of the first stage to disperse the loaded nanostructures.The plating process of the Ni-P-MOF coating was illustrated in Scheme 1.

Schema 1.
2.4.Characterization of the MIL-53(Al)
The morphological image of the MOF nanostructures was provided using the SEM instrument(LEO 1430 VP).Also,the Perkin Elmer Spectrum Rx1 instrument was used to record the corresponding FTIR spectrum.The average pore size and total surface area of the synthesized nanostructures were analyzed by the BET analyzer (Belsorp mini II).Thermal stability of the MOF was studied by the TGA analyzer (Linseis STA PT-1000) from 25 to 700 °C with a heating ramp of 10 °C min-1under the air atmosphere.To determine the pH of the MIL-53(Al) at Point of Zero Charge (PZC),the salt adding technique was used.For this purpose,10 series of 0.1 M NaNO3solution with the volume of 10 mL was prepared and pHs of the solutions (pHi) were adjusted from 3.0 to 12.0 by adding either 0.1 M HNO3or NaOH.Then,50 mg of the synthesized nanostructures were added to each prepared solution and the resultant suspensions were centrifuged after shaking for 24 h Afterward,the pH values of each sample were measured again and denoted as pHf.The plot ofΔpH(pHf-pHi) versus pHiwas developed to obtain the pHPZC[44,45].
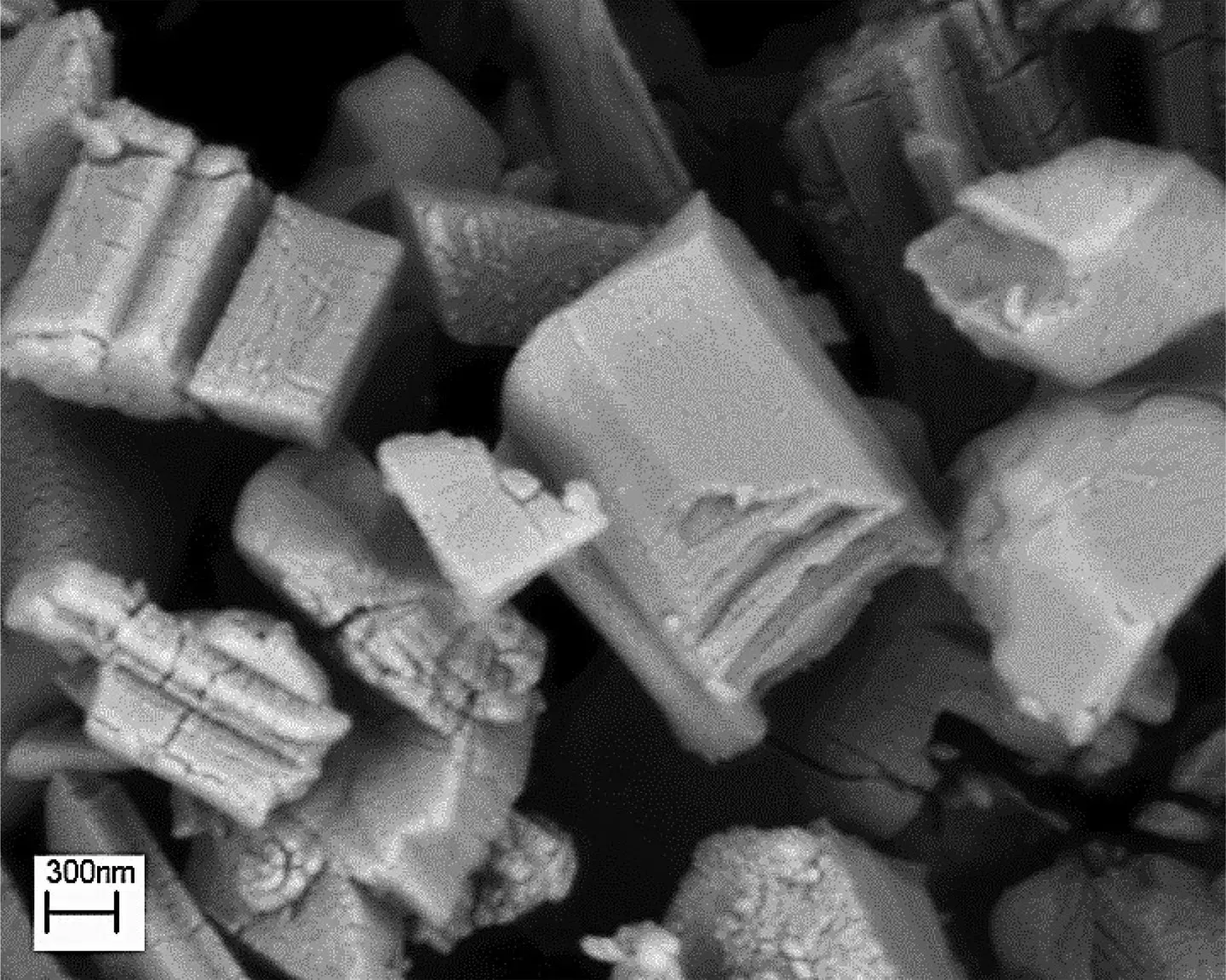
Fig.1.Surface morphology of synthesized MIL-53(Al) nanostructure.
2.5.Characterization of the coatings
The morphological image of zirconium pretreatment layer was assessed by the SEM (LEO 1430 VP).The FESEM instrument (MIRA3TESCAN-XMU) was employed to observe the morphological images of the plain and composite coatings together with the corresponding cross-sectional images.The elemental composition of zirconium pretreatment layer and electrolessly plated coatings determined by the EDS (RONTEC Gmbh Germany).Besides,the elemental distribution of Ni-P-MOF composite coating from the cross-sectional view was provided by the EDS-mapping images.The 3D topographical images of the plain and composite coatings were carried out by the AFM instrument (CoreAFM Nanosurf).The microstructure of the plain and composite coatings before and after the heat treatment at 200 and 400 °C were studied by the XRD analyzer (Philips Xpert) by Cu Kαradiation (λ=0.154178 nm).
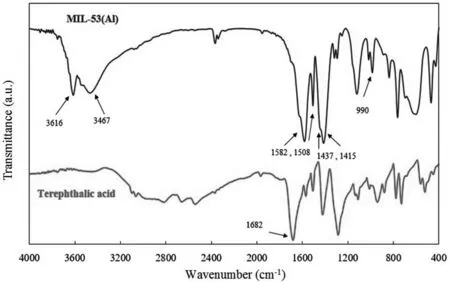
Fig.2.The FTIR spectra of the synthesized nanostructure and pure terephthalic acid.
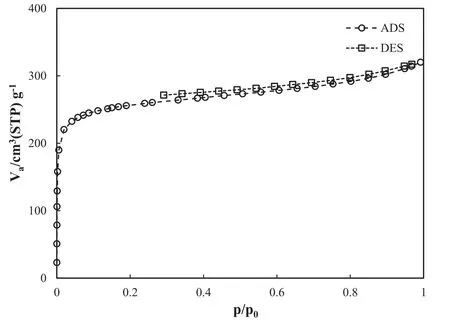
Fig.3.Nitrogen adsorption/desorption isotherm of synthesized MIL-53(Al)[Ref.53].
2.6.Corrosion tests
The electrochemical EIS and PDP tests were carried out by μAutolab3 instrument in sodium chloride solution (3.5 wt.%)at ambient temperature.Preparation of the test samples and experimental parameters can be found in our previous work[46].The test samples (with exposed area of 1 cm2) were immersed in the corrosive solution for 1 h prior to the EIS measurements.Also,the polarization experiments were immediately performed at the end of the EIS tests in each case.The PDP curves were analyzed by cathodic Tafel extrapolation in order to extract the polarization parameters.Each corrosion tests was repeated three times and then the results averaged.
