A review on recent advancements in biodegradable Mg-Ca alloys
2022-10-25ManasRanjanSahuSampathKumarUdayChakkingal
Manas Ranjan Sahu,T.S.Sampath Kumar,Uday Chakkingal
Department of Metallurgical and Materials Engineering,Indian Institute of Technology Madras,Chennai 600036,India
Abstract The binary Mg-Ca alloys are drawing increasing attention as temporary implant materials because of their excellent biocompatibility,biodegradability,and good mechanical properties.However,their applications are limited due to their high degradation rates in the human physiological environment,the consequent release of hydrogen gas,and rapid loss in mechanical properties.Furthermore,biocompatibility depends upon the degradability of the material.Various researchers have demonstrated that these issues can be addressed by control of Ca content,thermo-mechanical processing to obtain suitable microstructures,deposition of surface coatings,etc.In this manuscript,a detailed review of published literature on Mg-Ca alloys is presented.The challenges and future directions of research in this area are also described.
Keywords: Mg-Ca alloy;Degradation;Temporary implant;Biocompatibility.
1.Introduction
The commercially used metallic materials for medical implant applications are titanium,stainless steel,and cobaltchromium alloys because of their excellent biocompatibility and good mechanical properties [1].The main issue associated with using these alloys as temporary implants are stress shielding and the surgical intervention required to remove the implant post recovery [2].In the case of orthopedic implants,the large difference between the elastic modulus of the stiffer conventional metallic implant (elastic modulus of 100-200 GPa) and bone (elastic modulus of 10-30 GPa) results in the distribution of load on the implant rather than the bone tissue.This results in stress shielding and leads to early implant loosening,damage to the tissue healing process,skeleton thickening,and chronic inflammation[3-6].The second surgery required to remove the material after the tissue healing process leads to adverse consequences like pain and increased treatment costs [7].Hence there is an increasing focus on biodegradable metals that degrade progressively,are replaced by the growing tissue in the human body,and completely dissolve after the healing without producing any adverse effects [8].
In this framework,magnesium,iron,and zinc-based alloys are used for short-term implant applications.Among these biodegradable metals,magnesium is a particularly important implant material because of its biodegradability,biocompatibility,and mechanical properties.The elastic modulus and density of magnesium is close to that of bone and hence it helps in reducing the stress shielding effect in orthopedic implant application.However,the rapid degradation of magnesium implants in the physiological environment with the evolution of hydrogen gas makes its application limited in the medical field [9].The evolved hydrogen gas has a detrimental effect on the surrounding tissue because of the formation of gas pockets next to the implant which separate the tissue layer and delay the healing process [10].The low strength of magnesium is also a major concern because the implant needs to be strong enough to maintain its structural integrity during the degradation before the tissue is adequately healed.To overcome these limitations,magnesium-based alloys and specifically binary magnesium-calcium alloys,have been proposed as temporary implant materials [2,11].In addition,Ca is one of the most important elements in the human body.It is essential in chemical signaling with cells and Ca as alloying element in Mg also helps in healing the broken bone by producing the bone mineral hydroxyapatite [12].Furthermore,Ca has a low density (1.55 g/cm3),which facilitates the Mg-Ca alloys to have a similar density to the bone,and Ca also act as a grain refiner in Mg to strengthen the alloy [13].In this paper,the research work on the development of Mg-Ca alloy based biomaterials and their advantages and limitations have been critically reviewed.Various thermomechanical processing and surface treatment techniques are discussed.The effect of alloying,processing techniques,and surface treatment methodologies on the microstructure,mechanical performance,degradation behavior,and biocompatibility have been discussed.The results of in vitro and in vivo investigations on these alloys have also been presented.
