In vitro evaluation of degradation,cytocompatibility and antibacterial property of polycaprolactone/hydroxyapatite composite coating on bioresorbable magnesium alloy
2022-10-25ZhngChunynChengLnLinJijiSunDongweiZhngJunLiuHuinn
Zhng Chunyn ,Cheng Ln ,Lin Jiji ,Sun Dongwei ,Zhng Jun ,Liu Huinn,∗
a School of Materials Science &Engineering,Chongqing University of Technology,No.69 Hongguang Avenue,Chongqing 400054,China
b Department of Bioengineering,and the Materials Science and Engineering Program,University of California,Riverside,900 University Avenue,MSE 205,Riverside,CA 92521,USA
Keywords: Mg alloy;PCL/HA coating;Corrosion resistance;Cytocompatibility;Antibacterial property.
1.Introduction
Magnesium (Mg) and its alloys have been increasingly researched as biomedical materials due to their great biocompatibility and biodegradability[1,2].However,a high degradation rate of Mg alloys leads in fast mechanical integrity loses before the injured tissues completely healed,which seriously limits their clinical application [3,4].
A protective coating can effectively delay the degradation of Mg alloys [5,6].In past decades,hydroxyapatite (HA,Ca10(PO4)6(OH)2) has attracted widely studying as a biological protective coating on Mg alloys due to its good osteoconductivity [7-11].HA coating can delay the degradation of Mg alloy to some extent,however,the loose structure and easy cracking of HA coating lead to the infiltration of body fluids and the local corrosion of Mg alloys.Therefore,HA coating alone is not fully competent of protecting the Mg alloys and meets a long service life.In recent years,further modifications of HA coating have become the research focus,such as HA/polymer coating [12,13],HA/inorganic composite coating [14,15] and ion-doped HA composite coating [16,17].As the biomedical polymers have good biocompatibility and biodegradability [18],HA/polymer composite coating not only could further improve the protective effect against Mg alloys,but also endow some biological properties of HA coating [12,13,19].
Polycaprolactone (PCL) is a nontoxic polymer,which has a slow degradation rate under physiological media and harmless degradation products (i.e.,carbon dioxide (CO2) and water (H2O)) to human body.Therefore,PCL has been used as tissue scaffolds and drug carriers [20].However,PCL used as protective coating for bioresorbable Mg alloys has been little explored because of its poor adhesion to Mg substrates.Bakhshag-Rad et al.[16] synthesized a FHA/ PCL coating on Mg-2Zn-3Ce alloy via a combination of electrodeposition (ED) and dip-coating methods.The results showed that the FHA/PCL coating had higher binding properties,more apatite nucleation sites and lower corrosion rate during the immersion process compared with the PCL coated and uncoated samples.Zomorodian et al.[20] prepared PCL/HA coating on AZ31 by dipping methods.The composite coating consisted of a polycaprolactone (PCL) matrix modified with nanohydroxyapatite (HA).The presence of HA enhanced the cell proliferation and cell viability of PCL layer,while the barrier effect of the composite coating was enhanced by the increase of PCL concentration.Abdal-Hay et al.[21] mixed HA nanoparticles with PCL to produce a colloid and a porous film was formed via immersion the AM50 Mg alloys into the PCL/HA composite colloid.The MTT cytotoxicity test results showed that PCL/HA composite coated Mg alloy has better cytocompatibility than the PCL coated Mg and non-treated Mg alloy.Although the biological and degradation properties of Mg alloy can be improved by the composite coating to a certain extent,the binding strength between organic polymers and HA was not satisfied because of the different properties,such as solubility,surface polarity and water absorption performance.Consequently,the interface delamination of PCL and HA often occurred duringin vitrodegradation experiments and resulted in the reduction of their protective effects.
Three strategies could be employed to solve the weak binding strength between the organic polymers and HA: (1)adding an intermediate layer [22];(2) etching the organic phase [23];(3) constructing a rough coating to form a “mechanical lock” to lock HA and organic phase together [24].γ-Aminopropyl triethoxysilane (KH550) hydrolyzes to alkaline,which could effectively improve the interfacial adhesion between two phases with different surface activities.Fang and Feng [25] used KH550 as the interface adhesive between HA and PLLA,which not only improved the surface chemical compatibility,but also achieved an improvement of the compressive strength of HA/PLLA stents.
In this work,we synthesized a PCL/HA composite coating on AZ31 alloy that exhibited excellent interface binding strength between the PCL and HA and corrosion resistance.Firstly,HA coating with micro-nano scale pores was synthesized by a simple hydrothermal method,then it was modified by KH550 to improve the interfacial adhesion between the organic and inorganic phase.Finally,the modified HA coating was dipped into PCL solution to make the PCL filling into the pores and form a dense and interlocked PCL/HA composite coating onto the AZ31 alloy.
In addition to good coating adhesion and low degradation rate,high biological activity of coating has also been desired for biomaterials.The early adhesion and growth of cells on the surface of implant materials is necessary for the cells to further secrete the extracellular matrix and synthesize related proteins.Studies have shown that factors such as the microscale morphology,surface energy,roughness,chemical composition,and structure of the material surface may affect osteoblasts [26-28].Furthermore,colonization of orthopedic devices caused by MRSA can raise difficulties to treat infections that require additional medical treatment and extended time for patient healing [29].For this reason,the cytocompatibility with BMSCs and the antibacterial property against MRSA of the PCL/HA composite coating were evaluated.
2.Experiments
2.1.Preparation of PCL/HA composite coating
An AZ31 alloy rod (Dongguan Kangji Magnesium Industry Co.LTD;Al: 2.88 wt.%,Zn: 0.81 wt.%,Mn: 0.30 wt.%,Si: 0.24 wt.%,Mg: Bal.) was sliced into disks with a diameter of 10 mm and thickness of 1 mm.All raw AZ31 disks were polished using silicon carbide (SiC) abrasive paper in a sequence with #600 grit,#800 grit,#1200 grit in ethanol,followed by ultrasonically cleaned for 15 min in ethanol.The AZ31 disks,referred as “AZ31” samples,were then immersed in a solution mixed of 0.25 M Ca-EDTA (C10H12N2O8Na2Ca) (Tianjin Guangfu Fine Chemical Research Institute) and 0.25 M KH2PO4(Chengdu Cologne Chemicals Co.LTD) in a Teflon container,and maintained at 363 K for 4 h to prepare the HA coating.The pH value of the hydrothermal solution was adjusted to 9.0 with 0.1 M NaOH solution at room temperature.Then the HA coating was modified by dipping the HA coated AZ31 disks into 100 g/L KH550 (NH2CH2CH2CH2Si(OC2H5)3) (Huaian Heyuan Chemical Co.LTD) and 90% ethanol aqueous(the volume ratio of ethanol and deionized water was 9:1) for 5 min,withdraw at a constant speed and dried at 150 °C for 20 min.The HA coated AZ31 samples,referred as “HA” samples,were further modified by KH550 solution and were referred to as “M-HA”.Finally,the “M-HA” samples were soaked into 30 g/L PCL dichloromethane solution(Mw=80,000 g/mol,Solvay Fine Chemical Co.,Ltd.China)for 10 min and then dried at room temperature for 24 h to prepare the sample with PCL/HA composite coating,which was designated as “PCL/HA” samples.
2.2.Evaluation of coating adhesion
The adhesion strength of PCL/HA coating to the Mg substrate was qualitatively assessed by tape test according to Chinese national testing standards(GB/T 9286-1998).In the tape test,the PCL/HA coating was cross engraved into a lattice pattern of 1 mm × 1 mm.Then a pressure-sensitive adhesive tape(P99,Permacel,USA)was applied over the coatings with lattice patterns.Finally,both ends of the tape were removedat the same time in the direction perpendicular to the sample surfaces.The adhesion strength was evaluated by comparison with descriptions and illustrations in the standard (GB/T 9286-1998).
2.3.Evaluation of the corrosion resistance
2.3.1.Electrochemical measurement
The electrochemical tests were performed in Hank’s solution to compare the electrochemical corrosion properties between HA coating and PCL/HA coating.Electrochemical measurements were conducted using a potentiostat (Gamry 3000) in a traditional three-electrode cell configuration,platinum was used as the counter electrode,saturated calomel electrode (SCE) was used as a reference electrode and the specimen was used as a working electrode.The specimens of“AZ31”,“HA” and “PCL/HA” samples were firstly immersed in Hank’s solution for 30 min to acquire a stable open circuit potential (OCP).Then electrochemical impedance spectroscopy (EIS) was scanned in the frequency ranging from 100 kHz to 0.01 Hz under 10 mV amplitude of the perturbation signal.The potentiodynamic polarization (PDP) test was conducted at the scan rate of 0.5 mV·s-1so that the corrosion reaction can approach a steady state [30,31].The initial polarization of cathodic potential is from -0.5 V versus OCP.Considering the passivation behavior of the anode,the anodic scanning potential ends at 1 V versus OCP.The corrosion currents were determined using Tafel extrapolating.The chemical composition of Hank’s solution was given in Table 1.The pH value of Hank’s solution is 7.4 and doesn’t need to be adjusted.