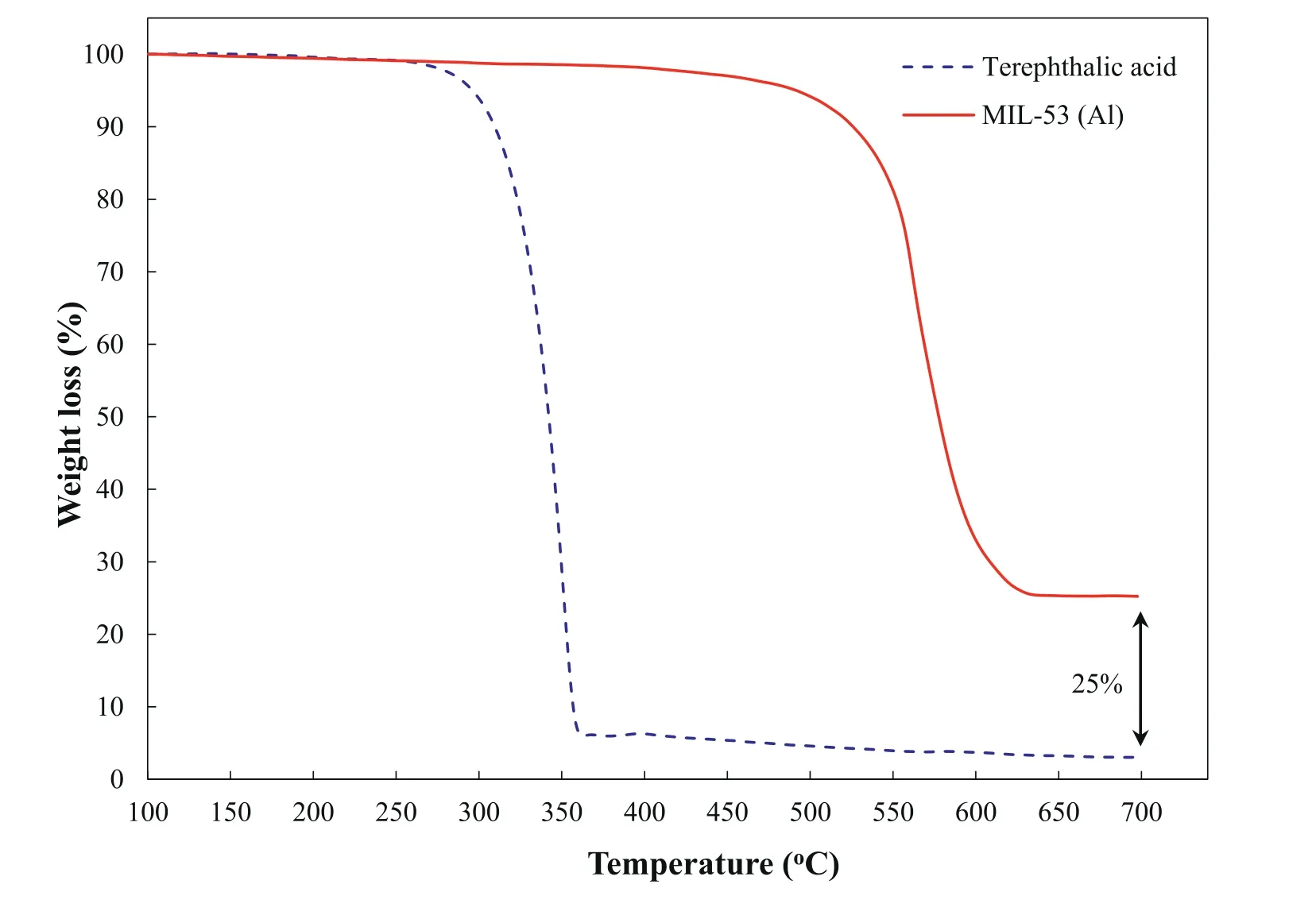
Fig.4.TGA curves of pure terephthalic acid and MIL-53(Al) nanostructure.
2.7.Micro-hardness and wear behavior
The micro-hardness measurements of the plain and composite electroless coatings were done according to the ASTM B578 [47].The measurements were done under a load of l00 g for 10 s and it was repeated 6 times for each test sample(the results were averaged).Moreover,the effect of the heat treatment (at 200 and 400 °C for 2 h) on the micro-hardness of both coatings was investigated.
The wear rates of the plain and composite coatings were calculated by the pin-on-disk machine (Ariana Modern Industry Co.,Iran).The sliding counter pin was made of steel(AISI 52,100) and the test samples were as disk.The used steel pin has Rockwell hardness of 60 and a diameter of 6 mm.The wear performance of the coatings was estimated under the load of 3 N.The wear track diameter was 0.4 cm and the speed of the wear test was 0.15 m s-1.Also,the disk was rotated with the rate of 358 rmp and the total length of the test was 300 m.To estimate the wear rate of the samples,the mass loss of the test samples was measured using a digital balance (±0.0001 g).Also,the wear tests were performed for the heat-treated plain and composite coatings (at 200 and 400 °C).After the wear test,the specific wear rates were obtained according to the Archard's equation [34]:

In this equation,K is the specific wear rate (Kg N-1m-1),ΔP is the mass loss (Kg),F is the applied force (N),and L is the sliding distance (m).After the tests,the wear tracks of the samples were observed by the SEM.
2.8.Adhesion test
To check the adhesive strength of the plain and composite electroless layers,the thermal shock method was used.The tests were performed according to ASTM B733-04 [48].For this purpose,the samples alternatively were exposed to the thermal stress (24-200 °C) for 20 cycles to evaluate the formation of any crack,blister,or detachment on the coatings.
3.Results and discussion
3.1.Characterization of the MOF
Fig.1 presents the morphological image of the synthesized nanostructures.As can be seen,layered semi-cube crystals of MIL-53(Al) nanostructures were successfully formed.The size of the cube edge of these crystals is about 20-60 nm.
Fig.2 illustrates the FTIR spectrums of the synthesized nanostructures and pure terephthalic acid.Comparison of the FTIR spectra demonstrates that the absorption band of the terephthalic acid and 1682 cm-1,which is attributed to-C=O stretching of the free carboxylic acid group,was not observed in the synthesized nanostructures.The FTIR spectrum of the nanostructures shows -CO(O) asymmetric stretching bands at around 1508 and 1582 cm-1and -CO(O) symmetric stretching bands at 1415 and 1437 cm-1[49,50].Also,Al-O bond in the structure of the synthesized MIL-53(Al) was revealed by the broad band at 990 cm-1.The stretching vibrations of water within the pores of the nanostructures were shown by two prominent broad bands at around 3467 and 3616 cm-1[51-53].
Fig.3 shows the size distribution and volume of the pores together with the surface area of MIL-53(Al)nanostructures,which were investigated using the adsorption/desorption isotherm (at 77 K) of nitrogen.The plot showed type I isotherm with a small hysteresis loop,indicating the micropore structure for the synthesized MIL-53(Al) which is in accordance with Langmuir monolayer adsorption model [54].The BJH and BET methods revealed that the surface area,average diameter of the pores,and total volume of the pores were 985.72 m2g-1,2.00 nm,and 0.49 cm3g-1,respectively[53].