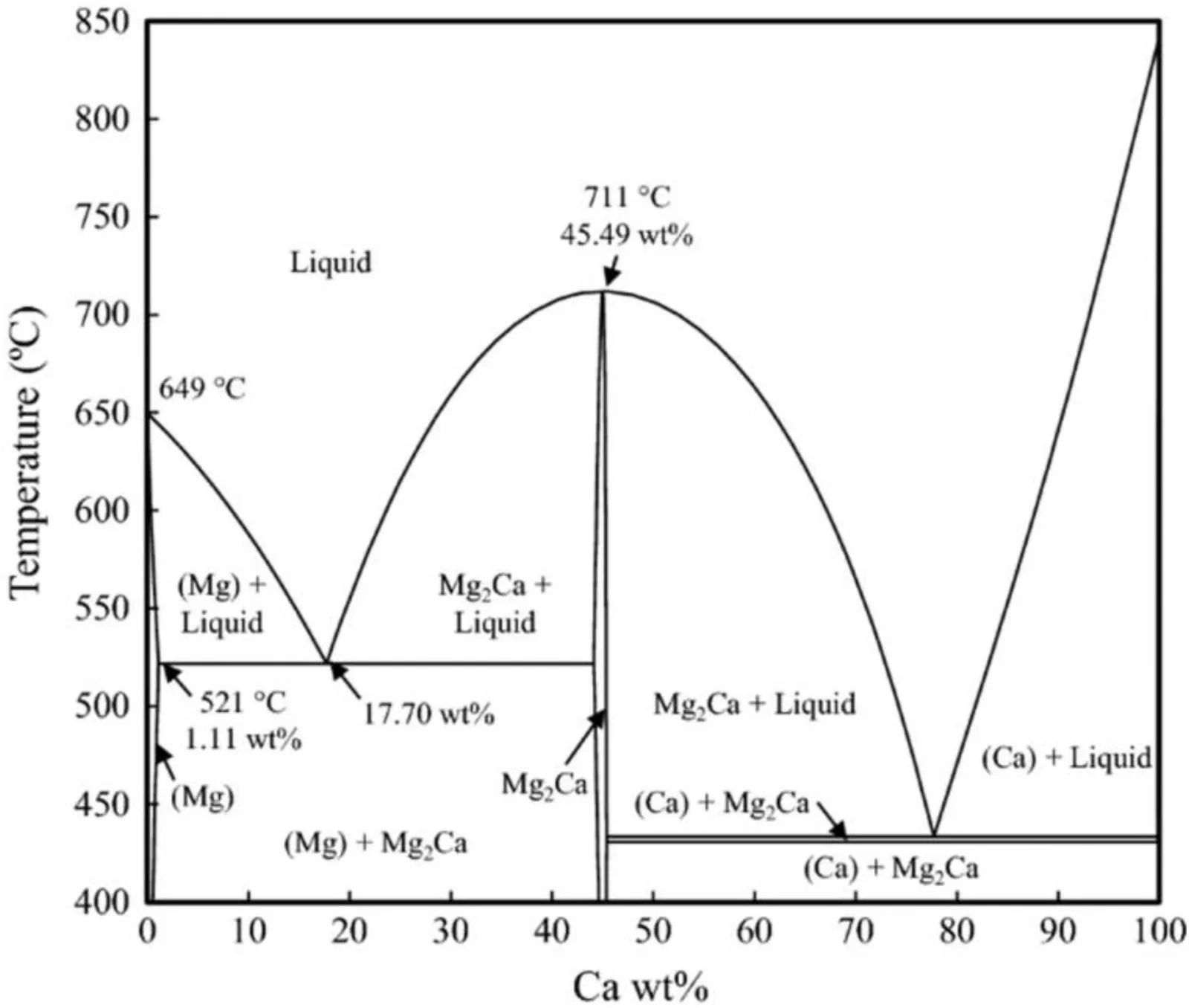
Fig.1.The binary phase diagram of Mg-Ca alloy.Reprinted by permission from Springer Nature: Oxidation of metals,the ignition behavior of Mg-Ca binary alloys: The role of heating rate,L.A.Villegas-Armenta,R.A.L.Drew&M.O.Pekguleryuz,vol.93,page no.545-558,Copyright 2020 [15].
2.Processing techniques and microstructure of Mg-Ca alloy
The binary Mg-Ca phase diagram (Fig.1) shows that the maximum solubility of Ca in Mg is 1.11 wt.% at 521 °C and it tends to zero as the temperature goes down to the room temperature.The solubility of Mg in Ca is negligible.The intermetallic Mg2Ca with 45 wt.% Ca,melts congruently at 711 °C.The microstructure of Mg-Ca alloy with Ca<45 wt.%consists of eutectic mixture of Mg and Mg2Ca phase,whereas Ca>45 wt.% consists of Ca and Mg2Ca phase.The intermetallic Mg2Ca has the same crystal structure (hexagonal)as the Mg itself,and the only difference is that the Mg2Ca has lattice parameters almost twice as large as theα-Mg [14].