Table 1 Chemical composition of Hank’s solution (g/L).
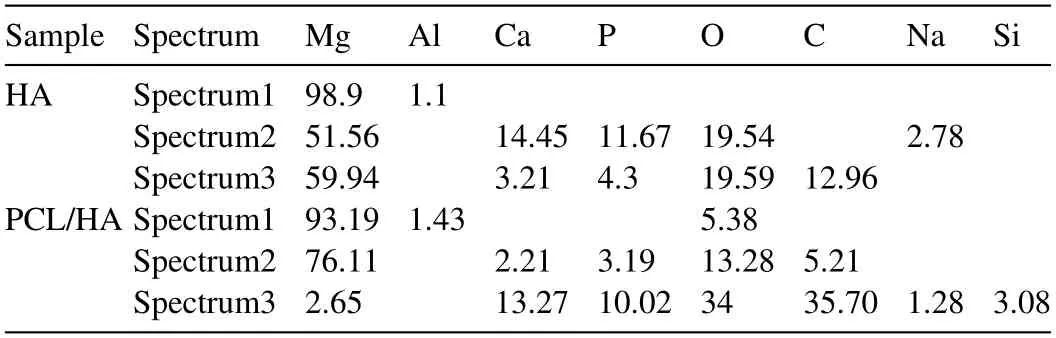
Table 2 EDS analysis (at%) of the Fig.3.
2.3.2.Immersion test
The immersion tests were carried out in Hank’s solution at 37.0 °C ± 0.5 °C to investigate the degradation performance of the samples.According to the ASTM G31-72 standard,solution volume-to-sample area ratio is between 20 ml/cm2and 40 ml/cm2,and the selected ratio of current immersion tests is 30 ml/cm2.The degradation behavior of the PCL/HA coating was investigatedin vitroby observing the changes in the surface morphology and compositions of the samples soaked at different prescribed time points.During the immersion test,the Hank’s solution was refreshed in every other day.After the immersion process,the samples were cleaned with deionized water,air-dried and analyzed.
2.4.Characterization
The surface morphologies of coatings were observed by scanning electron microscopy (SEM,ΣIGMA HDTM).The phase constitutions,chemical bonding groups and chemical composition of the coatings were analysed by X-ray diffraction meter (XRD,Empyrean DX-2500),Fourier transform infrared spectra (FTIR,NicoletIs10) and energy dispersive Xray spectroscopy (EDS,Oxford Isis).The surface roughness parameters of samples are measured by 3D laser scanning microscope (Keyence VK-X).
2.5.Cytocompatibility with BMSCs
2.5.1.Direct culture of BMSCs
Besides the “AZ31”,“HA” and “PCL/HA” samples,a reference group of “Glass” (Fisher Scientific,1 mm thick,10 mm in diameter) and a blank group “BMSCs” that had BMSCs alone in media without samples were included in the cell culture study.Before cell seeding,all the samples were placed in standard 12-well tissue culture treated plate and disinfected each side under ultraviolet (UV) radiation for 1 h.
BMSCs were harvested from the marrow cavity of the femur and tibia of rat and cultured in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum(FBS)and 1%penicillin/streptomycin(P/S)under standard cell culture conditions,according to the procedures published in reference [30].BMSCs of passage two (P2) were seeded onto the disinfected samples at a density of 1 × 104cells cm-2and incubated in 3 mL DMEM+10%FBS+1%P/S under standard cell culture conditions for 24 h.BMSCs cultured in DMEM+10% FBS+1% P/S served as the cell-only reference group.The DMEM group without BMSCs served as the media-only reference.After a 24 h of cell culture,the media was collected and the pH value was measured immediately using a pH meter (Symphony TM,Model SB70P,VWR).The concentrations of Mg2+and Ca2+ions were measured by inductive coupled plasma optical emission spectrometry (ICP-OES,Optima 8000,Perk in Elmer).
2.5.2.Evaluation of BMSC adhesion and morphology
The “AZ31”,“HA”,“PCL/HA” and “Glass” samples were transferred to new disinfected 12-well plates.BMSCs that adhered on the surface of samples and on the tissue cultured well plate around the samples were fixed with 4% formaldehyde,and then stained with Alexa Flour 488 (vendors) for Factin and DAPI (vendors) for nuclei.The BMSCs adhered on the surface of “AZ31”,“HA”,“PCL/HA” and “Glass” samples were marked as direct contact,while the BMSCs on the tissue cultured well plate around the samples were marked as indirect contact.The stained cells were imaged using a fluorescence microscope (Eclipse Ti,Nikon).At least six random fields were imaged to ensure the reliability of the cell statistical results.
Image J analysis tools were used to quantify the cell adhesion density,spreading area per cell and aspect ratio.Cell adhesion density was defined as the number of adherent DAPIstained cell nuclei (blue color) per square centimeter.The spreading area per cell was quantified by the area of cell skeletons (green color) stained with F-actin in the fluorescence image for each BMSC.The cell aspect ratio was defined as a ratio between the length of the major axis and the length of the minor axis of the elliptical fitting for a cell outline.
2.6.Antibacterial analysis with MRSA
For orthopedic infections,a minimal bacterial seeding density of 7.8 × 106cells/ml is the clinically relevant concentration [29].The actual seeding density in this study was 2.1 × 107cells/ml in the media of revised simulated body fluid (r-SBF) +10% FBS.The seeding density was determined by a serial dilution of the bacterial culture media to 1:10,000,and 100 μL of the bacterial culture media was plated onto Tryptic Soy Agar (TSA;Fluka Analytical,Sigma-Aldrich) and incubated at 37 °C for 24 h.
2.6.1.Bacterial culture and post-culture bacterial viability analyses
Before cell seeding,all the samples (“AZ31”,“HA”,“PCL/HA” and “Glass” group) were placed in standard 12-well non-tissue treated plate and sterilized under UV radiation for 1 h on each side.Additional controls included bacteriaonly and media-only groups.
To evaluate the bacterial density in the media that cultured with the samples and on the surfaces of samples,the bacterial culture with a volume of 3 ml were added to individual experimental samples in 12-well plates and incubated in a shaker incubator at 37 °C and 50 rpm for 24 h.After incubation,the media that contained the suspended bacteria were collected from each well and analyzed for pH and the concentrations of Mg2+and Ca2+ions.To determine the bacterial density in the media that cultured with the samples,the collected media were serial diluted and plated on their respective agar plates and the colony forming units (CFUs)was counted to calculated the bacterial density.Each cell culture was plated in triplicate.These plates were incubated at 37 °C for 24 h.Afterward,the concentrations of the respective bacteria in suspension were determined by counting the CFUs on each agar plates.To evaluate bacterial density on the surfaces of samples,the samples were washed three times using Tris (hydroxymethyl) aminomethane buffer (Tris buffer,pH 8.5;Acros,Sigma-Aldrich) to remove non-adhering bacteria.Then the individual samples were placed into 5 ml Tris buffer and vortexed for 5 s and sonicated for 5 min twice to dislodge the adhered bacteria.The Tris buffer containing the bacteria dislodged from the samples was diluted,plated,incubated and counted to determine the CFUs.
2.6.2.Characterization of bacterial adhesion and morphology
In preparation for SEM,one washed sample from each type of sample was placed into an individual well in a new well plate.The bacteria adhered on each samples was fixed using 3% glutaraldehyde in Tris buffer for one hour followed by dehydration by submersion in a series of ethanol solutions with increasing concentrations from 30,70 to 100%for 30 min each.Dehydration in 100% ethanol was repeated twice.The samples were sputter-coated with platinum/palladium (Pt/Pd) for 60 s,then characterized by SEM to observe the morphologies of MRSA.
2.7.Statistical analysis
All numerical data used in this study were obtained from experiments run in triplicate and examined using a one-way analysis of variance (ANOVA) followed by the Tukey posthoc test for detecting statistical differences.Statistical differences were considered at∗p <0.05,∗∗p<0.01,∗∗∗p<0.001.