The TGA curves of the terephthalic acid and synthesized nanostructures were shown in Fig.4.As is clear,the weight loss of the synthesized nanostructures takes place in one step and the temperature range of 480-620 °C up to 75%.This weight loss is probably related to the thermal degradation of terephthalic acid coordinated with the aluminum oxide clusters [55].The remained weight (about 25% of the initial weight) at 700 °C is related to the aluminum oxide.The results of the TGA curve confirmed that MIL-53 (Al) nanostructures have suitable thermal stability up to 480 °C.
The experimental curve of theΔpH versus pHiof MIL-53(Al) was presented in Fig.5.The minimum of this curve indicates that pHZPCof the synthesized nanostructure is about 4.0.As a result,the synthesized nanostructure has positive and negative surface charges at pH<pHZPCand pH>pHZPC,respectively [44,45].
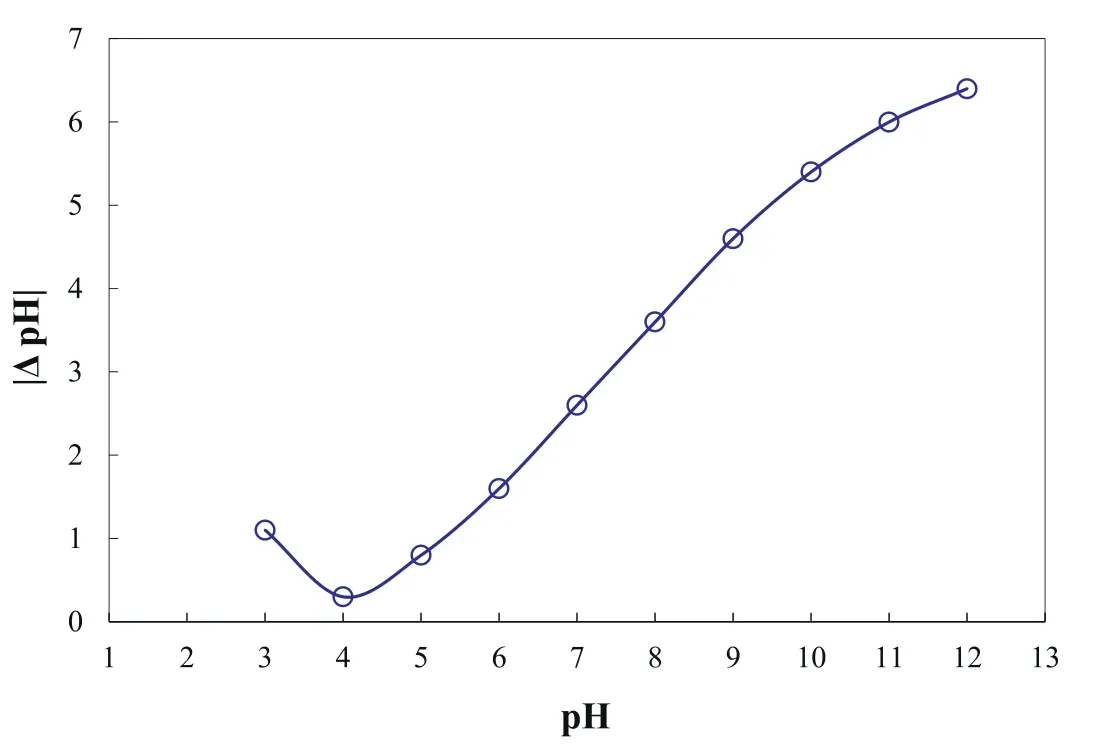
Fig.5.The zeta potential of MOF nanostructure in distilled water and electroless nickel plating bath.
3.2.Electroless plating
Surface morphology of zirconium conversion coating as the pretreatment layer can be seen in Fig.6.The morphological image revealed that the applied conversion layer was covered the whole surface of the substrate via a network-like structure,and micro-cracks distributed all over.The formation mechanism and EDS-mapping of zirconium conversion layer were described in detail in our previous work [15].The reported mechanism suggested the formation of MgO,MgF2,ZrO2,and ZrF4on the surface.Also,the EDS-mapping results confirmed that MgO and MgF2mostly exist in the cracked regions of the surface(probablyαphase)while the non-cracked places are mostly composed of ZrO2and ZrF4.
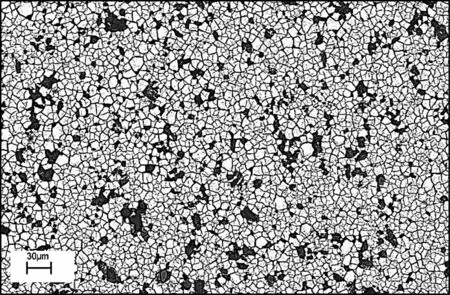
Fig.6.The SEM image of Zr conversion coating.
Fig.7 displays the FESEM images of the plain and composite coatings after 3 h plating.Microscopic images recorded at low magnification (Fig.7a,c) showed that the surface of the samples was completely covered with uniform and nonporous electroless layer.Also,the images recorded at high magnification (Fig.7b,d) confirmed that the created plain and composite coatings have a regular nodular structure with a cauliflower morphology and uniform size distribution.
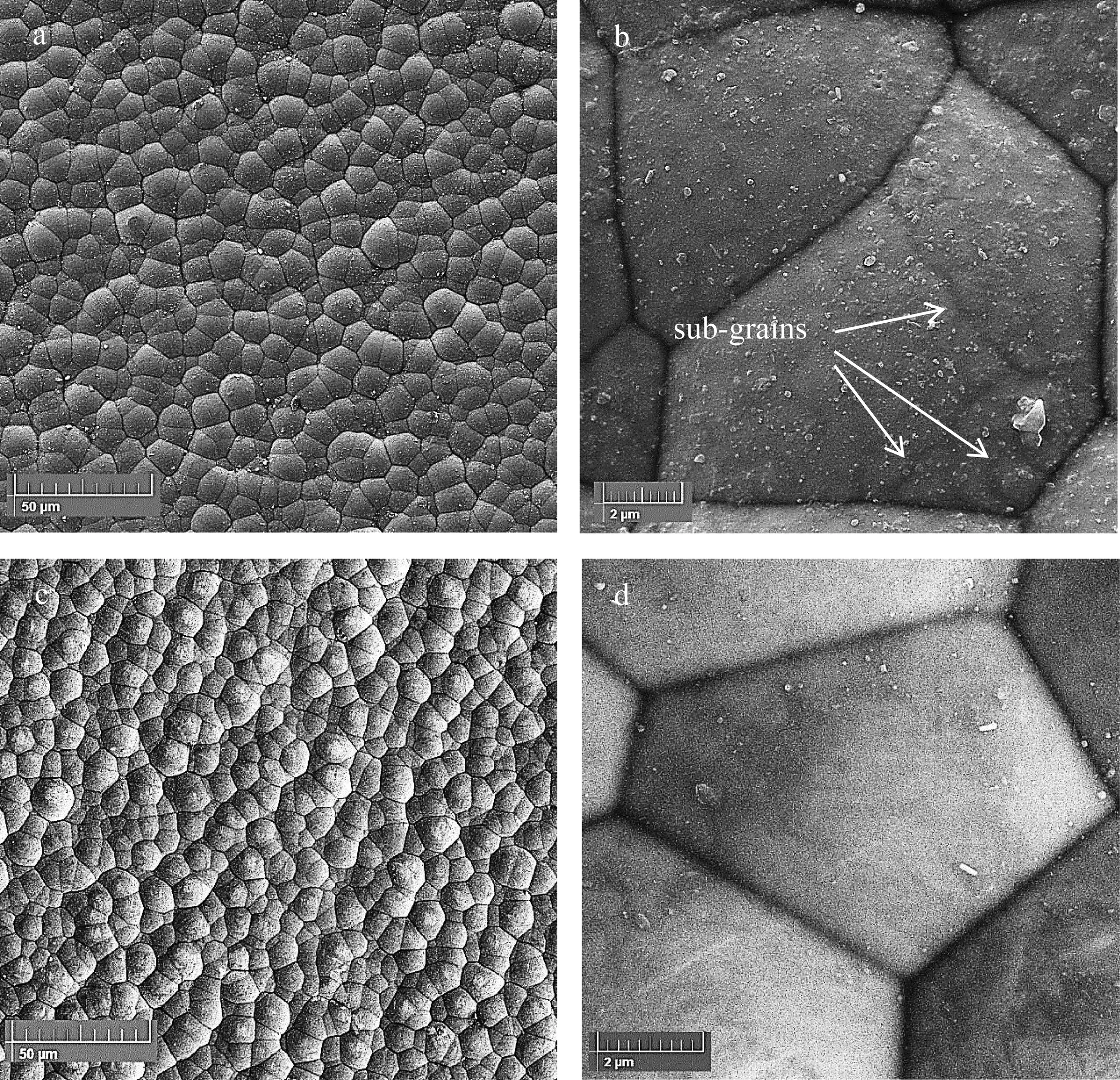
Fig.7.The FESEM images of electroless (a and b) Ni-P and (c and d) Ni-P-MOF.
