Pore structure of porous Mg-1Mn-xZn alloy fabricated by metal-gas eutectic unidirectional solidification✩
2022-10-25CnxuZhouGngqingLingYunLiuHuweiZhngXingChenYnxingLi
Cnxu Zhou ,Gngqing Ling ,Yun Liu,b,∗ ,Huwei Zhng,b ,Xing Chen,b ,Ynxing Li,b
aSchool of Materials Science and Engineering,Tsinghua University,Beijing 100084,China
b Key Laboratory for Advanced Materials Processing Technology,Ministry of Education,Tsinghua University,Beijing 100084,China
Abstract Lotus-type porous Mg-1wt.% Mn-xZn (x=0wt.%,1wt.% and 2wt.%) alloys were fabricated by metal-gas eutectic unidirectional solidification (the Gasar method).Effects of Zn addition and the fabrication process on the porosity,pore diameter and microstructure of the porous Mg alloys were investigated.Zn addition from 0 wt.% to 1 wt.% and 2 wt.% to the Mg-1 wt.% Mn alloy decreased the porosity from 41.2% to 36.9% and 35.8%,respectively,with the same preparation processing.In the lotus-type porous Mg-1wt.%Mn-1wt.%Zn alloy,the porosities and average pore diameters changed with hydrogen pressures from 0.1 to 0.6MPa.Conical areas that were rich in elemental Zn existed below the directional pores,and precipitates were also found in conical areas.Homogeneous directional pores existed in the lower portion of the ingot,and coarser directional pores and finer non-directional pores formed in the upper part.A theoretical model of the change in porosity with hydrogen pressure agreed well with the calculated porosities in the steady bubble growing area.The compressive strength of Mg-1 wt.Mn-Zn alloys can be increased by around 20 MPa through rising Zinc content from 1 wt.% to 2 wt.%,which basically linearly decline with the increasing of porosity.This work provides the basis for Gasar Mg-Zn-Mn alloy synthesis in biological applications and shows that the Gasar process is a promising method to fabricate Mg-Zn-Mn alloys with directional pores and a controllable pore structure.
Keywords: Porous material;Mg-Mn-Zn alloy;Porosity;Gasar;Directional solidification.
1.Introduction
In the past few decades,magnesium has been considered a potential material for biodegradable orthopedic applications[1-3].Magnesium-based material,which facilitates new bone formation and osseointegration,can be applied as cardiovascular stents,bone fixation implants or bone fillers [2-4].Porous Mg scaffolds with an open porous structure can improve bone ingrowth into the pores and transport nutrients,which yields good therapeutic effects [5,6].Compared with other porous materials fabricated by the method of porous materials such as space holder (powder metallurgy),melt foaming method,preform infiltration,pattern casting and syntactic foam,materials with the lotus-type porous structure prepared by solid-gas eutectic unidirectional solidification (the Gasar process) present less stress concentration around the pores with stress along the longitudinal direction of the pores,and the lotus type porous whose pores are aligning in one direction shows superior compressive and fatigue properties [7].In addition,during the Gasar fabrication process,no residual spacer holder or internal salt exists in the material,so the materials would not be contaminated.Porous materials with a lotus-type pore structure that is fabricated by solid-gas eutectic unidirectional solidification (the Gasar process) shows superior mechanical properties compared with traditional porous materials [7].Because no residual spacer holder or internal salt exists,Gasar materials and exhibit better biocompatibility and corrosion resistance.Besides,the cost of Gasar process is much lower than that of additive manufacturing.Alvarez implanted lotus-type porous stainless steel in rats and confirmed the osteocompatibility of lotus-type porous austenitic stainless steels [8].Gu found that lotus porous pure Mg is promising as a degradable tissue engineering scaffold material,but because of the poor corrosion and mechanical properties of pure Mg,it does not meet practical application requirements [9].
Alloying has been used extensively to improve the corrosion and mechanical properties.Among the elements tested,Zn,Mn and Ca,which are essential elements for humans,were preferred in biodegradable materials.Zn can improve the corrosion resistance,and Mn can improve the saltwater resistance of magnesium alloy by removing iron and other heavy-metal elements [2,5].Zhang found that Mg-Zn-Mn alloys provide high mechanical properties and Zn could accelerate the formation of a passivation film,which provides good protection to the magnesium alloy against synthetic body fluid[10].Rosalbino studied the in vitro degradation performance of Mg-Zn-Mn alloy in Ringer’s physiological solution and found that the Mg-1.5Zn-1Mn alloy was a promising candidate material for the development of degradable implants[11].Jamesh et al.studied the corrosion behavior of ZM21 Mg alloy that was immersed in Ringer’s solution.A thickening of the CaCO3layer may improve the corrosion resistance of the ZM21 Mg alloy and suggests that the ZM21 Mg alloy is a promising candidate material for the development of degradable implants [12].
It is a pity that only pure Mg and a small amount of Mg alloys,such as AZ31 and AZ91D Mg alloys,have been fabricated by the Gasar process [13,14,15],but the non-uniform pore diameter/distribution and the existence of harmful alloying elements restrains their clinical application.In this study,Mg-1wt.%Mn-xZn (x=0wt.%,1wt.%,and 2wt.%)alloys with a lotus pore structure were prepared by using the optimized Gasar equipment process [16].The effects of element addition on the pore structure of Gasar Mg-1wt.%MnxZn (x=0wt.%,1wt.% and 2wt.%) alloys were investigated,which can provide a theoretical basis for the fabrication of Gasar Mg-Mn-Zn alloy as a degradable tissue-engineering scaffold material in biological applications.
2.Experimental methods
Lotus-type porous Mg-1wt.%Mn-xZn (x=0wt.%,1wt.%and 2wt.%) alloys of 99.9% purity were prepared by the Gasar process in hydrogen,and details on the apparatus can be found in our previous work [16-18].The alloying contents of the fabricated Mg alloy ingots are presented in Table 1.The melt temperature is 1023 K and the withdraw speed is 1mm/s.The pressures were controlled at 0.1-0.6 MPa to obtain lotus Mg alloys with various porosities.The ingots obtained were~80 mm in diameter and nearly 80-100 mm in height,which was dependent on the porosity.The overall porosity of the entire ingot was evaluated through the Archimedes’principle.Each cylinder ingot was cut into two parts along the central axis.The sample surfaces were scanned with a HP-G3010 scanner.The pore structural parameters wereevaluated by using image-analysis software.The specimen microstructures were studied by optical microscopy (Zeiss Axio lmager),scanning electron microscopy (SEM Zeiss GEMINISEM 500) and electron probe microanalysis (EPMA JEOL JXA8230).Samples for SEM and EPMA examination were ground with SiC emery paper to 4000 grade,and polished with diamond paste to 1μm.The samples for optical microscopy examination were etched in 5% citric acid monohydrate (C6H10O8).The compressive strength of cylindrical specimens (Φ5 mm×10mm,GB/T 7314-2017) was tested by Shimadzu AGX-V 20KN,and the strain rate was set at 10mm/min.
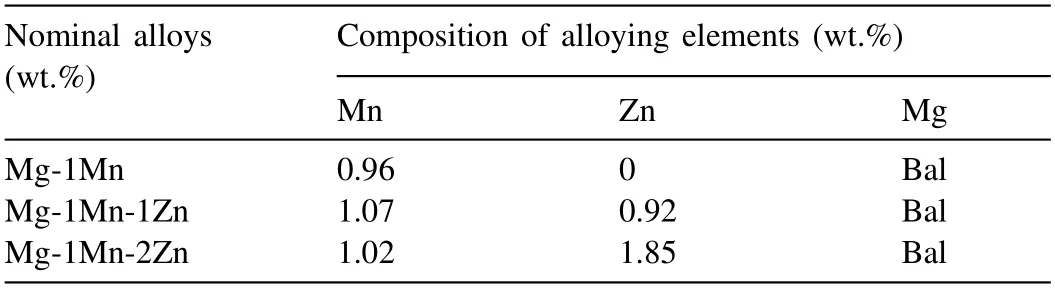
Table 1 Chemical composition of lotus-type porous Mg-1Mn-xZn alloys.

Table 2 Alloying composition of 1-3 areas in Fig.8c.