2.1.Casting
Wan et al.cast Mg-xCa (x=0.6,1.2,1.6,and 2.0 wt.%)alloy by melting pure Mg and Ca at 680 °C under a high pure argon gas atmosphere [16].After casting,the cast ingots were heat treated (solutionized at 550 °C for 8 h followed by aging at 150 °C for 7 h) in argon atmosphere and equiaxed microstructure were observed in all the Mg-Ca alloy compositions.In Mg-Ca alloys,small and separate Mg2Ca precipitates within the grains and discontinuous distribution of Mg2Ca precipitates along the grain boundaries are observed.Li et al.melted pure Mg and Ca under a mixture of SF6and CO2gas atmosphere and cast different Mg-Ca alloy with Ca content of 1.0,2.0,and 3.0 wt.% [17].It has been reported that the microstructure of all the cast alloys haveα-Mg primary grains with Mg2Ca precipitates along the grain boundaries.Kirkland et al.also cast Mg-xCa (x=0.4,0.8,1.34,5,10,16.3,and 28 wt.%) alloy by melting high-purity Mg (99.99%) and Mg-28 wt.% Ca master alloy at 750 °C under pure argon gas atmosphere [14].The melt was slowly cooled with an average cooling rate of 1 °C/s and resulted in a dendritic microstructure in the alloys.The microstructure consisted of primaryα-Mg dendrites and an interdendritic eutectic phase consisting ofα-Mg and Mg2Ca phase.There was an increased refinement ofα-Mg dendritic structure and Mg2Ca phase from 3 to 24 vol% with an increase in Ca from 0.8 wt.% to 28 wt.%in Mg-Ca alloy.Rad et al.[18] prepared Mg-xCa (x=0.5,1.25,2.5,5,and 10 wt.%) alloy under argon gas by melting pure magnesium ingot and Mg-40Ca master alloy at 740 °C and observed a microstructure ofα-Mg matrix and Mg2Ca intermetallic phase in Mg-Ca alloy.In Mg-Ca alloys,with an increase in Ca content from 0.5 to 2.5 wt.%,there was a decrease in grain size with an increased amount of Mg2Ca phase both in the matrix and the grain boundaries (Fig.2).The microstructure of Mg-5Ca and Mg-10Ca alloy consists ofα-Mg dendrites and eutectic phase (α-Mg+Mg2Ca) and the dendritic cell size is reduced with increased Ca content from 5 to 10 wt.%.Rad et al.[18] have confirmed the presence of Mg2Ca phases in these alloys by XRD and shown that the intensities of the Mg2Ca phases increased with increasing Ca content (Fig.3)
2.2.Deformation techniques
Different deformation processing techniques have been used to modify the microstructure of the Mg-Ca alloy.Li et al.[17] reported a reduction in grain size of Mg-1Ca alloy by performing hot rolling at 410°C with a thickness reduction from 5 mm to 2 mm.Koleini et al.[19] performed hot rolling on Mg-1Ca alloy at three different temperatures of 330,370,and 410 °C with three different reduction percentages of 20,40,and 60 at each temperature.There was a decrease in the grain size and redistribution of the Mg2Ca phase from grain interior to grain boundary with an increase in rolling passes due to dynamic recrystallization (DRX).The grain refinement process was accelerated because of twinning leading to the presence of twin boundaries,which restricted the dislocation movement.Seong et al.performed a specialized rolling process called high ratio differential speed rolling (HRDSR) by setting the roll-speed ratio between the upper and lower rolls at 2 on Mg-xCa (x=0.4,1.0,2.0,and 3.0 wt.%) alloys[12,20].After casting,they homogenised (370 °C for 8 h) the Mg-Ca ingot and extruded it indirectly into a bar of 25 × 4 mm2at 350 °C with an extrusion ratio of 19.6.The extruded plate was preheated to 300 °C and conventionally rolled to a 2 mm thick sheet in 6 passes.Finally,the HRDSR process was performed on the conventionally rolled sample at 200 °C to reduce the thickness from 2 mm to 0.7 mm.The microstructure of HRDSR Mg-Ca alloy showed heavily deformed (unrecrystallized) grains,indicating that the presence of the Mg2Ca phase particles hindered the DRX process.The delay of DRX was attributed to the pinning effect of fine particles that exert a drag force (Zener drag) on the moving dislocations,which prevents the substructure from developing to the critical size for the formation of the DRX nucleus.Annealing at 350 °C after HRDSR resulted in a uniform fully recrystallized microstructure with a majority of Mg2Ca phase on the grain boundaries.Grain growth was observed in the HRDSR annealed Mg-Ca alloy after annealing.The HRDSR annealed Mg-Ca alloy having a higher content of Ca showed decreased grain size than the alloys with lower Ca content because of the presence of the more Mg2Ca phase on the grain boundaries which retarded grain growth during annealing by the Zener drag effect (Fig.4).