3.Results and discussions
3.1.Microstructure and composition of the coating
As shown in Fig.1(a1) and (a2),after 4 h of hydrothermal deposition,a uniform coating mainly composed of Ca,P and O elements was formed on “AZ31” samples.The coating consisted of a dense array of nanoscale rod-shaped crystals perpendicular to the substrate surface.Fig.2a and b are the XRD patterns and FT-IR spectra of samples.In addition to the diffraction peaks from substrate AZ31,peaks of coating at 25.9°,28.1°,28.9°,46.7°,and 49.5° and 53.2°are in good match with the characteristic peaks of (002),(102),(210),(222),(213) and (004) crystal planes of HA(Ca10(PO4)6(OH)2,PDF#09-0432).Moreover,the peak intensity from (002) plane is higher than that of other planes.The relative peak intensity from (002) plane was about 225% (I(002)/I(211)),significantly higher than 40% of JCPDS (PDF 09-0432).And the relative peak intensity from (004) plane(54%) was also higher than that of JCPDS (20%),indicating that the rod-shaped HA crystals grew oriented along the c axis orientation.Calculated using the Scherrer formula,the average diameter of the rod-like HA crystals was about 24 nm.FT-IR spectra of HA sample shows that the absorption peaks at 547 cm-1,601 cm-1and 1007 cm-1correspond to the PO43-groups of HA.Fig.1(a2) shows the atomic percentage of Ca and P elements on HA coating were 24.99 and 21.58%,separately.The atomic ratio of Ca/P was about 1.16,less than the theoretical atomic ratio of HA (1.67).And a small amount of Na (3.16 at%) was detected in HA coating.The results indicated that HA coating might be the calciumdeficient apatite and some Na+might dope into HA lattice.The SEM in Fig.1(b1) shows that the coating morphology of “M-HA” samples did not changed much after modified by KH550.The XRD pattern in Fig.2(a) shows there was no new phase formed.But the EDS result in Fig.1(b2) demonstrates that element C and Si were detected in “M-HA” sample.Fig.1(c1) displays that after soaked in the PCL solution,the space between the modified HA crystals was filled by PCL and a few tips of some rod-shape crystals still could be observed.EDS results in Fig.1(c2) shows the content of C and Si on the surface of PCL/HA composite coating was significantly increased compared with that on M-HA coating.The XRD pattern of PCL/HA displays that the peak intensity of Mg and HA decreased due to the coverage of PCL,but no organic peak of PCL was detected which might be caused by no heat treatment.According to the research,the corrosion resistance of the PCL layer is better without heat treatment [31].After coated with PCL,some new absorption peaks appeared in FTIR spectra (Fig.2b).The absorption peak at 1085 cm-1was attributed to the stretching vibration peak of the~Si-O-Si~group in KH550.Symmetric stretching vibration peak and asymmetric stretching vibration peak from -CH2-group appeared at 2995 cm-1and 2945 cm-1.The absorption peaks of 1362 cm-1and 1267 cm-1were the bending vibration peaks of -CH2-.Furthermore,the absorption peak at 1382 cm-1corresponds to the C-N stretching vibration peak in KH550 [32] and the absorption peak at 1453 cm-1was caused by the deformation of CH2.FTIR spectroscopy also showed strong stretching vibration peaks of C=O groups at 1755 cm-1and C-O at 1180 cm-1and 1086 cm-1.The characteristic peak of C-N-H bending vibration at 1561 cm-1represented that covalent bond was formed between KH550 and PCL.According to the literature[32,33],KH550 could form an ionic bond between~Si-OH and metal ions by hydrolysis into alkaline form.After the complete hydrolysis of KH550,the molecules formed can also be bonded to form~Si-O-Si~network structure.Therefore,the change of infrared absorption peak not only reflected the functional groups from PCL but also reflected the grafting behavior of KH550 with HA coating and its condensation reaction with PCL.
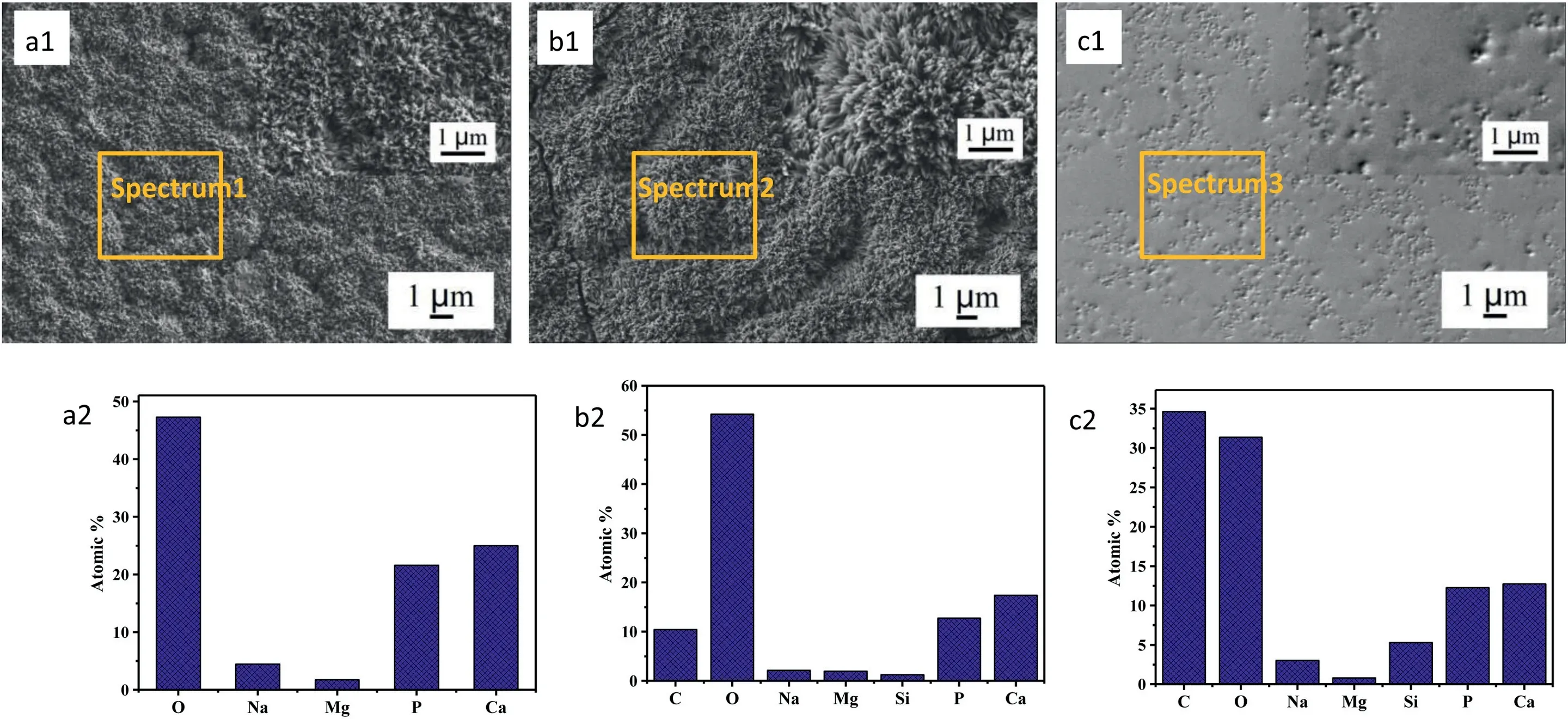
Fig.1.Surface SEM morphology (a1-c1) and corresponding EDS analysis (a2-c2) of the coating (a: HA;b: M-HA;c: PCL/HA).
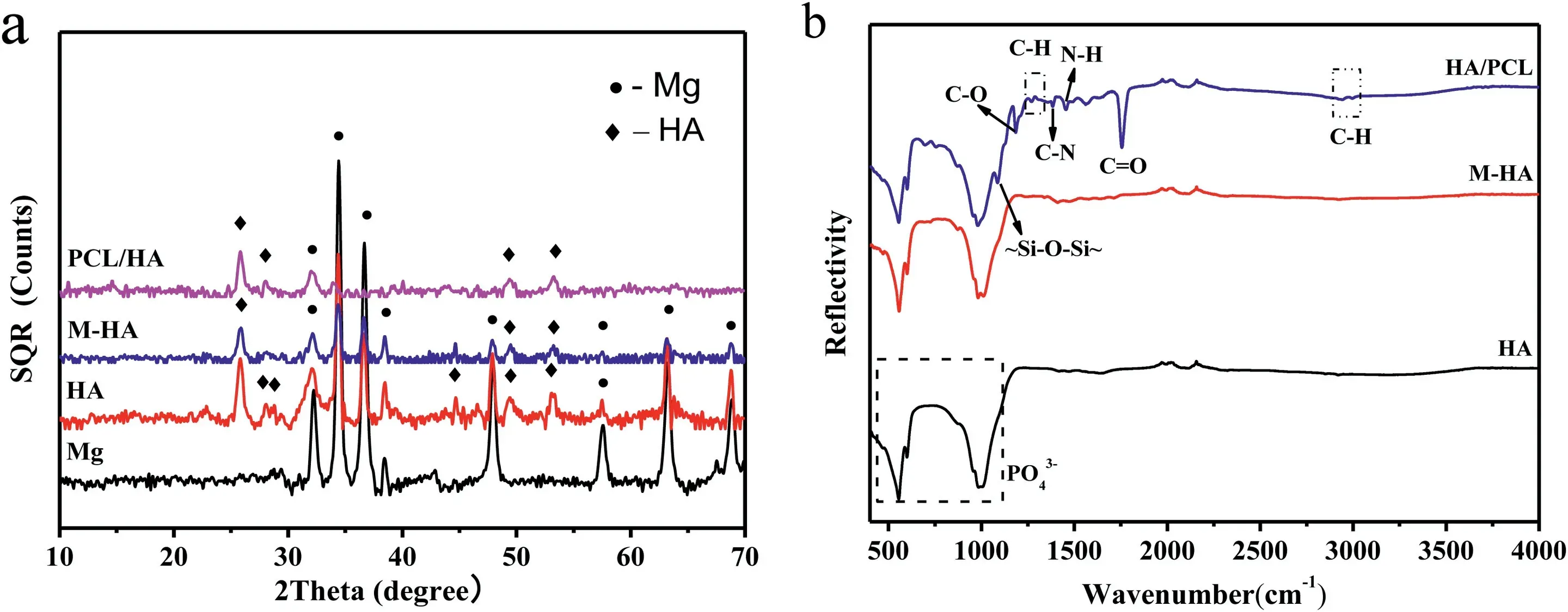
Fig.2.(a) XRD patterns and (b) FTIR spectra of samples.