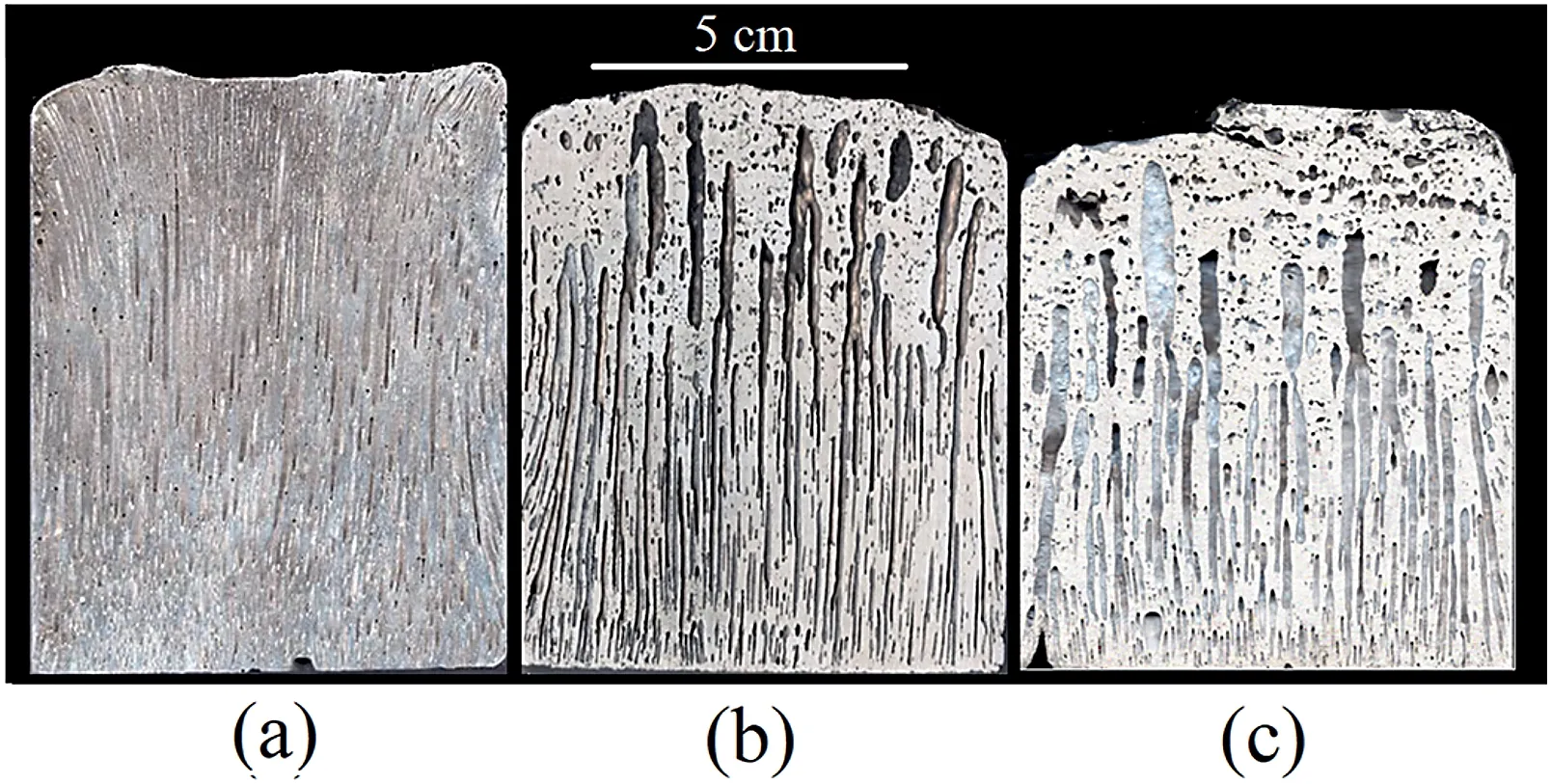
Fig.1.Pore morphologies of longitudinal section in (a) Mg-1Mn alloy,(b)Mg-1Mn-1Zn alloy,and (c) Mg-1Mn-2Zn alloy (T=1023K,PH2=0.4MPa).
3.Results
3.1.Effects of Zn addition on Mg-Mn-Zn alloy pore structure
Fig.1 shows the morphologies of the Mg-1Mn(Mg-1wt.%Mn alloy in Fig.1a),Mg-1Mn-1Zn (Mg-1wt.%Mn-1wt.%Zn alloy in Fig.1b) and Mg-1Mn-2Zn (Mg-1wt.%Mn-2wt.%Zn alloy in Fig.1c) alloys on the longitudinal section.The melt temperature was 1023K,the withdraw speed was 1mm/s and the H2pressure was controlled at 0.4MPa.The pore distribution in the Mg-1wt.%Mn alloy was approximately homogeneous,like that in pure Mg [17].The pore structure of the Mg-1Mn-xZn alloy exhibited different morphologies.Additional non-directional pores existed in the upper area,and the lower parts showed more homogeneous pore morphologies.The Mg-1Mn alloy porosity was 41.2%,and Zn addition from 1% and 2% to Mg-1Mn alloy decreased the porosity to 36.9% and 35.8%,respectively.The addition of Zn can decrease the porosity of the Mg-Mn alloy,which indicated that Zn alloying may have negative effects on the porosity improvement.
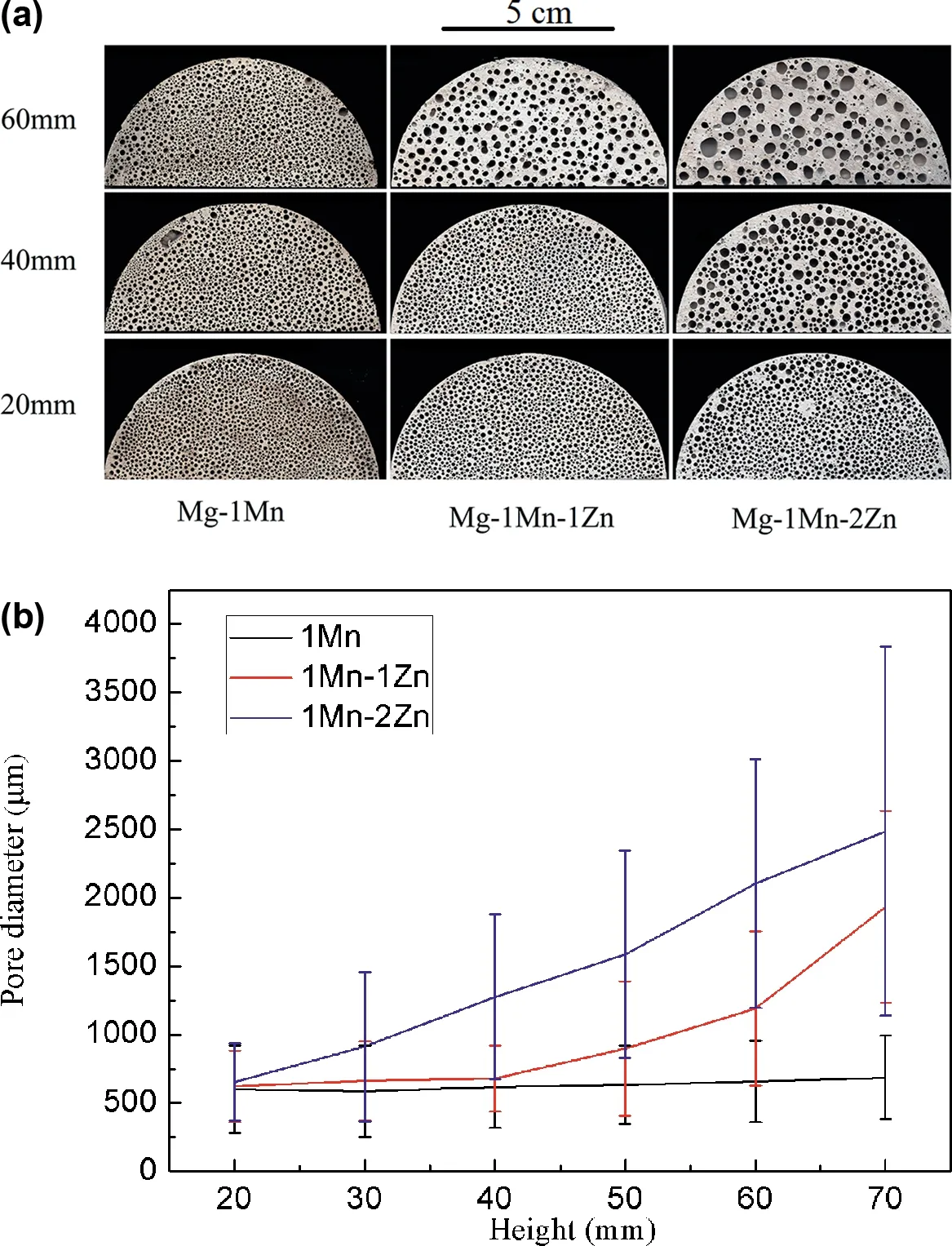
Fig.2.(a) Pore morphologies of transverse sections of Mg-1Mn,Mg-1Mn-1Zn and Mg-1Mn-2Zn ingots at heights of 20mm,40mm and 60mm.(b)Pore diameters of Mg-1Mn,Mg-1Mn-1Zn and Mg-1Mn-2Zn ingots on transverse section at different heights.