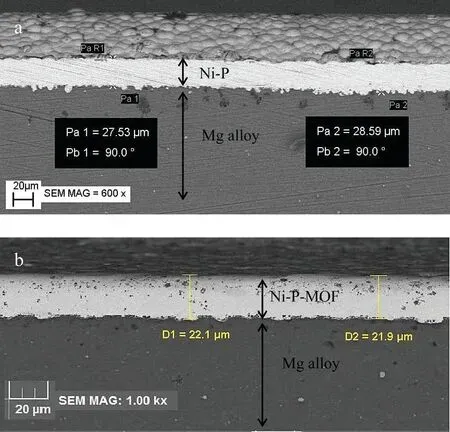
Fig.8.Cross sectional images of electroless (a) Ni-P and (b) Ni-P-MOF coatings.
Besides,the resulting coatings have high degree of compactness,so that,very little space is visible between the nodules.There is no remarkable difference in the morphology of the plain and composite coatings except in the sub-grains of their structure.As is clear,after incorporation of MOF nanostructure,the sub-grains of Ni-P coating were disappeared probably due to the increasing of the phosphorous content.The deposition mechanism of the electroless nickel plating was discussed in detail previously [15,43].The general reaction of the electroless plating could be indicated as follows[56]:
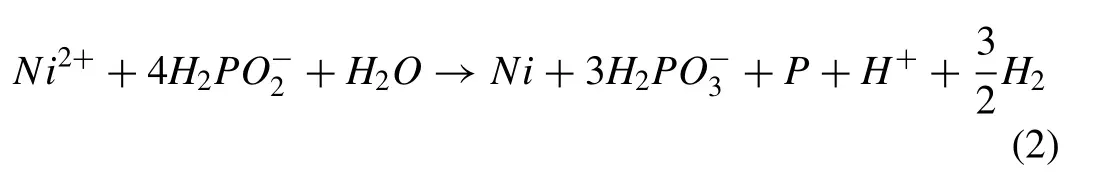
Also,Cu atoms were co-deposited in the coating according to the following reaction [15]:

To describe the entrapment mechanism of the particles in the composite coating,some theoretical models have been suggested,which are mostly based on the Guglielmi's mathematical model [57].According to Ashassi-Sorkhabi and Es’haghi [58],the formation of the composite coating could be explained by impingement and settling of the incorporated nanoparticles on the alloy surface and subsequently surrounding of them by the metal matrix.Balaraju et al.[59] mentioned that the impingement and the time that particles are held on the surface of the substrate are two main factors in the co-deposition process.The flux of the incorporated particles at the interface affects the impingement of the nanoparticles on the surface.However,the particle shape and hydrogen evolution determine the residence time of MOF on the surface.After addition of MOF into the plating solution,the particles are physically adsorbed on the surface because of the fluidal attack.Next,the adsorbed particles dehydrate by the strong electric field of Helmholtz layer of the electrode leading to the irreversible adsorption of the nanoparticles on the substrate.Finally,the adsorbed particles were embedded in the reduced metal [60].
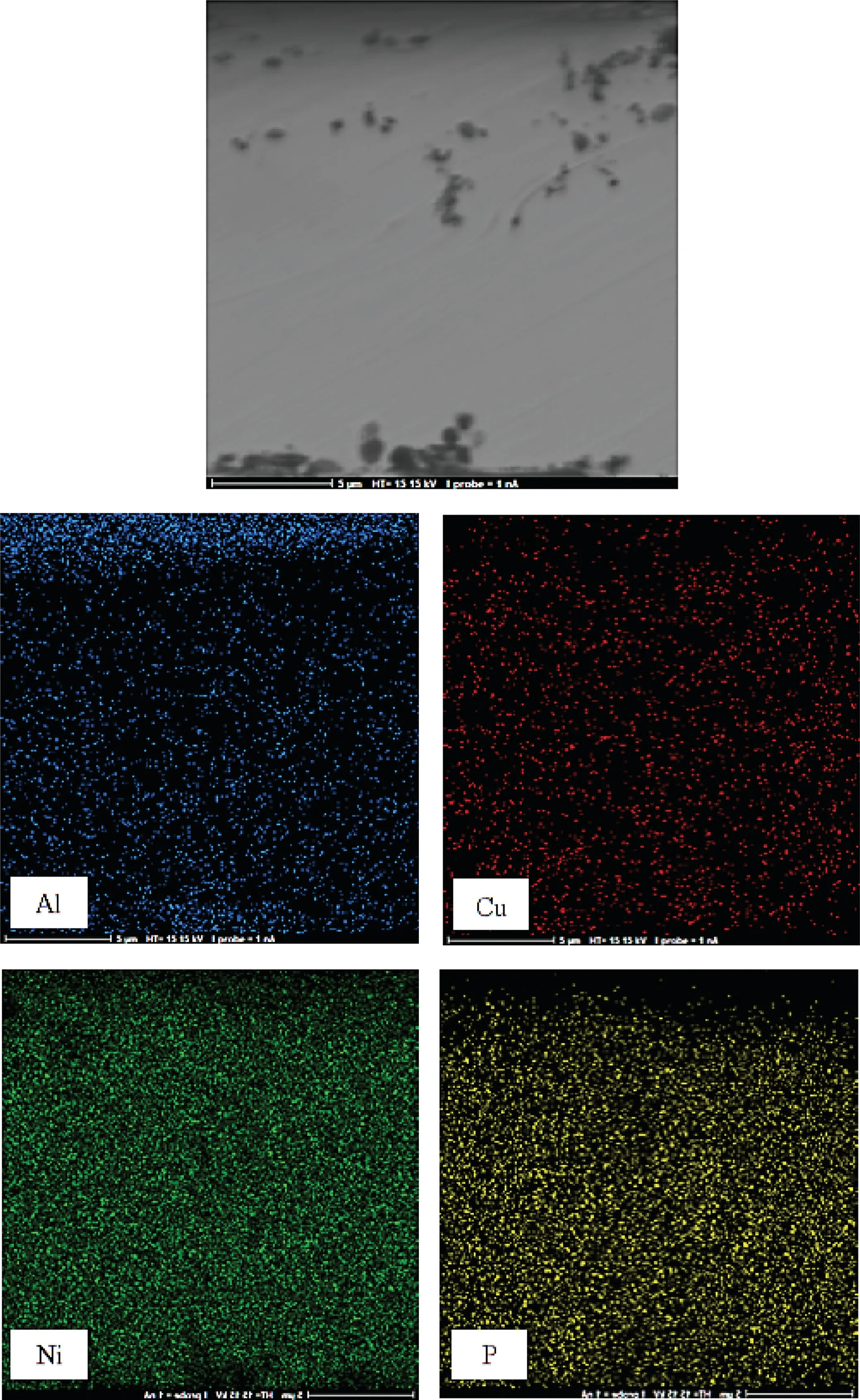
Fig.9.Cross-sectional EDS-mapping image of Ni-P-MOF electroless coating.