3.2.Coatings adhesion
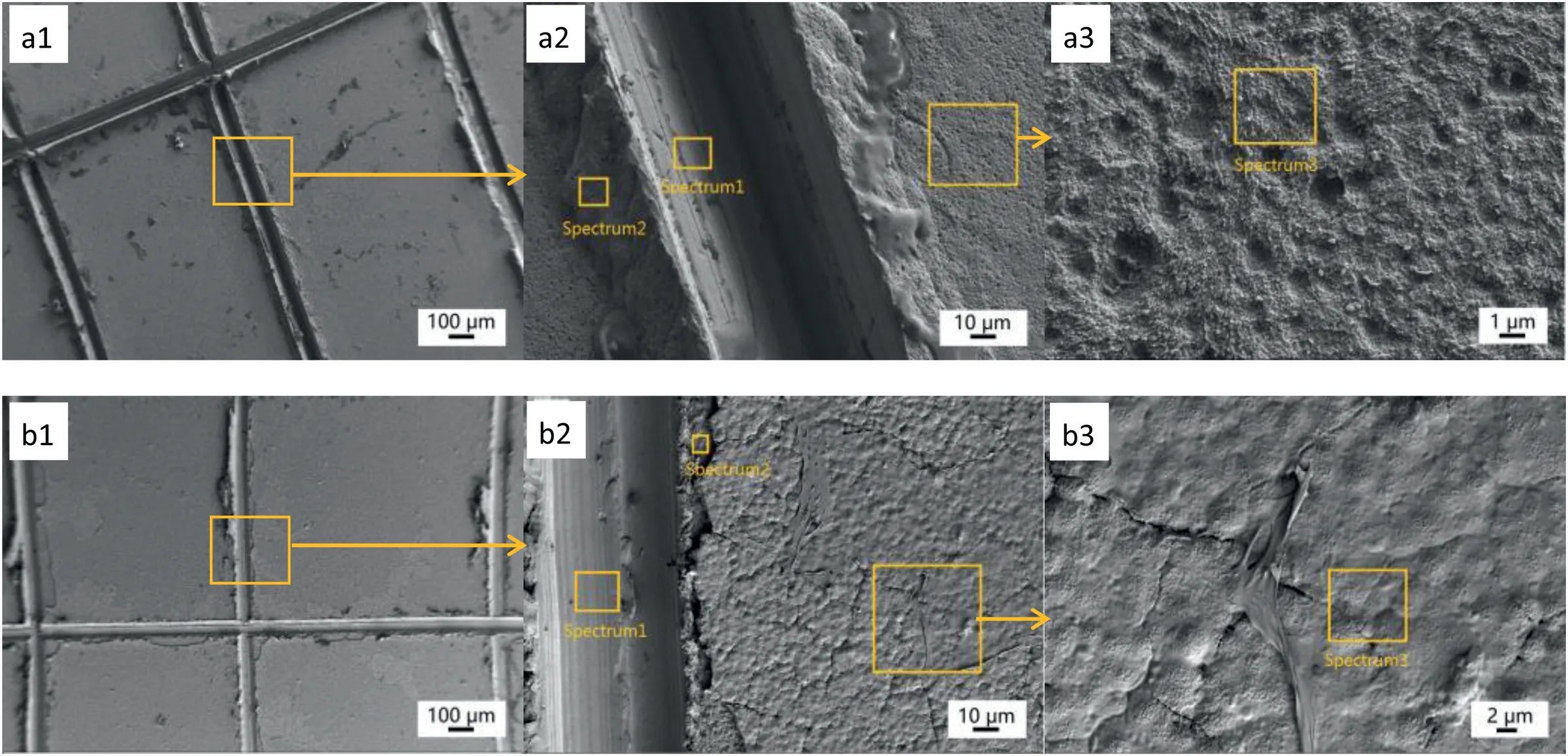
Fig.3.The SEM micrographs and corresponding EDS analysis (at%) of (a) HA coated AZ31 sample and (b) PCL/HA coated AZ31 sample after tape test.
The SEM images and corresponding EDS analysis of“HA” and “PCL/HA” samples are shown in Fig.3 and Table 2.After the tape test,the content of Ca and P on the surface of samples (Fig.3(a3) Spectrum3) was significantly decreased and the layer composed of rod-shape crystals was totally detached from the HA coated samples.The presence of element Ca and P on the surface and at the edge of the cut(Fig.3(a2) Spectrem2) of the sample indicated that there was a thin and compact Ca-P layer on the surface of “HA” sample.Fig.3(b) shows that the surface chemical composition of the “PCL/HA” sample (Fig.3(b3) Spectrum3) changed little compared with that before the tape test.No large-scale peeling off occurred of PCL/HA composite coating except at the edge of the cut(Spectrem2).Therefore,it could be considered that there was a strong adhesion between HA and PCL and the overall bonding performance of PCL/HA coating with AZ31 substrate were satisfied.
3.3.Corrosion resistance of the coatings
3.3.1.Electrochemical corrosion analysis
Fig.4a shows the open circuit potential (OCP) curves of the specimens.The OCP curve of three samples increased rapidly in the first 200 s,then rose up slowly and reached a constant value.Fig.4b shows the potentiodynamic polarization curves of samples in Hank’s solution.The corrosion potential (Ecorr) and corrosion current density (icorr) are listed in Table 3.The corrosion potential of the “HA” and “PCL/HA”samples were similar to that of the uncoated AZ31 alloy,indicating that the coatings has little effect on the corrosion tendency of Mg alloys.Theicorris used to determine the average corrosion ratePi(mm/year) [34].
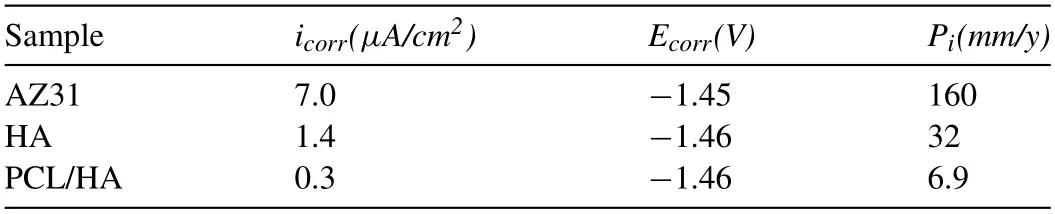
Table 3 Parameters obtained from the polarization curves.
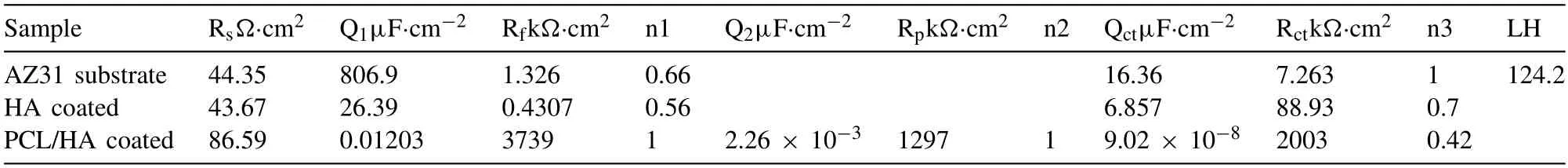
Table 4 Fitting parameter of the equivalent circuit.