Fig.2a shows the cross-section that is perpendicular to the solidification direction of Gasar Mg-1Mn,Mg-1Mn-1Zn and Mg-1Mn-2Zn alloys at heights of 20mm,40mm and 60mm.The pore distribution becomes more nonuniform in the Zn-containing ingots than that in the Mg-Mn along the solidification direction.Fig.2b shows the dependence of the average pore diameter on the different Mg-1Mn,Mg-1Mn-1Zn and Mg-1Mn-2Zn ingot heights.With an increase in height,the pore diameters increased slightly in the Mg-1Mn ingot.A steady pore growth occurred for the Mg-1Mn-1Zn ingots at a 40mm height along the solidification direction.When the height increased to 40mm or above,the increase in pore diameter changed significantly.For Mg-1Mn-2Zn ingot,it shows an obvious porosity increment in each height,which indicates that,when the Zinc content is above 2wt.%,the pore diameter tends to increase linearly as the increasing of height.The difference of pore diameters along the solidification direction increases as the rising of Zinc content in Mg-Mn alloy system.
3.2.Effect of Zn addition on Mg-Mn-Zn alloy solidification

Fig.3.Simulated time to solidus during solidification of Mg-1Mn,Mg-1Mn-1Zn and Mg-1Mn-2Zn alloy.
To study the differences,Postcast software was used to simulate the solidification process.Although the gas phase was induced to the solidification process of the Gasar method,it has been found that pores do not significantly affect the solidification [16].The physical model,heat boundary conditions that were applied in the simulation,the simulation assumptions and the major parameters that were used in the simulation are described in our previous studies [16].Physical and thermodynamic parameters correlations for the Mg-1Mn,Mg-1Mn-1Zn and Mg-1Mn-2Zn alloys were calculated by using the Postcast software.The sample size wasφ80mm×90mm,the melt temperature was 1023K and the withdrawal speed was 1mm/s.Fig.3 shows the simulated time to solidus of different heights on the longitudinal sections of the Mg-1Mn,Mg-1Mn-Zn and Mg-1Mn-2Zn alloys.Because of the axial symmetry property of the temperature field,only half of the isochrones maps are presented.The addition of 1wt.%Zn in Mg-1wt.%Mn can increase the time to solidus significantly,especially the upper parts of the ingots.Fig.4 shows the simulated solid fraction with different heights for the Mg-1Mn,Mg-1Mn-1Zn and Mg-1Mn-2Zn alloys.Almost no mushy zone (over 0.8) at the growth front was found during the solidification of the Mg-1Mn alloy.For Mg-1Mn-xZn,the mushy zone width increased with an increment in Zn content,and the existence of the mushy zone may affect the pore growth direction of the alloys.
Fig.5 shows the simulated width of the mushy zone with a solid/liquid interface at varying heights during the solidification (from the initial to the final time) of the Mg-1Mn,Mg-1Mn-1Zn and Mg-1Mn-2Zn alloys.The increment of width of the mushy zone at different heights exhibits a similar trend to the increase in pore diameter (Fig.2),which shows that pore growth is related to the formation of the semi solid mushy zone.
3.3.Microstructures
Fig.6 shows the microstructure of the longitudinal and transverse sections of the Mg-1Mn-1Zn alloy at 20mm and 60mm heights.Directional columnar dendritic crystals (6a and 6c) were found in the lower part of the ingots,but this microstructure changed to equiaxed dendritic in the upper part(Fig.6b and d).The change may have been caused by the change in solidification rate and temperature gradient at different solidification heights and have affected pore growth.
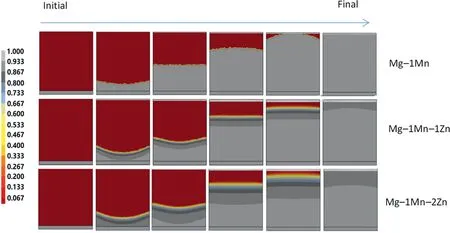
Fig.4.Simulated ratio of solid phase at different solidification times of Mg-1Mn,Mg-1Mn-1Zn and Mg-1Mn-2Zn alloy.
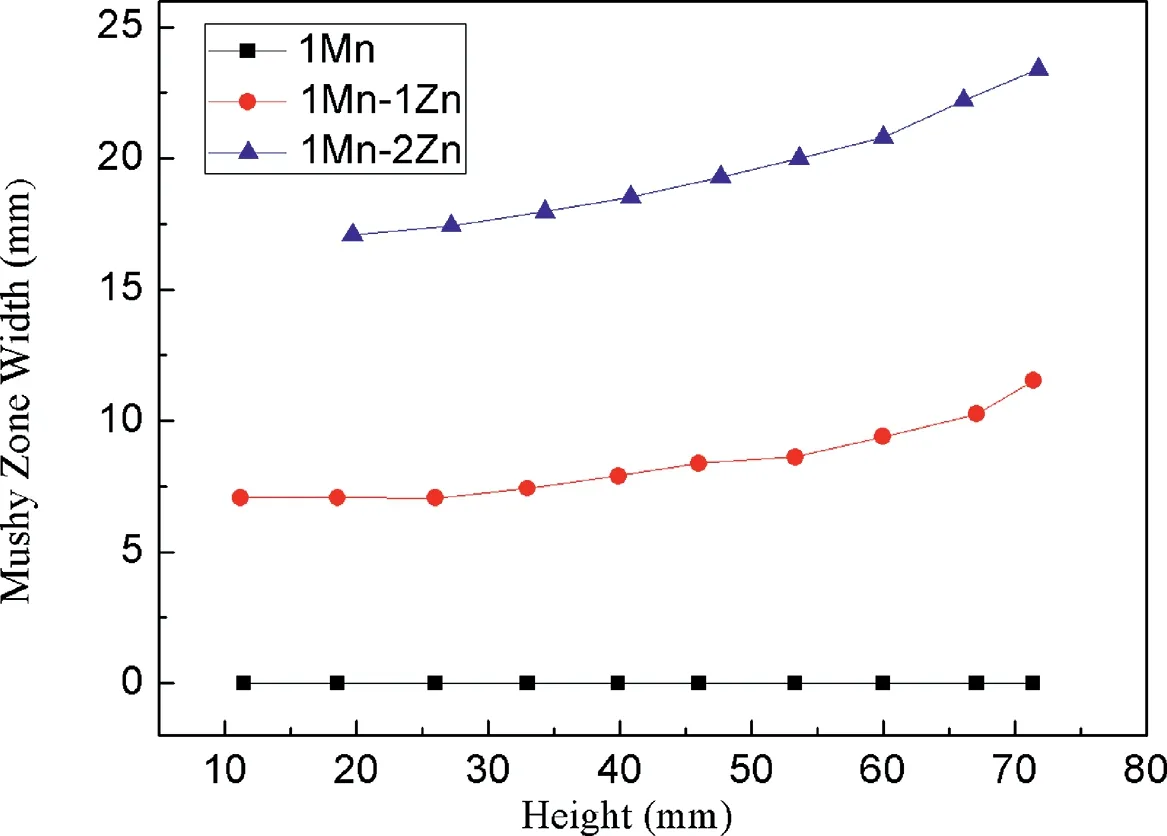
Fig.5.Simulated width of mushy zone for varied solid/liquid interface heights during Mg-1Mn,Mg-1Mn-1Zn and Mg-1Mn-2Zn alloy solidification.
Fig.7(a-f) shows the 2D tomography image slice perpendicular to the solidification direction from the lower to the upper part along the solidification direction of the Mg-2Zn-1Mn ingots.The white arrows represent a round higher-atomicnumber element aggregation area,and most of the aggregation areas are located on the metal matrix between the pores.Furthermore,a few aggregation areas around the small pores are observed,which indicates that the aggregation areas are formed at the tail of pores along the solidification direction.With the increasing of height,pores were found on the top of at these areas,as shown by the yellow arrows.Pore coalescence (red arrows) occurred along the solidification direction,resulting increment of the pore diameters.