Fig.8 displayed the cross-sectional views of the plain and composite coatings.The plain coating thickness was about 28 μm (Fig.8a) while it was decreased to about 22 μm after the introduction of MOF nanostructures (Fig.8b).Probably,the difference in the thickness is related to the electrophoresis of Ni2+ions during the plating.As was mentioned above(Fig.5),the pHZPCof MIL-53(Al) is equal to 4.0 and so the synthesized nanostructure has a negative surface charge at pH of the plating bath (pH~6.4) [44,45].So,it is expected that Ni2+metal ions adsorbs on the negatively charged MOF nanostructures.Obviously,due to the low mobility of MOF-Ni2+nanostructures,this process slows down the rate at which metal ions reach the metal surface,resulting in a relative reduction in the plating rate and a decrease in the coating thickness [61].Also,the images show that both coatings mechanically interlocked to the substrate and defects or cracks were not observable in the interface of the coating/substrate.
The elemental EDS analysis from the surface of the plain coating confirmed the existence of Ni,P,and Cu with the weight percentages of about 91.49,7.06,and 1.45,respectively.For the composite coating,the co-deposition of the synthesized nanostructures was confirmed by the presence of Al which revealed by the elemental analysis.The obtained data indicated that the composite coating contains Ni,P,Cu,and Al with the weight percentages of about 90.54,8.51,0.70,and 0.25,respectively.The EDS results revealed the relative increment of P content of the coating after the incorporation of the synthesized MOF nanostructure.Increasing the amount of the phosphorus may reduce the crystallinity of the plain coating.This prediction was confirmed due to the disappearing of the sub-grains in the relevant SEM images of the Ni-P-MOF coating.
Also,the cross-sectional EDS-mapping images of the composite coating were displayed in Fig.9.The images show that Ni,P,and Cu were distributed evenly in the inner part of the coating and their amounts were decreased in the outer part of the electroless layer.In contrast,Al content was increased in the outer part of the layer because MOF nanostructure were added to the bath after 1 h plating and their amount will be more in the outer part.In other words,the detection of Al in the EDS quantitative analysis of the composite coating and corresponding EDS-mapping images confirms that the MOF nanostructures were successfully incorporated in the coating.
The nodular structure of the electroless coatings was confirmed using AFM analysis (Fig.10).The recorded images also showed the composite coating with a relative higher uniformity compared to the conventional coating.The averaged surface roughness(Ra)values of the plain and composite coatings were about 309 and 222 nm,respectively.Thus,incorporation of the synthesized nanostructure in the coating leads to the decreasing in the surface roughness.
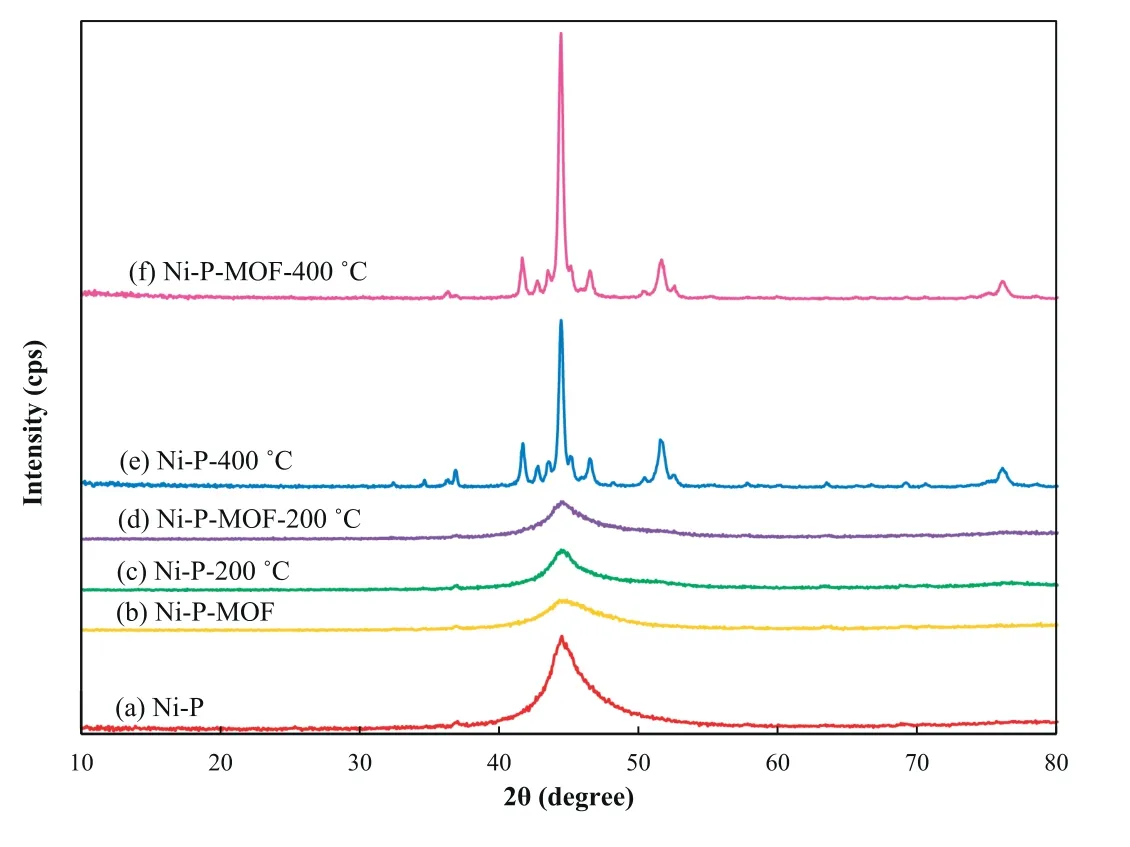
Fig.11.The XRD patterns of Ni-P and Ni-P-MOF electroless coating before and after heat treatment at 200 and 400 °C.
Fig.11 displays the XRD patterns of the plain and composite electroless coatings before and after the heat treatments.The results show that the characteristic peak of Ni(111) (JCPDS Ref.no.: 04-0850) was observed at about 45°for both plated samples without any shift [15].Comparison of Fig.11a,b revealed that the introduction of MIL-53(Al)causes the broadening of Ni diffraction peak.In other words,the broadening of Ni peak signifies that the crystallinity of Ni-P-MOF composite coating is lower than the plain coating.This result was in accordance with the SEM images in which the sub-grains were disappeared and EDS result that P content was increased after MOF introduction.The XRD patterns of the electroless coatings were not changed remarkably after heating at 200°C and just a distinct broad peak correspondingto Ni(111)was observed(Fig.11c,d).This result implies that the phase transition was not taking place for the heat-treated samples at 200 °C.But,new XRD peaks appeared after heating of the samples at 400 °C indicating precipitation of the second phase and crystallization.Also,the nickel diffraction peaks became sharper and their intensity was increased for both coatings.Also,the intensity was sharper for the Ni-PMOF electroless coating relative to Ni-P.These diffraction peaks are related to the stable intermetallic nickel phosphide(Ni3P) phase (JCPDS Ref.no.: 01-074-1384) which is consistent with the other reports [62,63].