Pi=22.85icorr
As can be seen that the uncoated AZ31 alloy had the largesticorr(7.0 μA/cm2) and corrosion rate (160 mm/year).The corrosion rate of “HA” sample was about 5 times lower than that of “AZ31” samples.While the “AZ31” and“PCL/HA” samples exhibited the smallesticorr(0.3 μA/cm2)andPi(6.9 mm/year),nearly 23 times lower than those of“AZ31”samples.The results indicated HA coatings could improve the corrosion resistance of AZ31 alloy slightly,while PCL/HA composite coating significantly reduced the corrosion rate of “AZ31” samples.Electrochemical polarization measurement is the process of accelerating corrosion of applying voltage.Due to the small area of the magnesium anode and the large area of the coating as the cathode,pitting corrosion can be observed obviously on the sample surface after measurement,and the Mg substrate under the coating was corroded seriously.The magnesium matrix will be accelerated corrosion.Therefore,the corrosion rate of coating sample calculated by the equation used by Atrens et al.is higher than that of the immersion test.

Fig.4.(a) The open circuit potential and (b) potentiodynamic polarization curves of samples in Hank’s solution.
Fig.5a and b show the Bode and Nyquist plots of EIS of the samples in Hank’s solution.Barrier properties of the coating could be evaluated by the impedance modulus and the diameter of the capacitive loop.As can be seen,the value of impedance polarization resistance (the Zmodvalue at the frequency of 0.01 Hz) and the capacitive reactance arc diameter of AZ31,HA coated and PCL/HA coated AZ31 increased obviously in turn.This indicates that the corrosion resistance of AZ31 alloy was improved by HA coating,while PCL further enhanced the protective property of HA coating.The change of phase angle (Fig.5a) with frequency is mainly caused by the change of capacitance of each part of the system.The high frequency corresponds to the coating response.The closer the value of phase Angle to 90° in a broader frequency range,the more similar the coating to pure capacitance,indicating that the better the insulation of the coating is and the less likely it is to be permeated by ions.According to the position and the number of phase Angle peaks,as well as the diameter of capacitive arc or inductive arc,the corresponding circuit elements can be estimated to match the coating structure and the appropriate equivalent circuit diagram can be selected to simulate the electrode reactions process.Fig.5c shows the equivalent circuit diagram of samples.The fitting results are listed in Table 4.Parameters related to the coating corrosion resistance mainly include coating resistance (Rf) and capacitance (Qf),reaction resistance (Rct) and electric double-layer capacitors (Cdl) and inductance (L).Due to the "dispersion effect",the constant phase Angle element Q is used instead of the pure capacitance C.Because of the existence of passivation film and HA coating,the EIS of the AZ31 and HA coated AZ31 alloy exhibit two Phase Angular peaks and two capacitance loops at high and medium frequencies separately,corresponding to the information of coating (Rfand Qf) and interface between coating and substrate (Rctand Qct),respectively.The existence of inductive arc in fourth quadrant of AZ31 alloy demonstrates that reactions of gain and loss of electrons on the surface of AZ31 alloy occurred.This also indicates the occurrence of pitting on the surface of AZ31 substrate which will cause stress concentration and fracture failure of implant materials.The phase angle and Rfof HA coated sample was lower than those of AZ31 substrate due to the porous nature of the HA coatings.After modified by PCL,the “PCL/HA” sample presented a phase angle peak in high frequency and two phase angle peaks in low frequency region.The maximum phase angle of PCL/HA sample was shifted to the right and was close to 90° in a range of frequencies from 103to 105Hz.The increase of phase angle peak value in high frequency region indicates that the PCL/HA layer could provide more effective shielding performance than HA coating alone.Rf,Rpand Rctof the corresponding equivalent circuit,respectively represent the outer PCL/HA coating resistance,inner compact Ca-P layer resistance and reaction resistance.According to the fitting results of equivalent circuit (Table 4),the Rctof HA coated sample was 12 times that of AZ31 alloy and was further increased 23 times after modified by PCL,indicating that the charge shielding performance and corrosion resistance of AZ31 alloy was significantly improved by PCL/HA composite coating.The high Rfvalue of “PCL/HA”sample show that PCL/HA coating has strong ability to prevent solution ion penetration.
3.3.2.Immersion test
Fig.6 shows the SEM images of the “PCL/HA” samples after immersing in Hank’s solution for different days.The surface compositions of the sample after immersing for different days were listed in Table 4.During the first 8 days of immersion,the PCL covering the surface of HA coating was dissolved.The exposed rod-shaped HA crystals and the filled PCL are visible obviously.EDS results in Table 5 show that the content of C on the surface of the sample decreased after immersed for 13 days and the content of Mg,Ca and P increased slightly (Spectrum 4).After further immersion,the height of the exposed rod-shaped crystals gradually decreased and the rod-shaped crystals were no longer obvious,the coating became denser.The overall surface morphologies of the sample immersed from 13 days to 38 days changed little except that there were some scattered spherical precipitates and a few pores caused by the degradation of PCL on the surface of coatings.The composition of the coating did not change much and XRD pattern (Fig.S1) shows that the coating was still mainly composed of HA.The results above indicated the degradation rate of PCL slow down after initial degradation and there was no significant degradation of the PCL/HA coating.Therefore,the change of coating morphology during the immersion may be due to simultaneous action of the two processes,which involves the dissolution of rod-shaped HA crystal and the adsorption of the Ca2+and PO43-ions in solution.The various cracks appeared on the surface of coating might be caused by vacuum dehydration of the coating as well as the impact from the electron beam.Fig.6i shows the PCL/HA coating was stable and adhered to the surface of AZ31 substrate after 38 days of immersion,demonstrating that the PCL/HA composite coating has satisfied adhesion strength and low degradation rate.

Fig.5.EIS plots of samples (a.Bode;b.Nyquist) and the equivalent circuit of simulated electrode process for each sample (c1:R(QR)(QRL)(AZ31);c2:R(QR)(QR)(HA);c3: R(QR)(QR(QR))(PCL/HA).
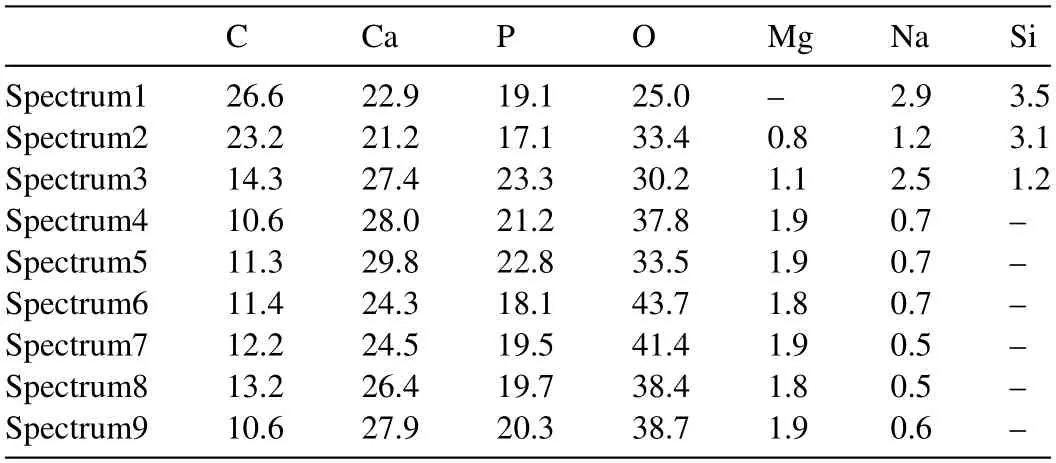
Table 5 Chemical compositions (at%) in the regions of Fig.6.
3.3.3.Corrosion resistance mechanism of PCL/HA coating
The compactness of coating and the bonding property between coating and substrate are two important factors to protect magnesium alloy from corrosion.The KH550 could covalent bond onto HA coating,and the amino groups of KH550 could condense with PCL through acetylation,which improved the surface chemical compatibility between HA and PCL and achieved a “chemical bonding”.The schematic diagram of chemical bonding between coatings is shown as Fig.7.Moreover,the micro-nano spaces of between the HA crystals constructed a rough surface for the penetration of PCL and form a “mechanical lock” between HA and PCL.The combination of “chemical bonding” and “mechanical lock” improved the adhesion strength of the composite coating.This might be one of the reasons why the PCL/HA composite coating remained intact on the surface during the entire immersion test period.
Normally,pure PCL coating on the substrate could almost completely block the seeping of the body liquid and protect the substrate from degradation in a scheduled time [35].PCL between the HA crystals filled the porous of HA coating and prevented the solution from penetration and corrosion of the magnesium alloy.Compared with single HA coating,the high impedance modulus and the large diameter of the capacitive loop of PCL/HA were attributed to the filling of PCL in the pores of HA coating.