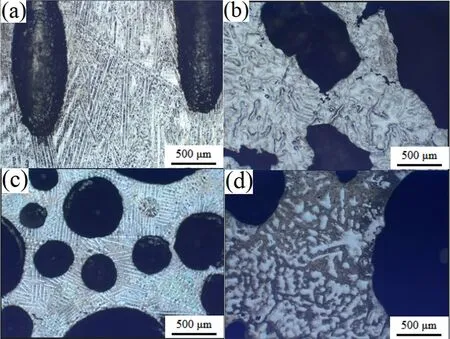
Fig.6.Microstructure of Mg-1Mn-1Zn alloy near pore area at different heights (a) longitudinal section at 20mm height,(b) longitudinal section at 60mm height,(c) transverse section at 20mm height,(d) transverse section at 60mm height.
Fig.8(a) shows a two-dimensional (2D) image tomography slice in Mg-1Mn-2Zn ingots parallel to the solidification direction.Conical areas that were considered higher-atomicnumber atom enrichment areas and with some precipitates were found under the pores.Fig.8(b-d) shows that this conical area is rich in elemental Zn,and some precipitates that were rich in elemental Zn or Mn existed in this area.The alloying element composition in different areas of ‘1’ to ‘3’in Fig.8(c) are shown in Table 2,and the precipitates in Fig.8(b) are considered to be Mg51Zn20andα-Mn.
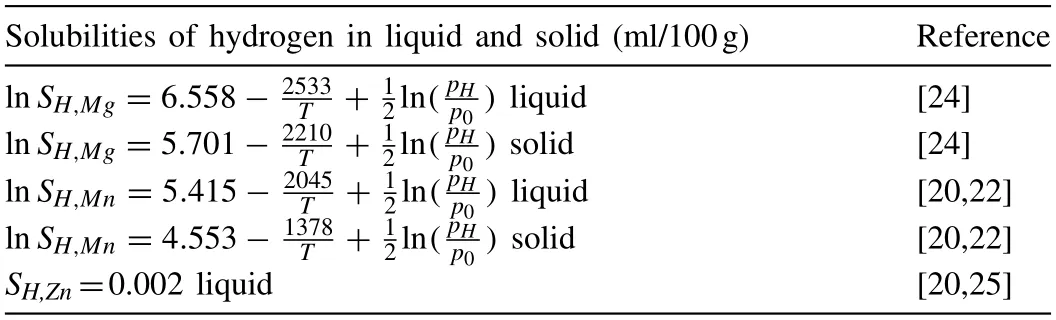
Table 3 Solubilities of hydrogen in liquid and solid of pure metal.
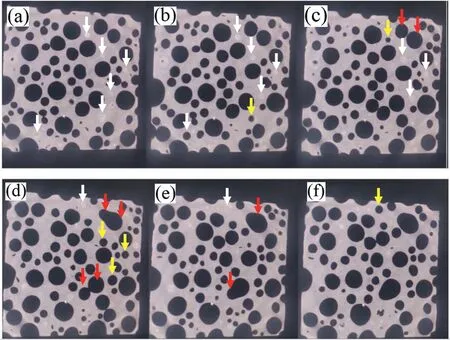
Fig.7.2D even slice images of tomography perpendicular to solidification direction from lower(a)to upper(f)parts along the solidification direction of the Mg-1Mn-2Zn sample from about 25mm height to 27mm height.(Scan section area: 10mm×10mm) (white arrow: higher atomic number element aggregation area,yellow arrow: pore formation,red arrow: pore coalescence process).(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.)
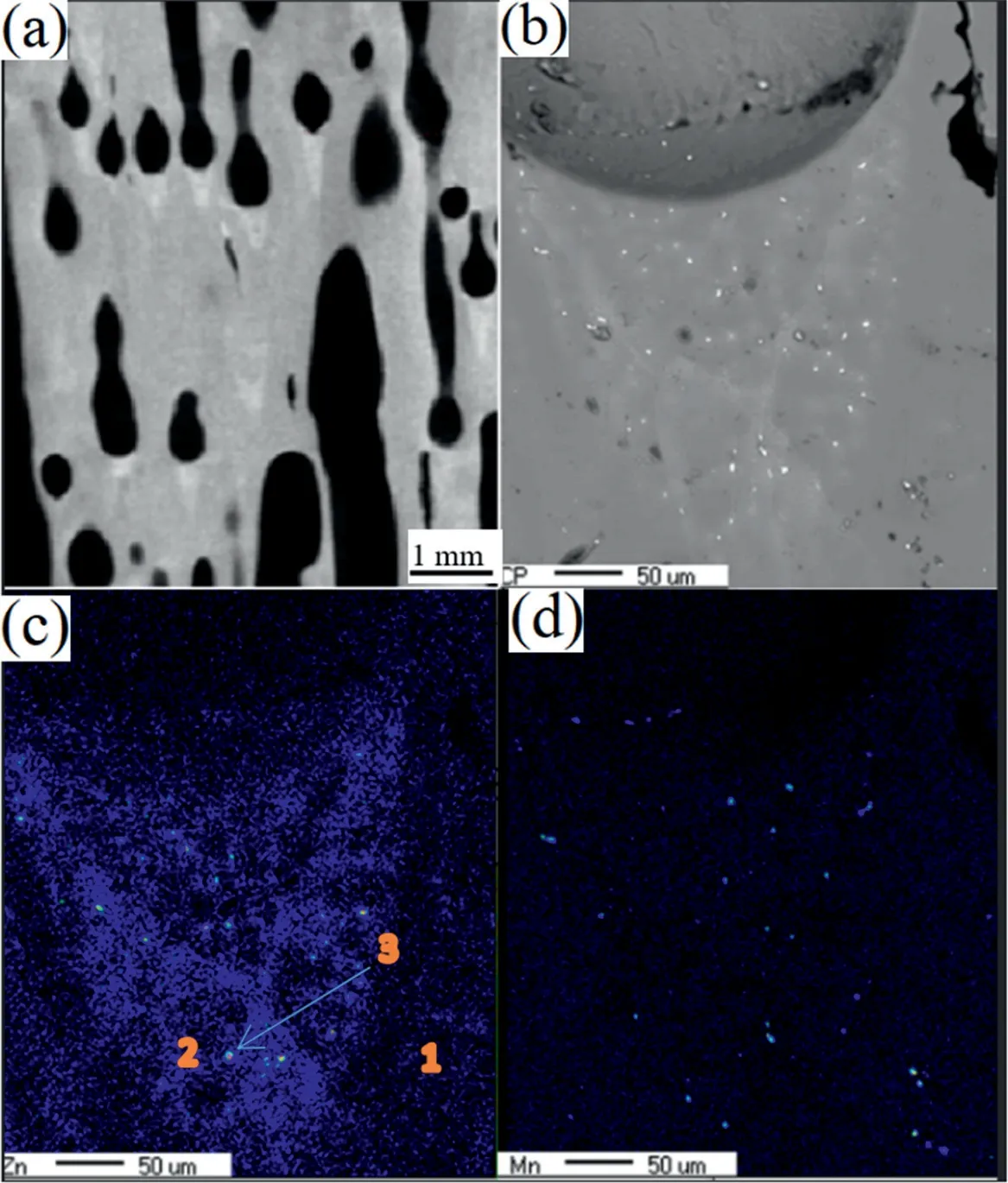
Fig.8.Microstructure of cross-section parallel to solidification direction in Mg-1Mn-2Zn alloy (a) 2D image slice of tomography parallel to solidification direction,(b) backscattered image of pore bottom area,(c) Zn element distribution by EPMA,(d) Mn element distribution by EPMA.(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.)
3.4.Effects of H2 pressure on pore structure of Mg-1Zn-1Mn alloy
Fig.9 shows the typical pore morphologies at different heights of the lotus-type porous Mg-1Mn-1Zn ingots with different H2pressures.The average pore diameters decrease as the hydrogen pressure increases from 0.1 to 0.2,0.4 and 0.6MPa and the overall porosities were 47.1%,43.3%,38.2%and 34.6%,respectively.Fig.10 shows the average pore diameters (a) and surface porosities (b) of the lotus-type porous Mg-1Mn-1Zn with different hydrogen pressures from 0.1 to 0.6MPa as a function of solidification height.Below a 40mm height,the pore growth was stable.As the hydrogen pressure increased from 0.1 to 0.2,0.4 and 0.6MPa,the average pore diameters of the stable pore growth area were 2512,1826,682 and 537μm,respectively.As the ingot height increased from 40mm,the pore diameters of ingots that were fabricated at a hydrogen pressure of 0.2,0.4 and 0.6MPa increased,but the ingot that was fabricated at a H2pressure at 0.1MPa displayed different characteristics.