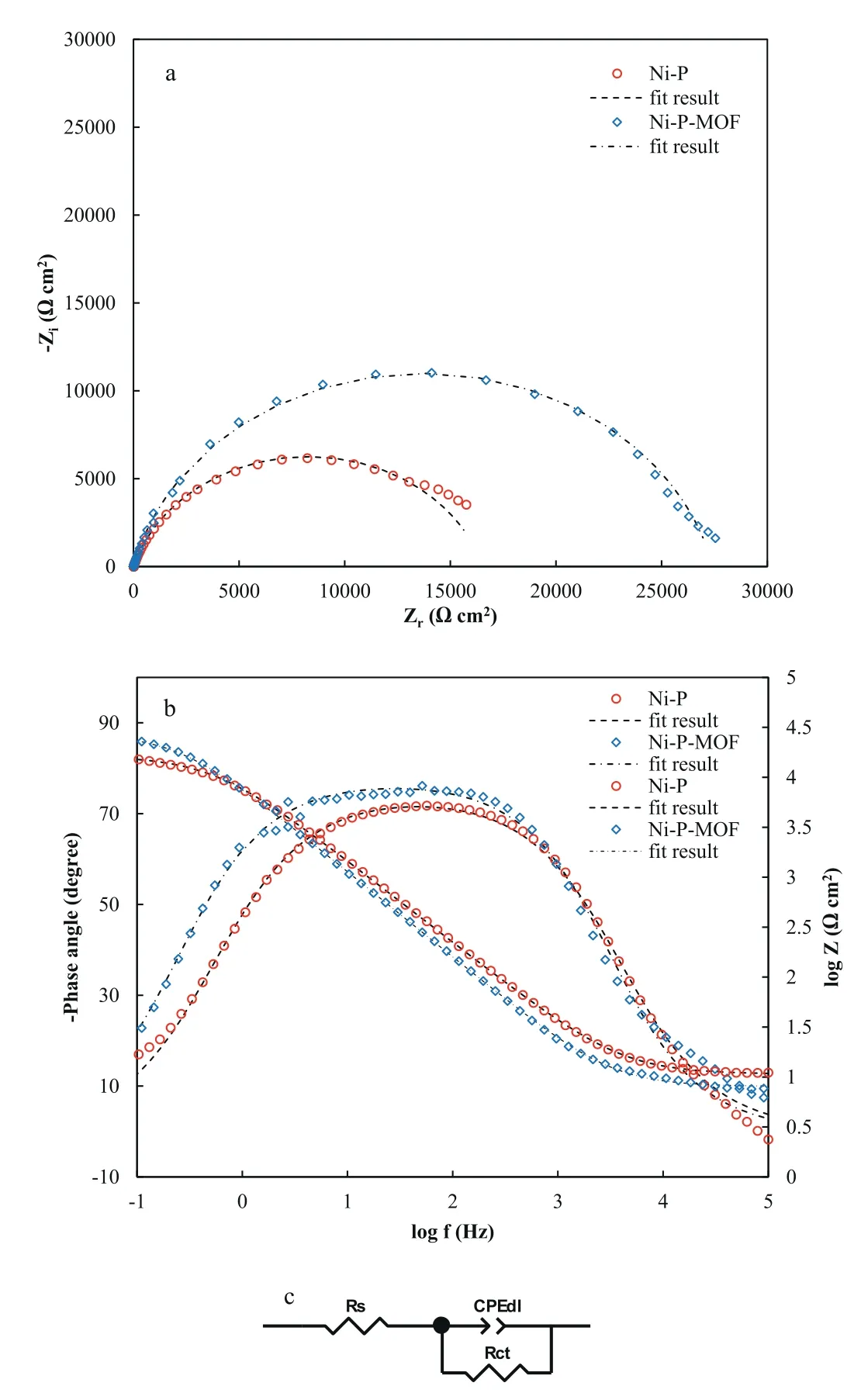
Fig.12.The EIS responses of Ni-P and Ni-P-MOF electroless coatings as(a) Nyquist and (b) Bode modulus/phase Bode plots in 3.5 wt.% sodium chloride solution.
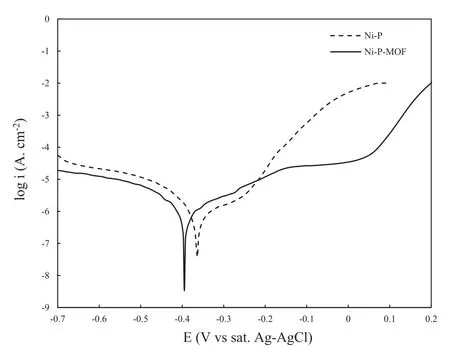
Fig.13.The PDP curves of Ni-P and Ni-P-MOF electroless coatings in 3.5 wt.% NaCl solution.
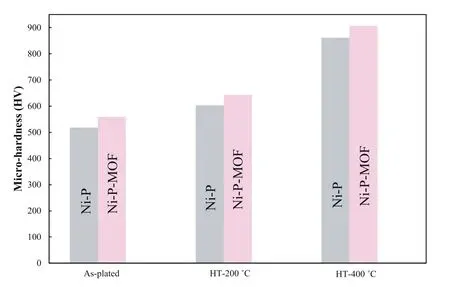
Fig.14.The micro-hardness of Ni-P and Ni-P-MOF electroless coatings before and after heat treatment at 200 and 400 °C.
3.3.Corrosion tests
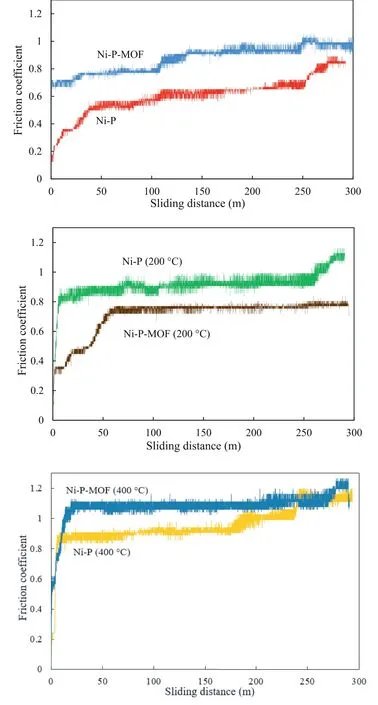
Fig.15.The friction coefficient variations of as-plated the Ni-P and Ni-PMOF electroless coatings.