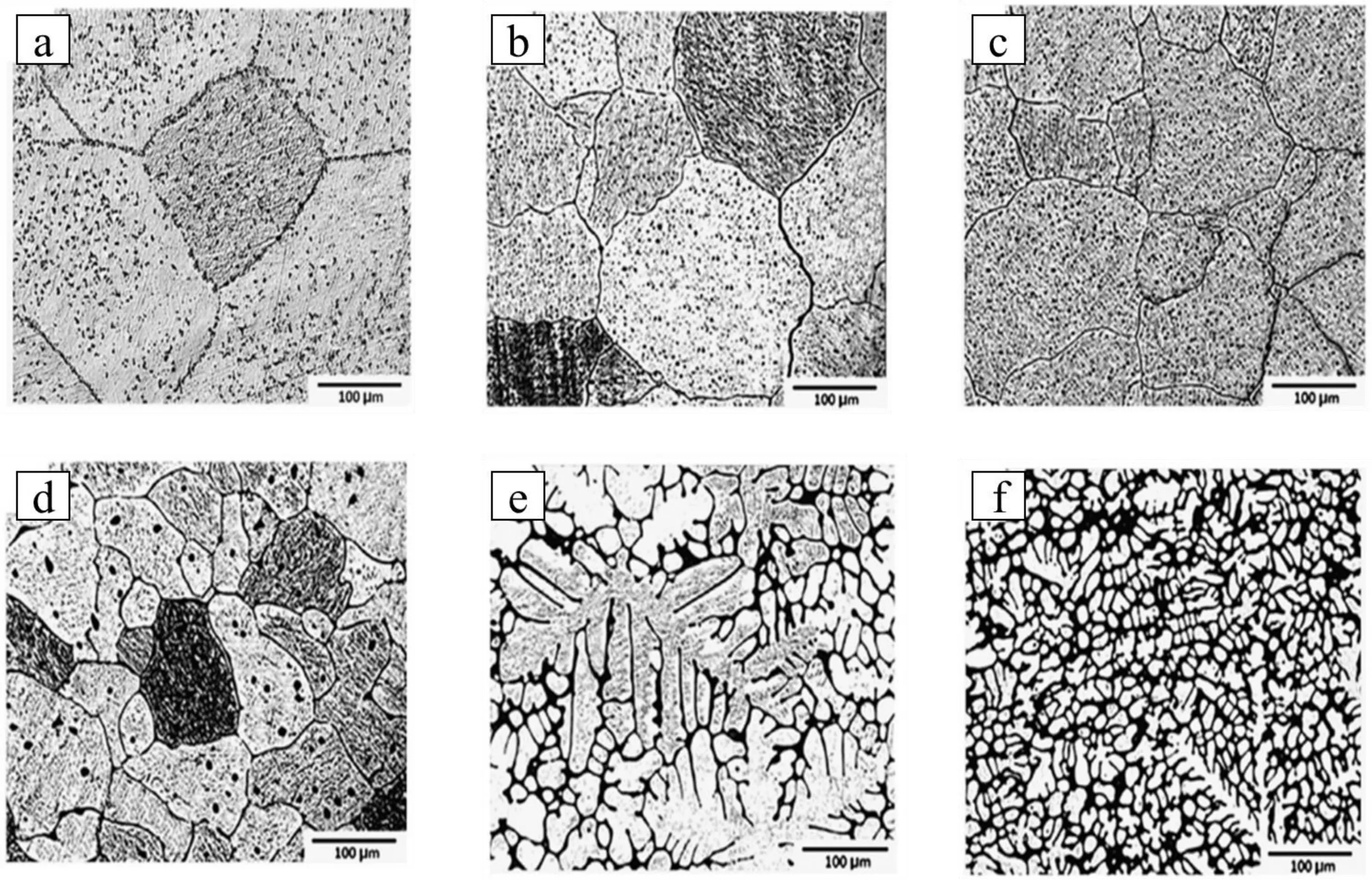
Fig.2.Optical microstructure of (a) pure Mg,(b) Mg-0.5Ca,(c) Mg-1.25Ca,(d) Mg-2.5Ca,(e) Mg-5Ca,(f) Mg-10Ca alloy.Reprinted from Materials &Design,vol 33,H.R.B.Rad,M.H.Idris,M.R.A.Kadir,and S.Farahany,Microstructure analysis and corrosion behavior of biodegradable Mg-Ca implant alloys,page no.88-97,Copyright 2012,with permission from Elsevier [18].