3.5.Effects of porosity on compressive strength
Fig.11 shows the compressive strength of Mg-1Mn-1Zn(red line) and Mg-1Mn-2Zn (blue line) with different porosity.Given that the specimens were collected from diverse parts of the Mg-1Mn-1Zn and Mg-1Mn-2Zn ingot,those discrete data can be fitted as regression lines.Basically,with the linear declining of porosity,thanks to the increment of Zn content (from 1 wt.% to 2wt.%),the compressive strength that parallels to the pore direction is significantly promoted(~20MPa) considering the solid solution and grain refining,since Zinc can reduce the grain size effectively by hindering the movement of grain boundaries during recrystallization.The Mg-1Mn-2Zn reaches the maximum compressive strength at 147.6MPa with porosity at 33.5%,while Mg-1Mn-1Zn peaks at 129MPa with porosity at 34.53%.
In Fig.12,for both Mg-1Mn-1Zn(a)and Mg-1Mn-2Zn(b)alloy,a number of clustered twins around the pores facilitate the ductility during the plastic deformation,while this phenomenon failed to be observed for those grains away from the pores.The hexagonal close packed (HCP) structure of Mg fails to meet the five conditions of Von-Mises criterion,as {0002}<110>basal slip can only provide two independent slip systems,lacking sufficient slip systems during plastic deformation,leading to poor ductility of Mg alloys in room temperature.However,twinning behaviors render increment of ductility possible.
4.Discussion
4.1.Porosity
Liu et al.[13,19] and Jiang [20] established a theoretical model and predicted the porosity of Gasar pure Mg and Cu alloys.The model that was used by Jiang [20] is shown by:
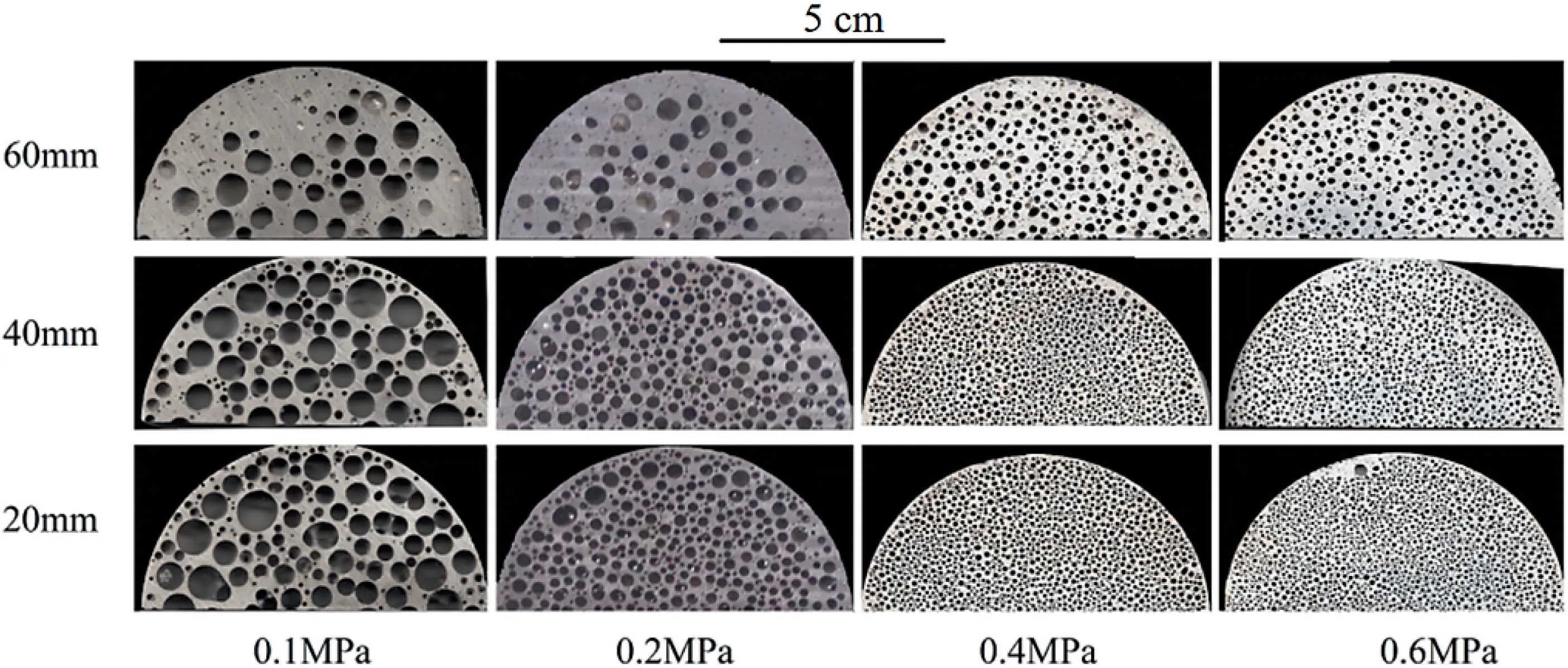
Fig.9.Variation of pore morphology of Mg-1Mn-1Zn alloys at different cross-sectional heights with different pressures.
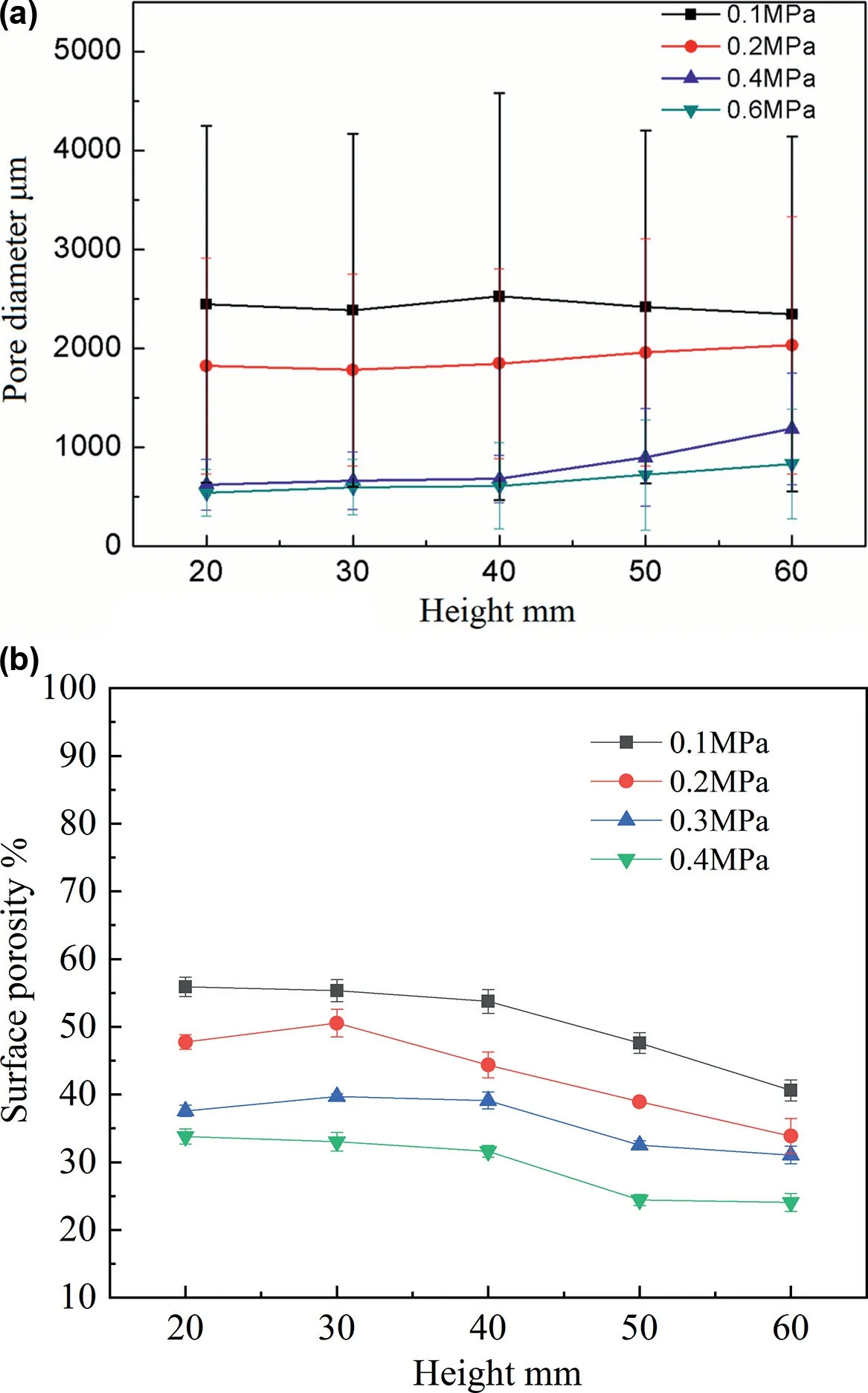
Fig.10.Pore diameters (a) and surface porosities (b) on transverse section at different Mg-1Mn-1Zn alloy heights.