Fig.12 shows a typical EIS response of the composite coating compared with the plain electroless coating as Nyquist and corresponding Bode plots in 3.5 wt.%sodium chloride solution.As can be seen,the Nyquist plots of both applied coatings contain one capacitive loop in the measured frequency range that was confirmed by the Bode plots,too.The presence of one time constant is probably due to the compactness of the coatings and lack of the penetration pathways for the corrosive electrolyte through the grain-boundaries of the coatings.However,it should be mentioned that even though the similar shape of the Nyquist plots for these two coatings,the diameter of the corresponding semicircles is different.This means that the coatings have a similar corrosion behavior but different corrosion rates.A comparison of the diagrams shows that the impedance value of the plain coating was considerably increased after incorporation of MOF nanostructures in the coating,indicating the enhancement in the corrosion resistance.This result confirms that the corrosion resistance of the electroless layer has improved,which is due to the incorporation of MOF nanostructure and reduction of its crystallinity,as discussed earlier.An equivalent circuit model (Fig.12c) was utilized to fit the EIS experimental results.The equivalent circuit of these coatings contains Rs(solution resistance),CPEdl(a Constant Phase Element for modeling of the capacitance behavior of electrical double layer in the coating-electrolyte interface),and Rct(charge transfer resistance).It should be noted that the CPE was employed in place of pure capacitance to represent the non-ideal behavior of the capacitor in this electrochemical system.The impedance value of the CPE can be calculated as follows [28]:

where Q is constant of the CPE element,j=ωis the angular frequency,and n is the exponent of the CPE element (the n value is between 0 and 1).The obtained EIS plots of three different tests for each coating were fitted by Zview2 software and the averaged impedance parameters are given in Table 1.The presence of only one time constant in the EIS plots of plain and composite electroless coatings in the test period reflects the occurrence of a single electrochemical reaction on the surface of the electrodes.The averaged fitting results showed that Rctvalue for Ni-P coating was about 19 kΩcm2which was increased to about 29 kΩcm2after the addition of the nanostructure.This higher Rctvalue of the composite coating implies an improved corrosion protection performance.Also,Qdlvalue of Ni-P coating decreased from about 26.0 μsnΩ-1cm-2to 23.8 μsnΩ-1cm-2after incorporation of MIL-53(Al) nanostructures.As has been repeatedly cited in literatures,the Qdlvalue reflects the porosity of the coating [64,65].Thus,the low Qdlvalue proves that the applied composite coating is comparatively less porous in nature.In this respect,it could be concluded that the increasing of Rctand decreasing of Qdlvalues indicating the improvement of corrosion protection performance of applied composite coating because the incorporated nanostructure block the electrolyte penetration pathways.These results are comparable with those reported in similar studies.In the study by Wang et al.[66],the Cdlvalue of Ni-P coating was about 37.51 μsnΩ-1cm-2while it was decreased to 36.66 μsnΩ-1cm-2in Ni-P-SiC composite coating and also,the Rctwas increased from 18.45 to 31.32 kΩcm2.Furthermore,Sadreddini at al.[64] reported the Cdland Rctvalues of Ni-P coating about 26.92 μsnΩ-1cm-2and 4.12 kΩcm2,respectively;while these values were reached to 17.12 μsnΩ-1cm-2and 5.02 kΩcm2in the optimum concentration of SiO2in composite coating.It seems that MIL-53(Al) nanostructures prevent the corrosive electrolyte from easily reaching on the alloy surface by being placed in the potential pores or intergranular spaces of the electroless coating,resulting in less corrosion.The presence of MIL-53(Al)nanostructures in these locations,even if it does not completely prevent the penetration of the electrolyte,will certainly causes to the formation of long winding paths that eliminate the chance of the corrosive solution reaching the surface of the alloy in the short term(Scheme 2).It has also been shown that MOF nanostructures have a high ability to absorb water[67].Therefore,it is expected that a part of the penetrated aqueous electrolyte will be absorbed by the dispersed MIL-53(Al) nanostructures inside the coating structure,leading to the better corrosion protection.Another reason for the higher corrosion resistance of the composite coating is the increased chemical inertness of the coating surface due to the presence of MIL-53(Al) nanostructures.

Table 1The EIS parameters of Ni-P and Ni-P-MOF electroless coating in 3.5 wt.% NaCl.

Schema 2.

Table 2The PDP parameters for Ni-P and Ni-P-MOF electroless coating in 3.5 wt.% NaCl.
The PDP curves of the plain and composite electroless coatings were displayed in Fig.13.In both coatings,the cathodic reaction is H2evolution and the anodic reaction is the dissolution of deposited Ni.Also,a pseudo-passivation region appeared in the anodic branch of the composite coating represents its better corrosion protection performance with respect to the plain coating.The estimation of the data was done by cathodic Tafel extrapolation [68] of three different samples and the averaged parameters,including corrosion current density (Jcorr),cathodic Tafel slope (bc),and corrosion potential (Ecorr) were collected in Table 2.The results show that despite the decrement of Jcorrafter applying the composite coating (from 2.08 to 1.23 μA cm-2),the Ecorrwas not changed significantly.Since the Jcorris associated with the corrosion rate of the coating,the low Jcorrof the applied composite coating represents the better corrosion protection performance than the plain coating.This is probably a result of the more compact structure of the composite coating.
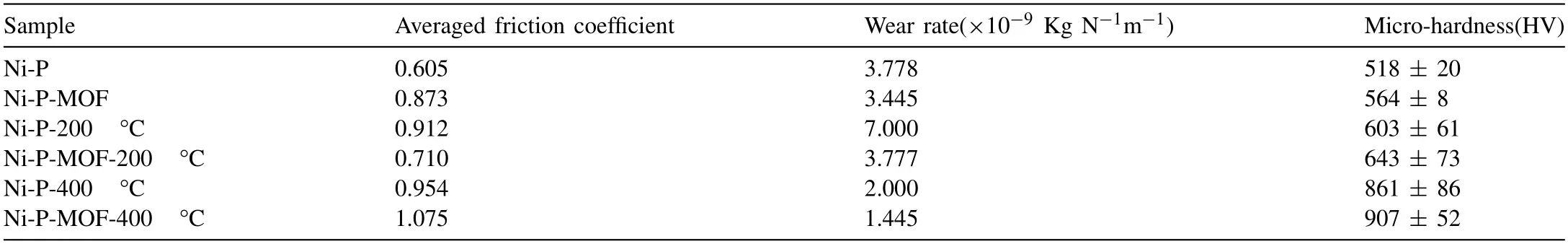
Table 3The wear parameters of Ni-P and Ni-P-MOF electroless coating before and after heat treatment.
3.4.Micro-hardness
Fig.14 shows the micro-hardness values of the plain and composite electroless coatings before and after the heat treatment (at 200 and 400 °C).The results show that the microhardness value of Ni-P-MOF coating is higher than the plain coating both for as-plated and heat treated samples.According to the literatures [15,34],the reported micro-hardness values of the both coatings have sufficient amount.The higher microhardness values of the composite coating could be ascribed to the barrier effect of the nanostructures against the plastic deformation and preventing of the dislocation movement.Also,the result indicated that the heat treatment at 200 °C slightly changed the micro-hardness of both coatings.According to Ma et al.[69],this increase in the micro-hardness is due to the relieving of the internal stress and hydrogen embrittlement.By extending the heat treatment temperature to 400 °C,the micro-hardness increased significantly relative to the as-deposited coatings.This remarkable increase at high treatment temperature is related to the precipitation of hard intermediate Ni3P phases which was previously confirmed by the XRD.