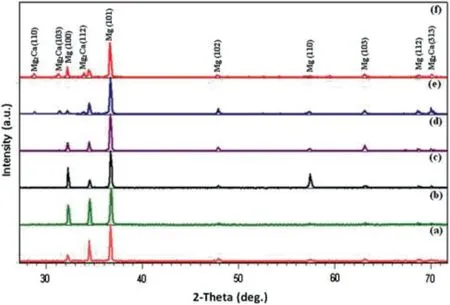
Fig.3.X-ray diffraction patterns of (a) pure Mg,(b) various Mg-Ca alloys with different calcium content: 0.5,(c) 1.25,(d) 2.5,(e) 5,(f) 10 wt.%.Reprinted from Materials &Design,vol 33,H.R.B.Rad,M.H.Idris,M.R.A.Kadir,and S.Farahany,Microstructure analysis and corrosion behavior of biodegradable Mg-Ca implant alloys,page no.88-97,Copyright 2012,with permission from Elsevier [18].
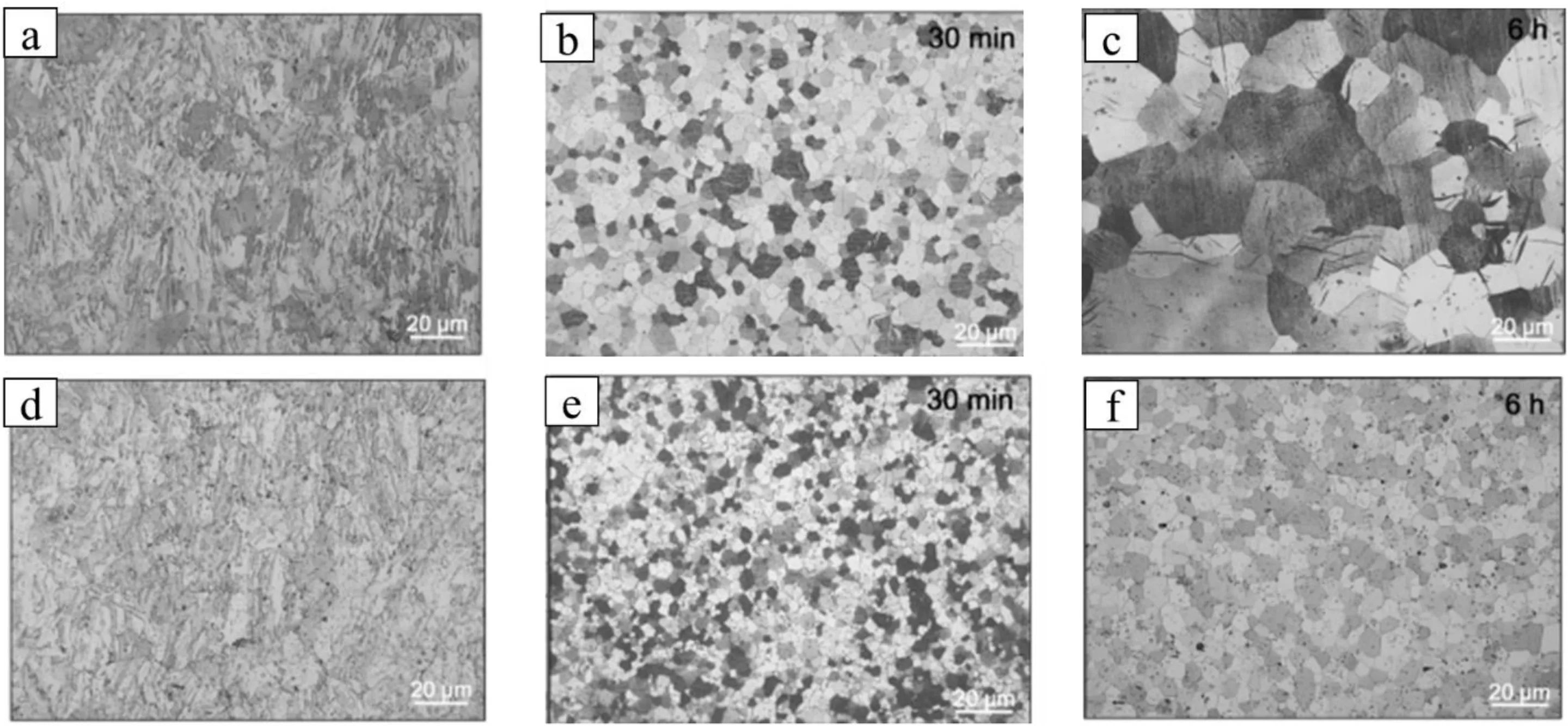
Fig.4.The optical micrographs of the (a and d) HRDSR Mg-0.4Ca and Mg-1Ca alloys respectively,and (b and c) Mg-0.4Ca and (e and f) Mg-1Ca alloys,which were annealed after HRDSR for different times (30 min and 6 h) at a temperature of 350 °C.Reprinted from Corrosion Science,vol 98,J.W.Seong,W.J.Kim,Mg-Ca binary alloy sheets with Ca contents of ≤1 wt.% with high corrosion resistance and high toughness,page no.372-381,Copyright 2015,with permission from Elsevier [20].