Fig.11.Compressive strength of Mg-1Mn-1Zn (red line) and Mg-1Mn-2Zn(blue line) with different porosity.(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.)
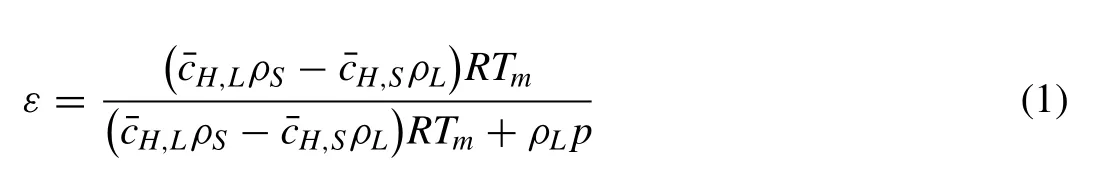
whereεis the theoretical overall porosity;Ris the gas constant;Tmis the metal melting point;ρsis the density (kg/m3)of the solid metal andpporeis the gas pressure(Pa)in the pore that is almost equal to the furnace atmosphere;andare the hydrogen concentrations in the liquid and solid metal(mol/m3),respectively;andρsandρLare the density of the solid metal near the melting point.Usually,hydrogen bubbles may escape from the melt,and the value ofshould be multiplied by a coefficient,such as(1-a),whereais the escape coefficient that is equal to the ratio of the escaping hydrogen and the original hydrogen concentration in the liquid metal.The key for porosity prediction is to determine the hydrogen solubility of the Mg alloy.For the Mg-1Zn-1Mn alloy,the hydrogen solubilitySH(ml/100g) can be expressed as [20,21]:
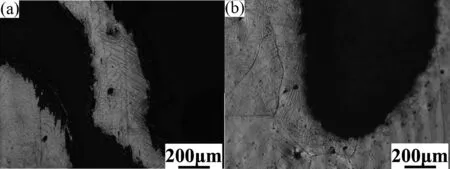
Fig.12.The twinning effect in Mg-1Mn-1Zn (a) and Mg-1Mn-2Zn (b).
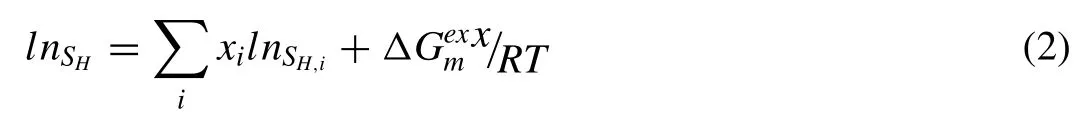
wherexiis the molar fraction of component i,is the excess molar free energy,andSH,iis the hydrogen solubility in liquid component i at temperature T.The solubilitiesSH,Mg/Mn(ml/100g) of hydrogen in liquid and solid pure element are expressed by the equations in Table 3.Moon derived a solubility value of 3×10-5at.% hydrogen in solid Zn at 200°C[22,23],which means that the H2solubility in solid Zn was very low.
For a ternary alloy i-j-k,the excess molar free energy of the alloy system could be described as:
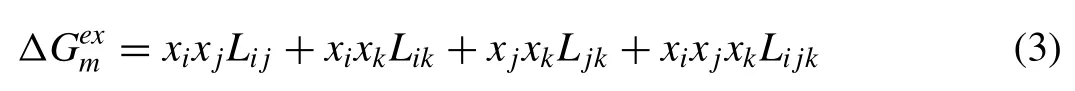
whereLi,j k(J/mo1) is a ternary interaction parameter.For the ternary Mg-1Mn1-1Zn alloy,few experimental thermodynamic works on the Mg-Mn-Zn system are available in the literature [26],and the atom percent of Zn and Mn in this alloy was low at less than 0.5%.The equation was simplified to approximate that of the Mg alloy (Mg-1Mn-1Zn)/H2system as follows:
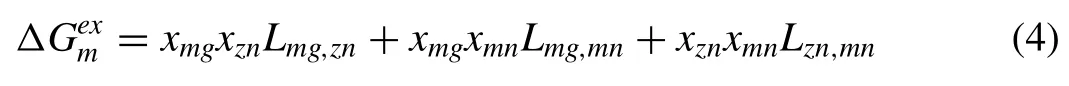
For the Mg-Zn-Mn alloy,the thermodynamic data are shown in Table 4.
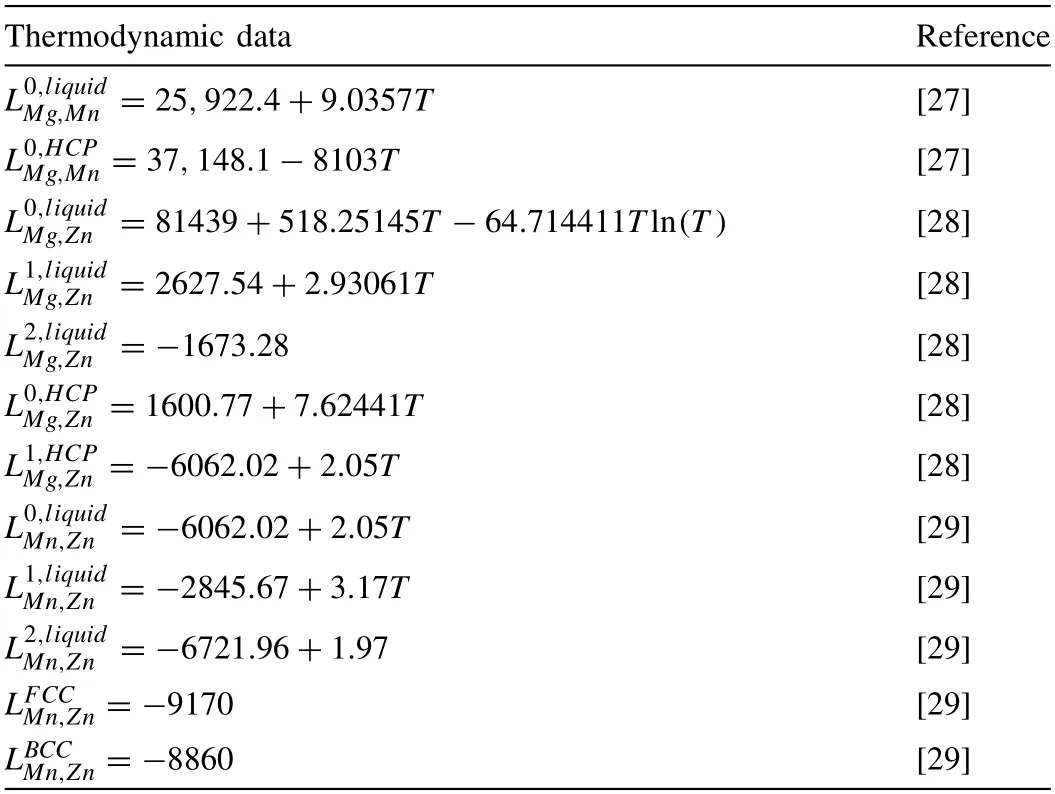
Table 4 Values of thermodynamic data.
After calculated the approximate value ofthe theoretical porosity can be obtained.More details exist in the literature [13,19,20].A comparison between the predicted porosities and the experimental results for the Mg-1Zn-1Mn/H2system is shown in Fig.13.The predicted porosities agree better with the experimental results for a porosity at stable area (nearly 20mm height along the solidification direction) of Mg-1Zn-1Mn alloy.