3.5.Wear behavior
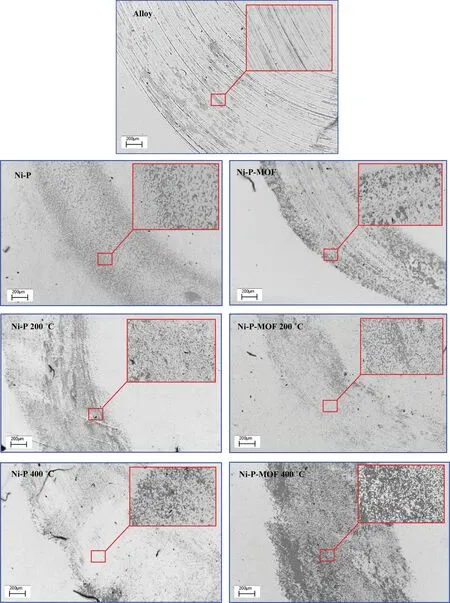
Fig.16.The SEM images of Ni-P and Ni-P-MOF electroless coating after wear test.
The friction coefficient variations of the plain and composite electroless coatings were depicted in Fig.15 and corresponding wear parameters,including averaged friction coefficient during sliding distance and wear rate were listed in Table 3.Generally,the interaction area between the test sample and counterpart is one of the main affecting factors in the friction coefficient.In fact,the surface roughness decreases by increasing the sliding distance and the friction coefficient rise due to the increasing of the contact region between the pin and the test sample surface [34].The SEM images of the samples after the wear test were shown in Fig.16.Traditionally,the wear mechanisms of the nickel electroless coating are adhesive and abrasive [70].The existence of the parallel scars at the surface of the sample indicates the abrasive mechanism which occurs due to the relatively higher hardness of the involved surfaces.Also,in the case of the low hardness of the test sample,worn tracks appear on the surface during the wear test indicating the plastic deformation and adhesive mechanism [34].As is clear from the SEM images,the alloy sample has an abrasive wear mechanism but the electroless coatings (before and after the heat treatment)have adhesive wear mechanism.Table 3 indicates that the friction coefficient of the plain coating was about 0.60 and it was increased to 0.87 after incorporation of MOF nanostructures.Increasing in the friction coefficient is probably due to the more contact area between the sliding pin and surface of the coating.In other words,the higher friction coefficient of the composite coating can be explained by the surface smoothness of this coating which was previously confirmed by the AFM.Also,the wear rate of the plain coating was decreased from 3.77 × 10-9Kg N-1m-1to 3.44 × 10-9Kg N-1m-1after applying the composite coating.This decrease in the wear rate of the Ni-P-MOF coating is probably because of the higher micro-hardness of the coating which was mentioned in Fig.14.Comparison of the results with the literatures revealed that the present coatings have a comparable wear rates with the similar studies [34,56,71].
Furthermore,comparison of the obtained results shows that after heat treatment at 200 °C,the nanocomposite coating has a lower coefficient of friction and wear rate.This result indicates that the composite coating has less contact area with the pin during the test and the wear rate is low due to higher micro-hardness.By extending the heat treatment to 400 °C,the composite coating showed lower wear rate and higher friction coefficient relative to the plain Ni-P coating due to the same reason which was mentioned about the coatings before the heat treatment.Therefore,it could be concluded that the introduction of MIL-53(Al) to the as-deposited Ni-P coating decreased the wear rate before and after the heat treatment because of an improvement in the micro-hardness.It is generally accepted that the wear rate of the electroless coatings decreases by the heat treatment due to the formation of Ni3P hard phase while their micro-hardness increases [72].However,in this study,the wear rate of both coatings was increased at 200 °C relative to the as-plated coatings while it was decreased significantly after the heat treatment at 400 °C.The wear behavior of the heat treated coatings is affected by two antagonistic factors.The first factor is the increase in the coating micro-hardness after the heat treatment which causes to a decreasing in the wear rate.Also,the friction coefficient of the coatings changes in accordance with the contact area between the sliding pin and the coated sample.The second factor is the formation of cracks during the heat treatment which result in formation of debris and increasing weight loss during the wear test [34].In the case of the sample subjected to the heat treatment at 400 °C,the first factor is predominant,as the increase in micro-hardness reduces the wear rate.However,in the case of the sample exposed to 200 °C,a negative effect of the second parameter on the wear rate was observed.
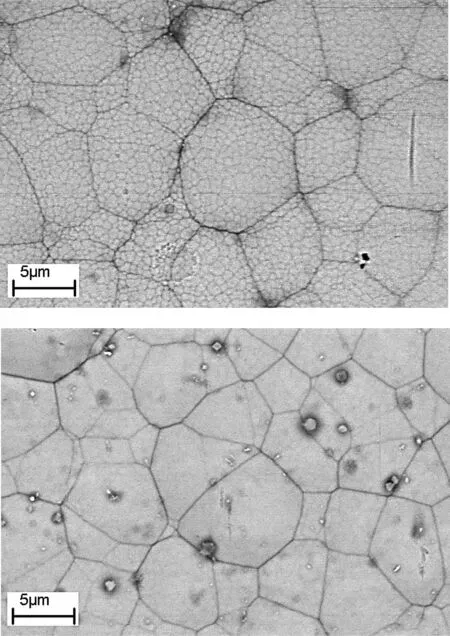
Fig.17.The SEM images of (a) Ni-P and (b) Ni-P-MOF electroless coating after 20 cycle thermal shock tests.
3.6.Adhesion test
The SEM images of the plain and composite coatings after thermal shock test were shown in Fig.17.As the observation and SEM images revealed,both coatings could resist against at least 20 cycles of the heating/cooling which indicates the suitable adhesion of the coatings to the substrate.No crack,blister,and detachment were observed on the samples over the test period.
4.Conclusion
(1) The MIL-53(Al) nanostructures synthesized hydrothermally and the TGA result confirmed that the nanostructures have suitable thermal stability up to 480 °C.
(2) Nodules with the cauliflower like structure were observed for the plain and composite coatings,which had a uniform distribution.After incorporation of MOF nanostructures,the sub-grains of Ni-P coating were disappeared.
(3) The EDS results indicated that the MOF incorporation increases the phosphorous content of the plain coating leading to the lower crystallinity.
(4) The broadening of the characteristic peak of Ni in the XRD pattern indicated that the crystallinity of Ni-P electroless coating was decreased after addition of MOF nanostructure.The heat treatment at 200 °C was not changed the XRD pattern of the coatings but at 400 °C,additional diffraction peaks were appeared due to the crystallization and second-phase precipitation.
(5) The EIS results revealed that the addition of MIL-53(Al)nanostructures was increased the Rctand decreased the Qdlvalues.This higher Rctvalue of the composite coating implies an improved corrosion protection performance.Also,the Jcorrof Ni-P coating was decreased after incorporation of MIL-53(Al) nanostructures.
(6) The composite coating has higher micro-hardness,probably due to the barrier effect of MIL-53(Al) nanostructures against the plastic deformation and preventing the movement of the dislocation.Although heat treatment at 200 °C had a small effect on the micro-hardness of the coatings while it was increased significantly after the heat treatment at 400 °C.
(7) The introduction of MIL-53(Al) to the plain coating decreased wear rate both before and after the heat treatment due to an improvement in its micro-hardness.
Declaration of Competing Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Acknowledgment
The authors wish to thank the Iran National Science Foundation (INSF) and University of Mohaghegh Ardabil for financial support of this study.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Improving the Young’s modulus of Mg via alloying and compositing -A short review
- Surface modifciation of magnesium alloys using thermal and solid-state cold spray processes: Challenges and latest progresses
- Preparation,structure and properties of Mg/Al laminated metal composites fabricated by roll-bonding,a review
- A review on recent advancements in biodegradable Mg-Ca alloys
- Analysis of element loss,densification,and defects in laser-based powder-bed fusion of magnesium alloy WE43
- Pore structure of porous Mg-1Mn-xZn alloy fabricated by metal-gas eutectic unidirectional solidification✩