After the initial degradation of PCL adhered on the outer surface of coating,the degradation rate of PCL/HA composition coating decreased significantly.The dissolution and reprecipitation of the HA during the degradation process resulted in the morphology change of the coating.The presence of HA crystals in PCL coating helped in enhancing biomineralization and developing CaP deposits on the surface,which resulted in a reduced degradation and provided satisfactory protection for longer immersion time.The HA particles in those coatings reduced the acidification of Hank’s medium due to PCL degradation.This buffering manner might benefit the slow degradation of PCL as well as avoid the probable inflammatory response owing to acidic degradation of the PCL polymer.The corrosion resistance mechanism of PCL/HA coatings was illustrated in Fig.8.
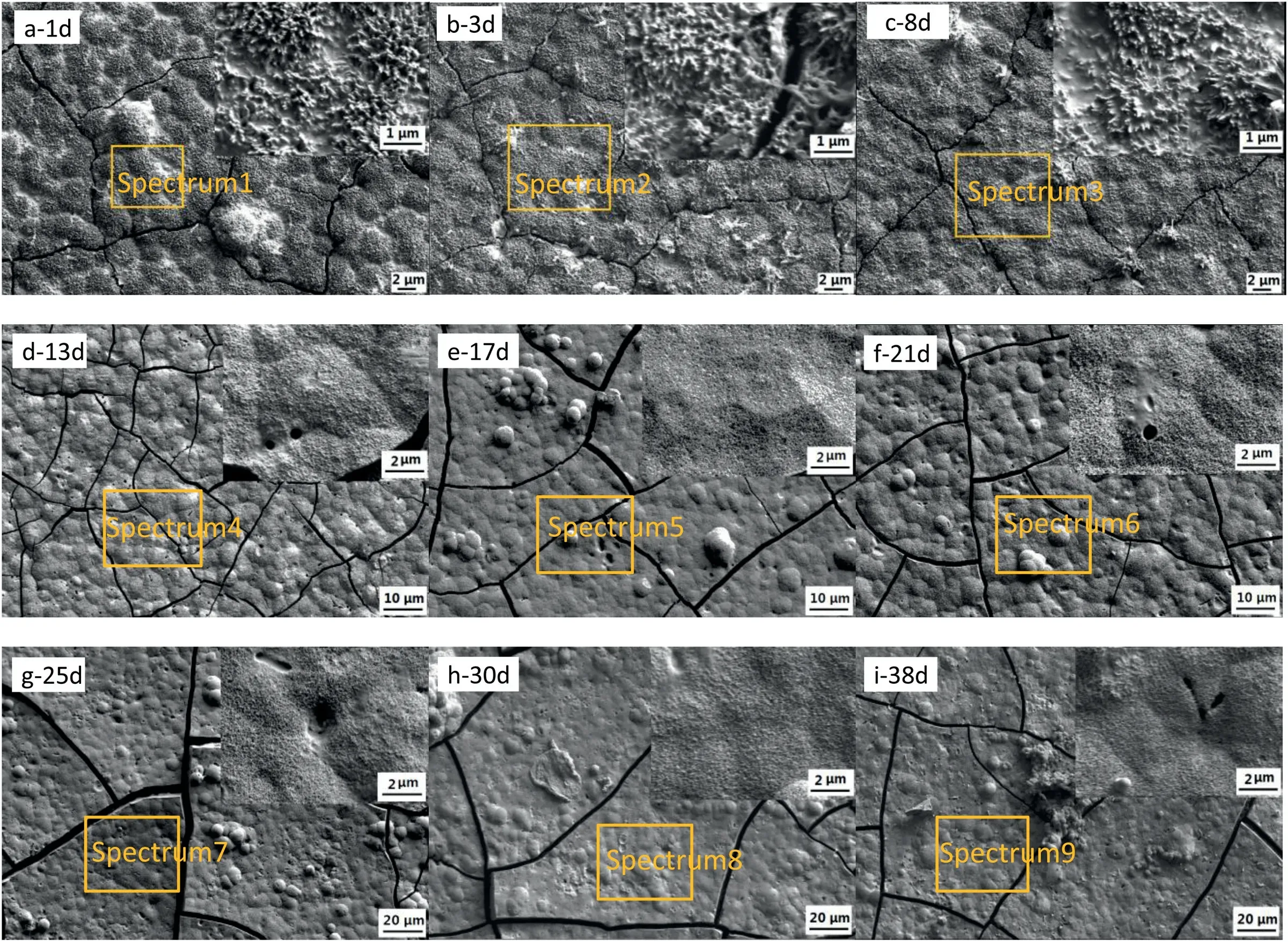
Fig.6.SEM micrographs of PCL/HA coated sample after immersed in Hank’s solution for different days (a: 1d;b: 3d;c: 8d;d: 13d;e: 17d;f: 21d;g: 25d;h: 30d;i: 38d).
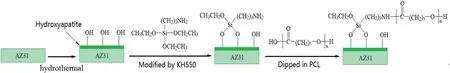
Fig.7.The schematic diagram of chemical bonding between coatings.
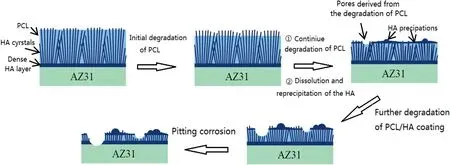
Fig.8.Diagram of degradation process of PCL/HA composite coating.
3.4.Cytocompatibility in direct culture
3.4.1.BMSC adhesion and morphology under direct contact and indirect contact conditions
The representative fluorescence images in Fig.9 demonstrated that under the condition of direct contact,the BMSCs that adhered on the reference group of glass and the BMSC control show the typical spreading cell morphology of non-proliferating cells (polygonal cell morphology) [36].The spreading of BMSCs on the surface of “HA” samples was greatly inhibited and cell spheroids could be observed.However,the BMSCs on “AZ31” and “PCL/HA” samples showed a spindle-shaped morphology,which is the typical result of BMSCs differentiation [36].Under the indirect contact condition,the morphology of BMSCs surrounding the HA coated samples appeared no difference from “AZ31”,“PCL/HA” samples and other reference groups.
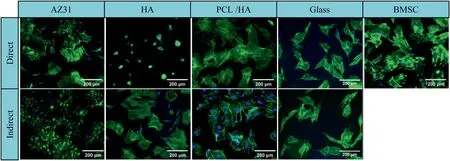
Fig.9.Fluorescence images of BMSC on the sample surface (direct contact with the sample),on the culture plate surrounding each corresponding sample(indirect contact with the sample) after in vitro culture for 24 h.
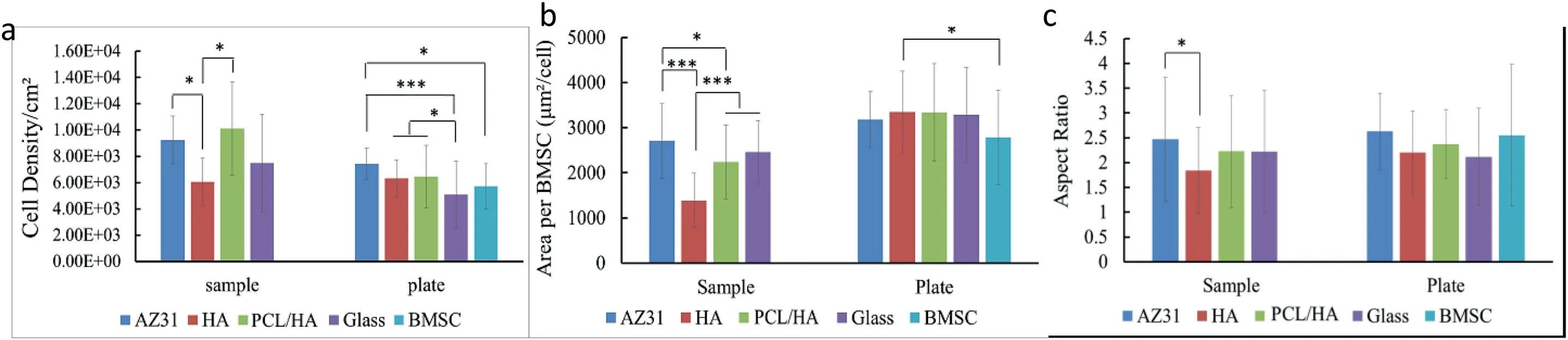
Fig.10.The quantitative analysis after BMSC culture for 24 h with the coated AZ31 alloys,controls (AZ31),and references (medium,BMSC and glass).(a) adhesion density of BMSCs,(b) spreading area per BMSC,(c) aspect ratio of BMSCs on the sample surface (direct contact with the sample) and on the culture plate surrounding each corresponding sample (indirect contact with the sample).The values are mean ± standard deviation (n=3).∗p<0.05,∗∗p<0.01,∗∗∗p<0.001.