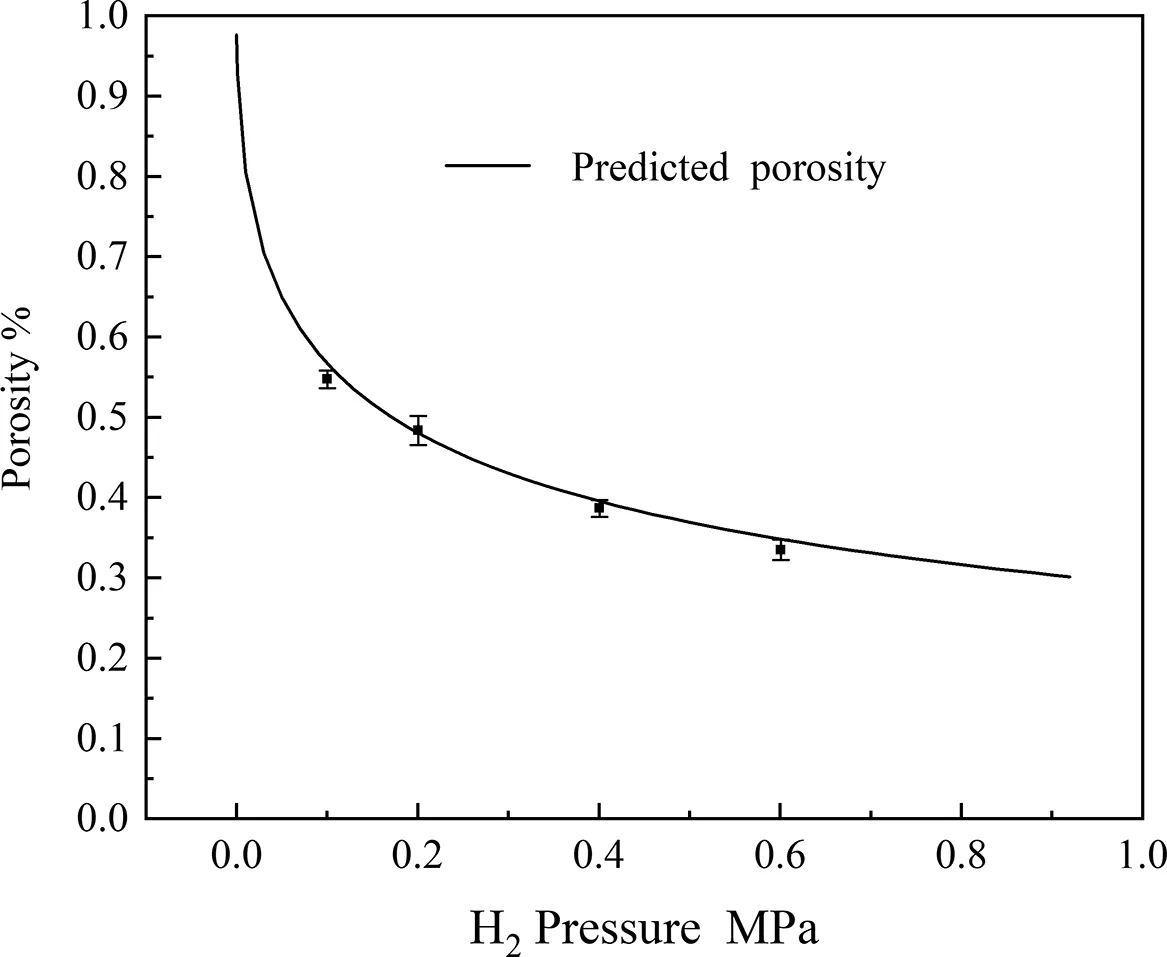
Fig.13.Comparison between predicted porosities and experimental values in Mg-1Zn-1Mn/H2 system.
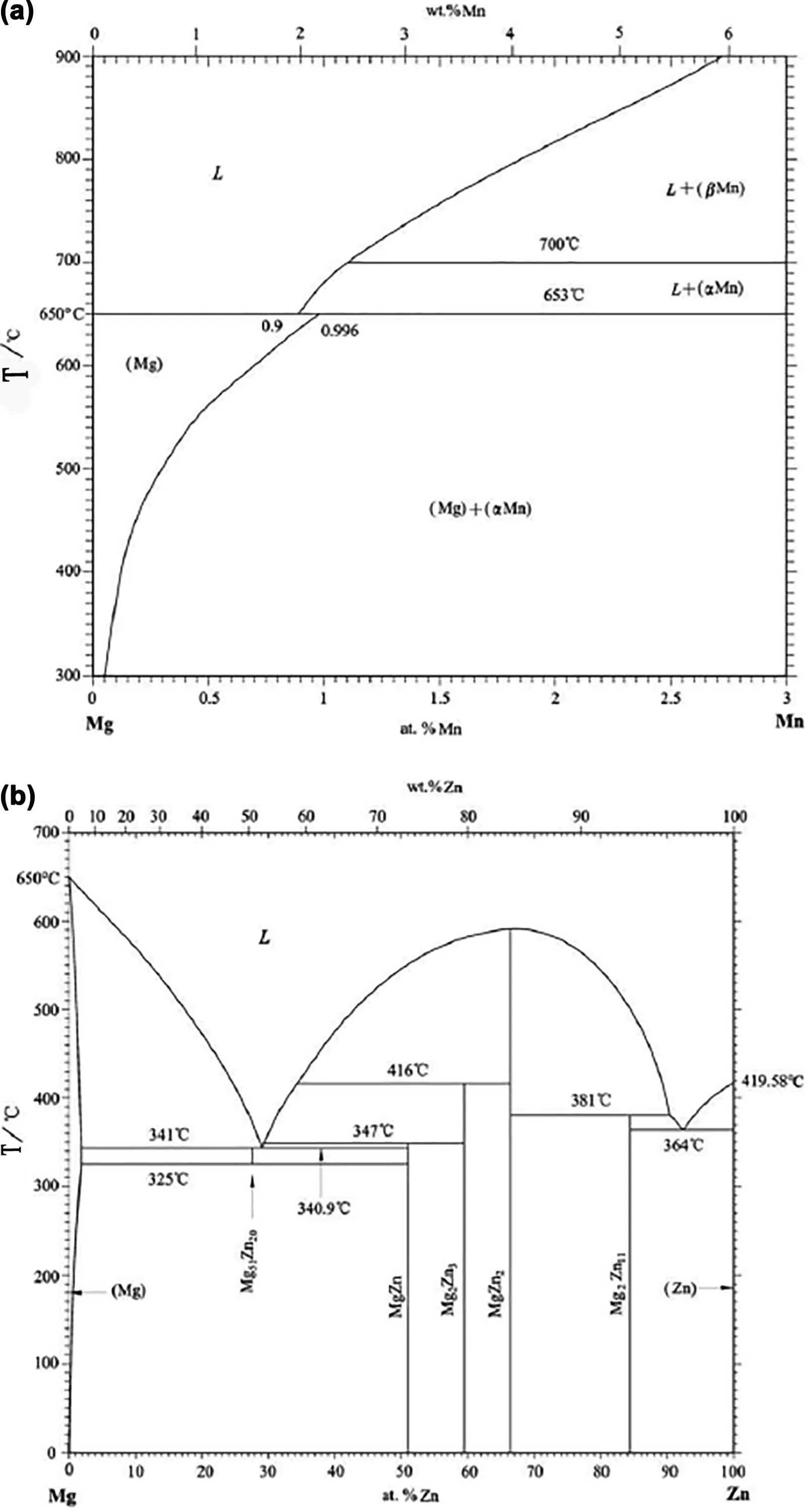
Fig.14.Phase diagram of Mg-Mn(a) and Mg-Zn alloy(b).
4.2.Pore structure
The pore structure of the Mg-Mn alloy differs from the Mg-Zn-Mn alloy.Fig.14 shows the binary phase diagram of the Mg-Mn and Mg-Zn alloys [30].The temperature range between the solidus and liquidus of the Mg-Mn alloy (Mn content less than 2wt.%) is narrow.Therefore,the pore structure of the Mg-Mn alloy is similar to that of pure metal,and the directional pore distribution of the ingot is uniform.The addition of Mn can decrease the thermal conductivity of Mg-Mn alloy,which can increase the pore diameter [31].Zn addition to Mg can widen the temperature range between the solidus and liquidus,and the wider mush zone formed during solidification in the Mg-Mn-Zn alloy,which means that the solidification process is different from that of pure Mg or Mg-1Mn alloys.Figs.3-5 show that,during solidification,as the Mg-Zn-Mn alloy height increased,the solidification time and mush zone width increased sharply,which indicates that in the upper part of the ingots,the solidification rate decreased.Equationv·L2=Fshows the relationship between solidification rate and pore spacing,and the detailed derivation process of the formulas and calculation method is described in our previous work [32].F is related to the alloy,melt temperature and gas pressure;vis the solidification rate andLis the pore spacing.For different parts of the sample,F was close to a constant,and from the equation above,the pore spacing increases with a decrease of temperature solidification rate.A larger pore diameter yields a larger pore spacing L [33].Hence,the pore diameters of Mg-Zn-Mn alloys increased as the height increased.While for the Mg-Mn alloy,the solidification rate did not change significantly along the solidification direction,so the pore diameters did not change visibly.