The Zener drag effect was also observed on hot forged Mg-1Ca alloy by Harandi et al.,where the grain size decreases with an increase in forging temperature from 250°C to 450 °C because of the precipitation of more Mg2Ca phase at grain boundaries [21].It was also mentioned that the equiaxed grains produced in the hot forging process also contains twins which confirms the deformation in Mg-1Ca alloy was dominated by twinning.
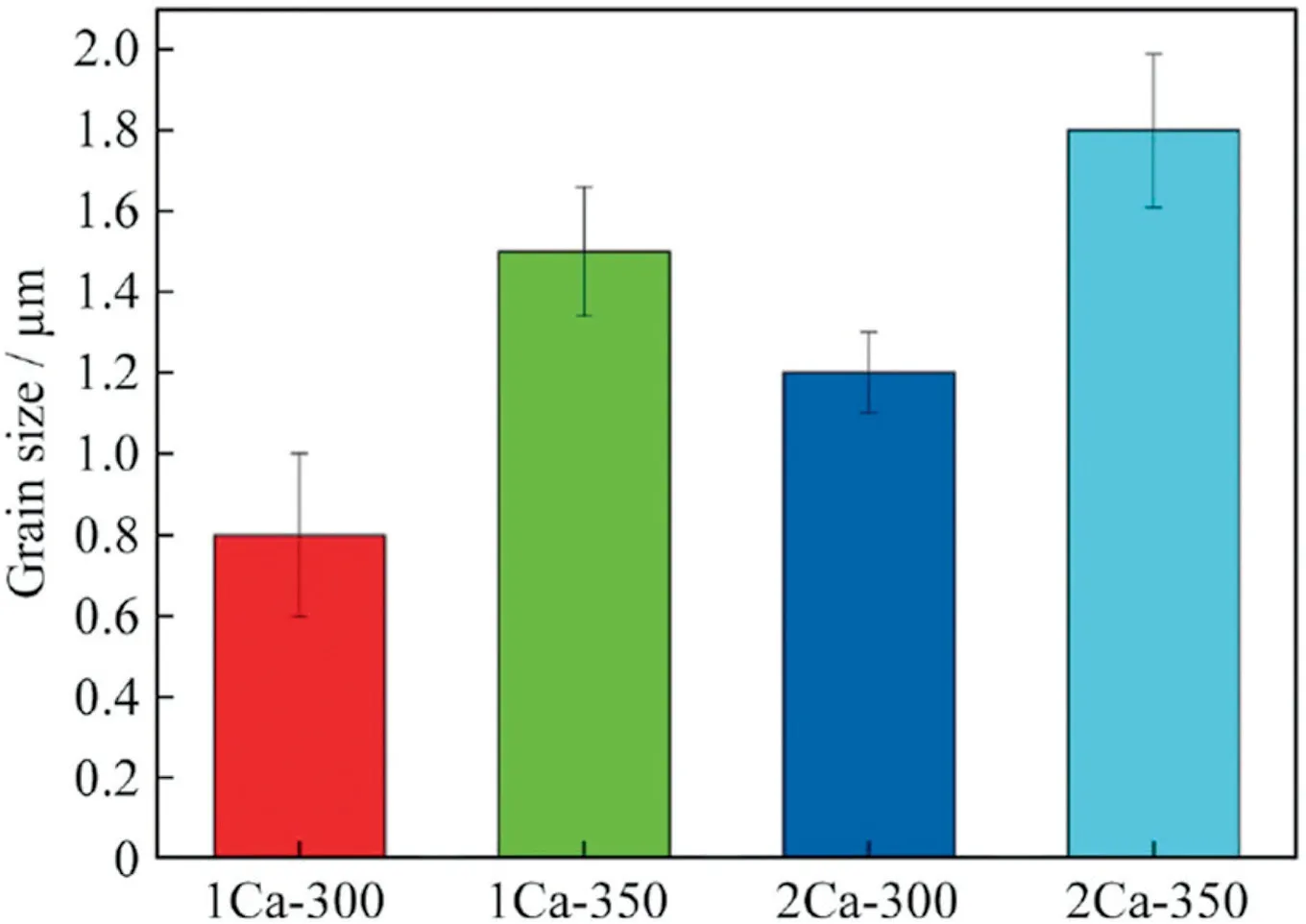
Fig.5.The average grain size for as-extruded Mg-Ca alloys.Reprinted by permission from Springer Nature: Rare Metals,Microstructures and biocorrosion resistances of as-extruded Mg-Ca alloys with ultra-fine grain size,Z.R.Xie,C.Zhang,H.C.Pan,Y.X.Wang,Y.P.Ren,and G.W.Qin,DOI:https://doi.org/10.1007/s12598-017-0945-2,Copyright 2017 [23].
Zheng et al.hot extruded the Mg-xCa (x=0.54,0.79,and 1.35 wt.%) alloys at an extrusion speed of 1 m/min and extrusion ratio of 19.7:1 by preheating the ingot and the mold of the extrusion machine to 300 and 350 °C respectively for 2 h [22].The fine and equiaxed re-crystallized grains and a considerable amount of dispersed fine Mg2Ca particles were observed in the microstructure of the extruded alloy.The grain size decreased and the volume fraction of the Mg2Ca phase increased with an increase in Ca content from 0.54 to 1.35 wt.% in the as-extruded Mg-Ca alloy.The homogeneity of the microstructure varies with Ca content and Mg-0.79Ca alloy showed the most homogeneous microstructure.Xie et al.performed indirect extrusion on Mg-xCa (x=1.0,and 2.0 wt.%) alloys at two different temperatures of 300 °C and 350 °C at a ram speed of 2 mm/s and extrusion ratio of 20 [23].There was an increase in the grain size and increased volume fraction of DRXed grains with an increase in the extrusion temperature from 300 °C to 350 °C.The as-extruded Mg-Ca alloy has partially DRXed microstructure with ultrafine grain (UFG) size.However,in contrast to Zheng et al.,Xie et al.reported increased grain size with an increase in Ca content from 1 to 2 wt.% for as-extruded Mg-Ca alloy(Fig.5).This can be attributed to the lower amount of dislocation density and other defects in Mg-2Ca alloy as compared to Mg-1Ca alloy.The Mg2Ca precipitates are sharply broken and distributed along the extrusion direction (Fig.6).