Fig.10 shows the cell adhesion density,spreading area and aspect ratio of BMSCs on the sample surface (direct contact)and the culture plate(indirect contact).As shown in Fig.10(a)under direct contact,the cell adhesion density of HA coating shows significantly lower value than that of “AZ31” and“PCL/HA” samples,while no significant difference from the glass reference.In the Fig.10(b),the spreading area per BMSC on HA coating was significantly lower than other groups,which was consistent with the results of fluorescence images.AZ31 alloy presented a statistically higher spreading area per BMSC compared with HA” and “PCL/HA” samples,and higher aspect ratio than HA coating.No statistically significant difference in the aspect ratio was found among the“HA”,“PCL/HA” samples and glass reference group.In addition,there is no significant difference in the cell density and aspect ratio of“AZ31”,“PCL/HA” samples and glass reference groups.Under indirect contact condition,the cell adhesion density of the HA and PCL/HA coating groups was significantly higher than the glass reference group but no statistical difference from the BMSC reference and AZ31 control groups.The cells under indirect contact show higher spreading area per BMSC than that of direct contact condition.Furthermore,the cells on the HA coating presented a statistically higher spreading area per BMSC than the BMSC-only control.No significant statistical difference of the aspect ratio was found among the coating,control and reference groups.The results of indirect contact condition indicated that HA and PCL/HA coating are nontoxic to BMSC.
3.4.2.Analyze the pH and ion concentration in post-culture media
Fig.11 exhibits the pH value,Mg2+and Ca2+ion concentration of the culture medium after 24 h of BMSC culture.The “AZ31” sample shows significant higher pH when compared with the DMEM-only group.Correspondingly,the Mg2+ion concentration of the “AZ31” samples was significantly higher than that of other groups,while the Ca2+ion concentration was significantly lower.The pH value,Mg2+and Ca2+ion concentration of“PCL/HA”samples,BMSC and glass reference groups have no significant difference when compared with DMEM-only group.While the pH value and Mg2+ion concentration of HA coating group were slightly higher and Ca2+ion concentration was slightly lower compared with those of “PCL/HA” samples and other reference groups.This may be due to the layer with nano rod-like structure on the surface of HA coating has a weak barrier to the culture fluid,and cannot effectively block the penetration of the culture fluid.However,no statistically significant differences of pH value,Mg2+and Ca2+ion concentration were found between the HA coating group and reference groups.
3.4.3.Possible factors affecting the adhesion and diffusion of BMSC
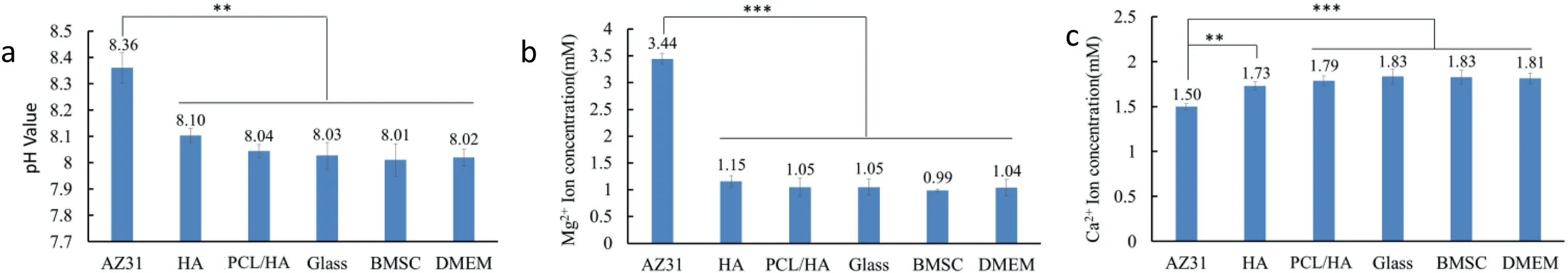
Fig.11.Post-culture media analyses after BMSC culture for 24 h with the coated AZ31 alloys (HA,PCL/HA),controls (AZ31),and references (DMEM,BMSC and glass).(a) pH values,(b) Mg2+ ion concentration,(c) Ca2+ ion concentration,Values are mean ± standard deviation (n=3).∗p<0.05,∗∗p<0.01,∗∗∗p<0.001.
Cipriano et al.[37] reported a pH increment of cell culture media from initial value to 9 would not affect the adhesion and growth of BMSC.The average pH value of the media varied in a range from 8.02 to 8.36 in this study.Under direct contact condition,“HA” samples showed significantly lower cell adhesion density of the BMSC and spreading area per BMSC than those of “AZ31” and “PCL/HA” samples.However,the cell adhesion density and spreading area of BMSC around the HA sample showed no significantly difference with those around “AZ31” and “PCL/HA” samples under the indirect contact condition at the same media pH value.Therefore the effect of media pH on the adhesion and spreading of BMSC was minimal.
The soluble ions released from degraded samples in the culture media may also affect cell adhesion and morphology on the sample surface (under direct contact condition),but may play a more important role around the sample (under indirect contact conditions).It was reported that when the Mg2+concentration was 5 mM,the extracellular matrix(ECM)mineralization of BMSCs was enhanced and when the Ca2+ion concentration exceeded 1 mM,the cell adhesion could be inhibited [38].The cell density of the BMSC around “AZ31”samples was significantly higher than other groups.Therefore,the increasing of Mg2+caused by Mg alloy degradation and the reduction of Ca2+in the medium caused by Mg-induced Ca2+deposition may be more helpful to improve the adhesion and differentiation of BMSCs.
HA coating showed a statistically low cell density and spreading area per BMSC compared with other groups under direct contact condition.It was speculated that the nano rod-like structure possessed by the HA coating prepared by the hydrothermal method could puncture the cells,leading to the death of the cells and not conducive to the adhesion and differentiation of the cells.Under indirect contact condition,cells around HA coating displayed a higher cell density and spreading area per BMSC than those of reference group,which indicated that the dissolution of HA coating in the culture media does not inhibit the proliferation and differentiation of the cells.Prakash et al.prepared a dense HA coating with nanoparticle-shapes on the surface of Mg alloy using a mixed discharge test device [10].The results ofin vitrobioactivity analysis showed that the HA-containing layer had excellent bioactivity and could promote the adhesion,growth and proliferation of human osteoblast MG-63.Therefore,the microstructure of the coating structure may have a more obvious effect on the adhesion of BMSC than the change of the composition in the medium.
Compared with “HA” samples,the BMSC adhesion density and spreading area per BMSC of “PCL/HA” samples under direct and indirect contact was statistically significantly higher.Moreover,the cell density on PCL/HA coating exceeded seeding density (104cell/cm2),indicating that BMSC cells proliferated in direct culture for 24 h.Washburn reported that cells are significantly sensitive to changes of topography and roughness in nanometer-scale [39].Fig.S2 shows the 3D and optical images of AZ31alloy,HA coating and PCL/HA composite coating.A microscopic bump topography of PCL/HA composite coated sample could be clearly observed.Compared with it,HA coating was more fine and dense.The surface roughness parameters of these samples listed in Table S1 show that AZ31 alloy has the lowest roughness value.The Ra (arithmetic mean deviation) and Rq (root mean square deviation) of HA coating were much smaller than that of PCL/HA composite coating.However,Ry (maximum profile valley depth) Rz (maximum height of profile)and Rp(the maximum profile peak height)values of HA coating and PCL/HA composite coating were similar,indicating that HA coating was more fine and sharp than PCL/HA composite coating.From the point of roughness,AZ31 alloy has the lowest Ra and PCL/HA has the highest Ra.But BMSC adhesion on AZ31 alloy and PCL/HA coating was more satisfied than that on HA.Jing Xia reported that increasing the roughness of nanoporous surface has no noticeable effect on cell attachment;nanopore size plays a more significant role than roughness in controlling BMSC behavior [40].The research result of Yogambha showed that surface topography of HA is one of the key factors in regulating interactions between materials and cells[41].Rehman et al.reported the cell morphology changes of HA nano-rods after 24 h treatment[42].The reason might be that the sharp topography on HA coating inhibited the adherent and proliferation of cells.After filling the PCL into the pores of HA coating,the number and height of nano-scale rod-shape HA crystals exposed to the surface decreased and PCL/HA coating presents a micronlevel unsharp structure.Therefore the roughness might have little impact on the BMSC adhesion here.The BMSCs adhesion and proliferation on PCL/HA coating might be benefited from the microstructure formed as well as the excellent biocompatibility of PCL and HA.
Fig.12.Representative images of viable bacterial colonies adhered on different samples and their corresponding culture medium after 24 h of MRSA culture.
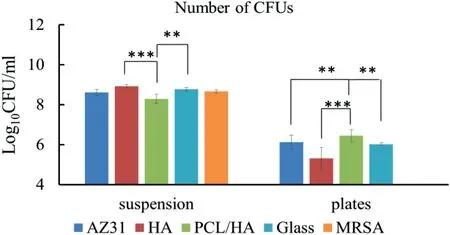
Fig.13.The quantitative analysis of the number of bacteria.The values are mean ± standard deviation (n=3).∗p<0.05,∗∗p<0.01,∗∗∗p<0.001.