Compared with the column grains due to large undercooling in Mg-1Mn-1Zn,at 40mm height,Mg-1Mn-2Zn prefers equiaxed grains,since the solidification rate decreased as the increasing of height.With the growth of columnar dendrites located in the lower parts,the constitutional undercooling occurs,which makes the undercooling zone extend from the front of the liquid-solid interface to the center of the melt,resulting the solute atoms in the liquid phase are increasingly more concentrated,and this leads to heterogeneous nucleation since the constitutional undercooling gradually increase in front of solid-liquid interface.In addition,the fine secondary dendrite drifts to the center of the liquid due to the temperature fluctuation.Consequently,the internal equiaxed dendrites will be formed,which allows wider mushy zone.These nucleated pores with a smaller pore size failed to grow as the equiaxed dendrites could inhibit the growth of the pores and these small non-directional pores were remained in the solid ingot,resulting discrete size and distribution of pores even coalescence.This discrete becomes server as the increasing content of Zn in upper part of the ingot,indicating that zinc can change the width of the mushy zone in the solidification of Mg-Mn alloy during grain refinement by transforming columnar grains into equiaxed grains.
Fig.15 represents that two types [34-36] of compression twins were observed with a compressive strain of 0.2 during the plastic deformation of Mg-1Mn-1Zn.{112}<113>/3 and {011}<102>twins were triggered along c-axis at the bottom of the pores,bringing about the increasing ductility of Mg-1Mn-1Zn alloy.The number of twins increase with increment of strain [34],but the critical resolved shear stress(CRSS) for activating {011} compression twins can be very large.Therefore,it basically generates at the later stage,requiring large deformation strain [35],which is in good agreement with the strain values in this experiment.
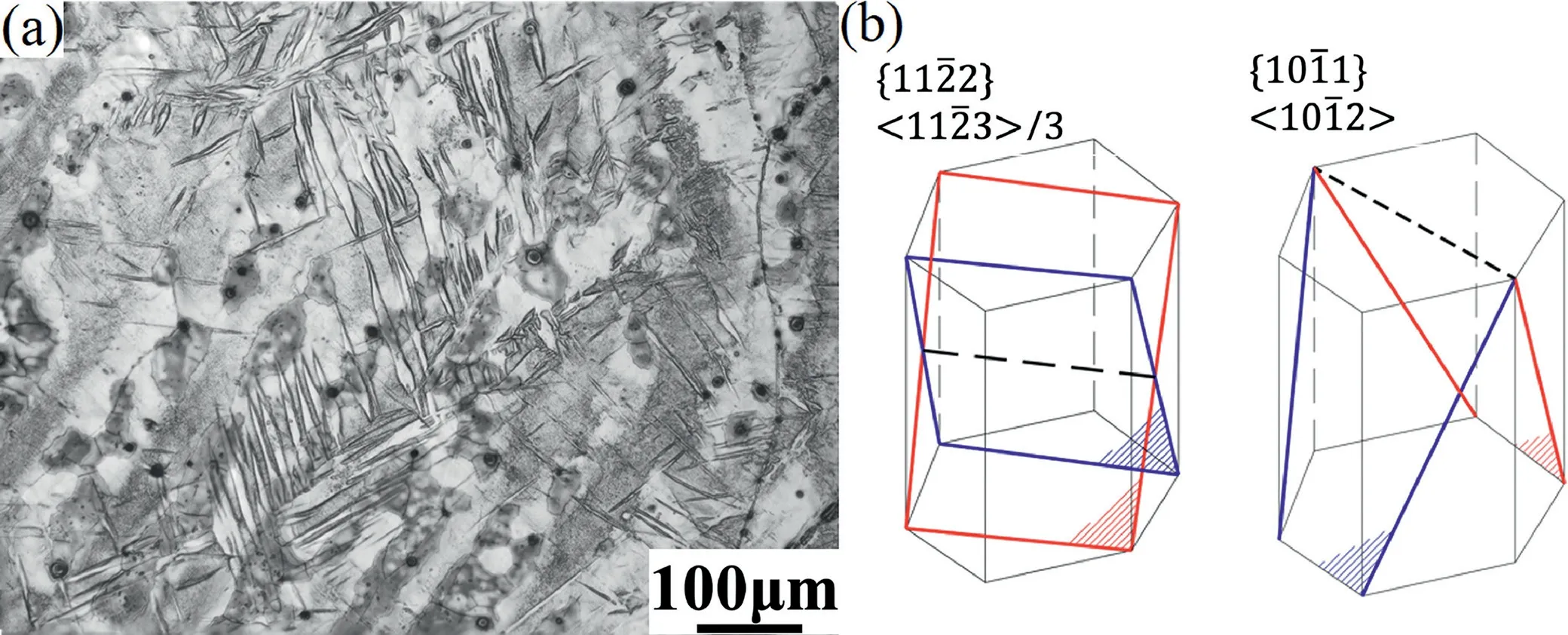
Fig.15.The microstructure of two types of compression twins (a),and {112} <113>/3 and {011} <102>twinning systems (b).
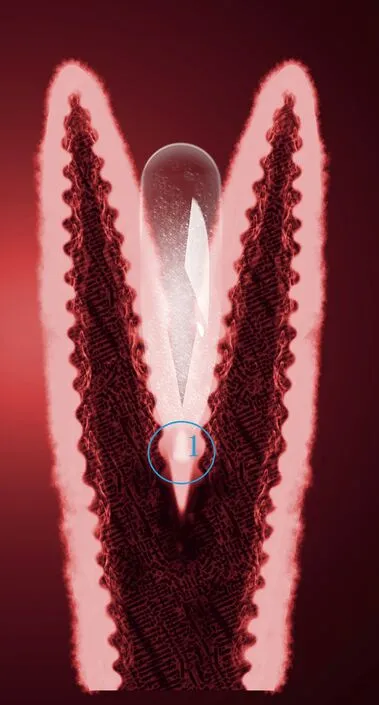
Fig.16.Schematic illustration of bubble nucleation at the conical area.
4.3.Nucleation of pores in Mg-Mn-Zn alloys
Conical areas that were rich in elemental Zn were found below the pore areas,and some precipitates that were rich in Zn or Mn were also found in these areas.In our previous work,through theoretical analysis,the heterogeneous nucleation of bubble nucleation in conical pits and cracks in impurities was found to be most feasible in the Gasar process [37].During the Mg-Mn-Zn alloy solidification,columnar dendrite grains grew with a certain crystallographic orientation,and the solute at the solid/liquid interface front was enriched in the inter-dendritic grain region,which conformed to conical area “1” in Fig.16.The solute atom aggregation area can afford fundamental bubble heterogeneous nucleation and as the bubbles grew,some melt with solute atoms drained to the bottom of the pore.As the solidification proceeded,the Mg-Zn phase and Mn particles formed in the solute aggregation area.Therefore,the solute-enrichment area can affect bubble nucleation and improve the precipitation of the Mg-Zn orα-Mn phase during the Mg-Zn-Mn alloy solidification for Gasar process.
5.Conclusions
The effects of Mn,Zn and hydrogen pressure on the porosity and pore diameters of directional solidified porous Mg-Mn-Zn alloys were investigated,and the results are as follows:
1.Mg-Mn-Zn alloys can be prepared by the Gasar process.A steady bubble growing area existed in the lower part of the ingot,and coarser directional pores and finer nondirectional pores formed in the upper part.The Mg-1Mn alloy porosity was 41.2%,and Zn addition from 1% and 2% to the Mg-1Mn alloy decreased the porosity to 36.9%and 35.8%,respectively.
2.Increasing zinc content (0wt.% -1wt.% -2wt.%) considerably promote the width of mushy zone in Mg-Mn alloy system,resulting discrete size and distribution of hydrogen pores.
3.The compressive strength of Mg-Mn-Zn alloys can be increased by around 20MPa through rising Zinc content from 1wt.%to 2wt.%,which basically linearly decline with the increasing of porosity.
4.Hydrogen pressure can influence the Mg-Mn-Zn alloy pore structure.The average pore diameters decreased as the hydrogen pressure increased and as the pressure increased from 0.1 to 0.2,0.4 and 0.6MPa.The porosities decreased from 47.1% to 43.3%,38.2% and 34.6%,respectively.
5.Conical areas that were rich in elemental Zn existed below the pores.The solute-enrichment area can affect bubble nucleation and improve the precipitation of the Mg-Zn orα-Mn phase during the solidification of Gasar Mg-Zn-Mn ingots.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Improving the Young’s modulus of Mg via alloying and compositing -A short review
- Surface modifciation of magnesium alloys using thermal and solid-state cold spray processes: Challenges and latest progresses
- Preparation,structure and properties of Mg/Al laminated metal composites fabricated by roll-bonding,a review
- A review on recent advancements in biodegradable Mg-Ca alloys
- Analysis of element loss,densification,and defects in laser-based powder-bed fusion of magnesium alloy WE43
- Role of bimodal-grained structure with random texture on mechanical and corrosion properties of a Mg-Zn-Nd alloy