It was reported that surface morphology,surface charge,crystal morphology and functional groups could affect cell adhesion and spreading on materials [28].Rehman et al.used KH550/HA to functionalize its surface and that the amine group of KH550 inhibited the growth of cells to some extent[42].However,Tan et al.reported that CaP coatings on silane coupling functionalized surfaces supported cell adhesion and proliferation [43].Our experiment results also showed that PCL/HA composite coating was more suitable for BMSC adhesion than HA coating alone.Therefore,neutralizing the surface amine by acetylation might significantly reduce the toxic of amine (-NH2) of KH550.However,how these characteristics collectively affect the adhesion and spreading of BMSCs on and around HA coating and PCL/HA coatings require further study.
3.5.Evaluation of antibacterial property
3.5.1.Bacterial activity after cultivation
The representative images of viable bacterial colonies grown on the agar plates for the bacteria in the suspension cultured with the samples and the bacteria adhered on the surfaces of samples after 24 h of culture were shown in Fig.12.The numbers of viable bacteria colony forming units were counted (Fig.13).After incubation,the number of bacteria in the suspension cultured with the “PCL/HA” samples was significantly lower than those of HA coating and glass reference.However,no statistically significant difference of the number of viable bacteria in the suspension was found between the“AZ31”,“HA”,“PCL/HA” samples and the MRSA group,indicating that the solubilized degradation products released from the samples had no antibacterial activity against MRSA.For the bacteria adhered on the sample surfaces,the number of bacterial colonies for the “PCL/HA” samples was statistically significantly higher than other groups.Fig.14 shows the SEM images of the samples,controls and references after MRSA culture for 24 h.After 24 h of bacteria culture,bacterial colonies was formed on the surfaces of glass and“PCL/HA” sample,demonstrating that PCL/HA composite coating have no antibacterial property against MRSA.However,only a few MRSA cells could be observed on the surface of AZ31 alloy and HA coating.
3.5.2.Analyze the pH and ion concentration in the post-culture media
Fig.15 shows the pH value,Mg2+and Ca2+ion concentrations in broth of the samples after MRSA culture for 24 h.As expected,after 24 h of bacterial culture,pH value and Mg2+ion concentration of the “AZ31” samples were statistically higher than other groups.There was no statistically significant difference in Ca2+ions concentration among all the sample groups.In addition,the Mg2+and Ca2+ion concentration of HA coating group was higher than those of “PCL/HA” samples and other reference groups.And the HA sample shows a statistically higher Mg2+ion concentration when compared with the media group.No statistically significant difference in Ca2+concentration was found among all the groups.
3.5.3.Possible factors affecting MRSA adhesion and activity
Media composition may affect MRSA viability in suspension and on the surface of sample.According to Wetteland et al.,the increase of pH value and Mg2+ion concentration will cause a certain reaction of bacteria,but when the pH was less than 10,no significant bactericidal effects was found [29].In this experiment,pH value and Mg2+ion concentration of the “AZ31” samples were significantly higher than other groups,but there was no statistically significant difference in the number of viable bacteria in the suspension cultured with the AZ31 alloy when compared with other groups.Moreover,under the condition of similar pH,the bacterial colonies on the surface of the HA coating samples were significantly lower than those of the PCL/HA coating,but the bacterial colonies in the suspension of the HA coating samples were significantly higher.The results indicated that the pH value and Mg2+ion concentration has a small effect on the adhesion and viability of MRSA.

Fig.14.After 24 h of MRSA culture,the surface morphologies of samples a: AZ31;b: HA coating;c: PCL/HA coating;d: Glass.
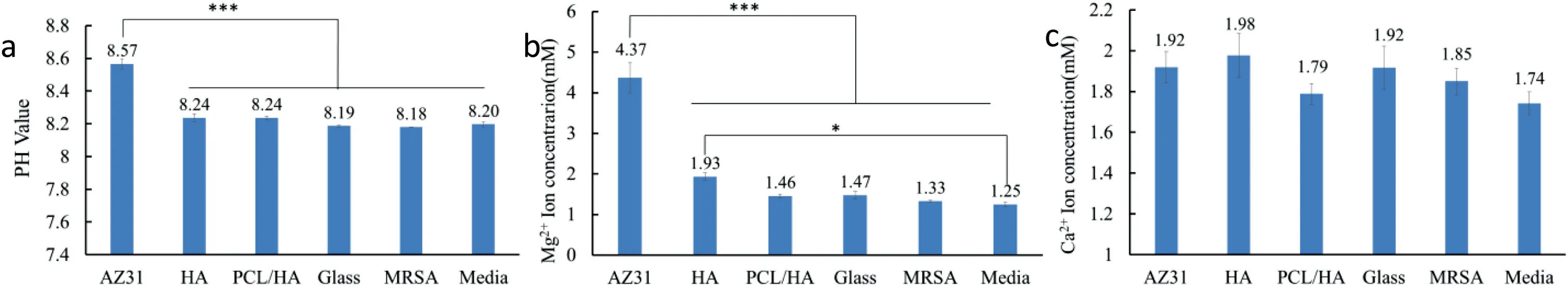
Fig.15.Post-culture LB broth analyses after MRSA culture for 24 h with controls (AZ31),the coated AZ31 alloys (HA and PCL/KH550/HA),and references(Glass,MRSA and Media).(a) Mg2+ ion concentration;(b) Ca2+ ion concentration;(c) pH values.The values are mean ± standard deviation (n=3).∗p<0.05,∗∗p<0.01,∗∗∗p<0.001.
Surface composition and microstructure of the sample may have an effect on the adhesion and viability of MRSA.It was reported that MgO could inhabited the growth of MRSA [44].Therefore the low density of MRSA cells adhere to AZ31 alloy might due to the MgO formed by the degradation of Mg.Al-Amshawee et al.reported that an effective biomass immobilization requires rough surfaces having edges,and peaks and valley.Rough and wetted surfaces ensure the initial adhesion of bacterial communities and provide robust protection from detachment [45].Fig.15 show the number of bacterial colonies for the “PCL/HA” samples was statistically significantly higher than other groups (AZ31alloy,HA coating and glass reference).The Ra (1.218 μm) of PCL/HA coating was the highest,so that the rough surface of PCL/HA coating might be one of the factor to form the bacterial biofilm.The number of MRSA colonies on HA coating was few,but there were a large number of MRSA colonies in the suspension of HA coating sample.According to Andrade et al.research results,HA coating with smooth surface does not show an inhibitory zone against bacteria,indicating that HA itself has no antibacterial effect [46].Therefore,it could be deduced that the small number of MRSA cells on the surface of the HA coating in this experiment might also be due to the sharp shape of the HA crystals.Consequently,it can be considered that the surface microstructure does have a significantly effect on the adhesion of MRSA.
4.Conclusion
The PCL/HA composite coating composed of nano-scale rod-shape HA crystals and PCL filled in their spaces was fabricated by the combination of hydrothermal and dipping methods.Compared with the single HA coating,the binding strength of PCL/HA composite coating with Mg substrate was obviously improved and the electrochemical corrosion rate was reduced by ten times.The PCL/HA composite coating could provide more effective barrier for Mg substrate against the ingress of corrosive ions than the HA coating alone and the PCL/HA coating was still stable and adhered to the surface of AZ31 substrate after 38 days of immersion in Hank’s solution.Both HA coating and PCL/HA composite coating were nontoxic to BMSC cells.The PCL/HA composite coating showed better BMSC cell compatibility,more suitable for BMSC adhesion than HA coating alone.The quantitative analysis of the number of bacteria indicated that neither HA coating nor PCL/HA composite coating showed the antibacterial property against MRSA.The fact that few BMSCs and MRSA bacteria adhered on HA coating might be caused by the sharp morphology of HA crystals.In summary,the PCL/HA coated AZ31 alloy has a potential application prospect as a biological materials due to its low degradation rate and good BMSC cell compatibility.However,from the perspective of clinical applications,the antibacterial property of PCL/HA composite coating needs to be further improved.
Declaration of Competing Interest
None
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grant No.51201192] and Natural Science Foundation of Chongqing [Grant No.cstc2018jcyjA2285cstc2018jcyjA2285].
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2021.07.014.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Improving the Young’s modulus of Mg via alloying and compositing -A short review
- Surface modifciation of magnesium alloys using thermal and solid-state cold spray processes: Challenges and latest progresses
- Preparation,structure and properties of Mg/Al laminated metal composites fabricated by roll-bonding,a review
- A review on recent advancements in biodegradable Mg-Ca alloys
- Analysis of element loss,densification,and defects in laser-based powder-bed fusion of magnesium alloy WE43
- Pore structure of porous Mg-1Mn-xZn alloy fabricated by metal-gas eutectic unidirectional solidification✩