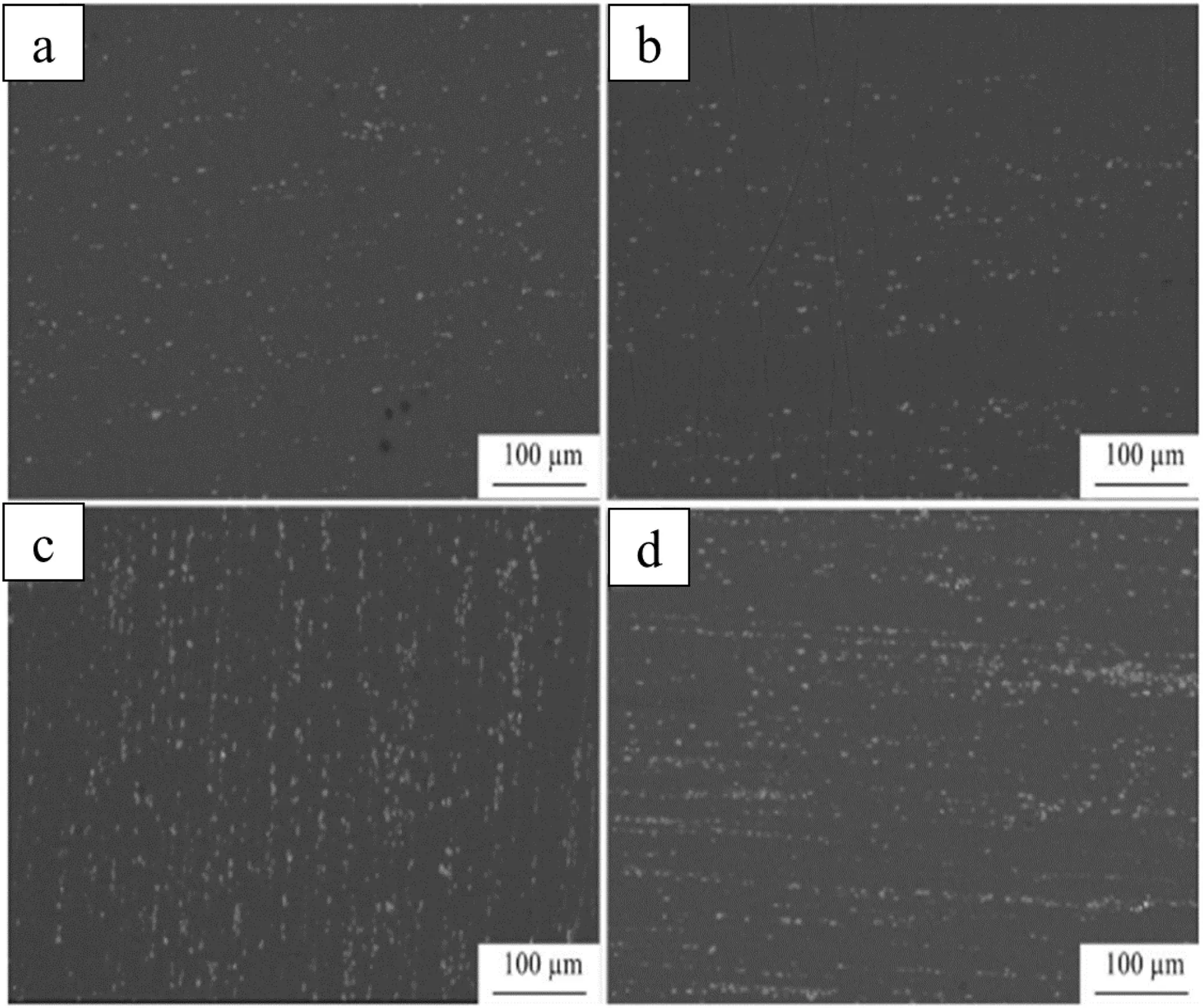
Fig.6.The SEM images of as-extruded Mg-Ca alloys: (a) 1Ca-300,(b) 1Ca-350,(c) 2Ca-300 and (d) 2Ca-350.Reprinted by permission from Springer Nature: Rare Metals,Microstructures and bio-corrosion resistances of as-extruded Mg-Ca alloys with ultra-fine grain size,Z.R.Xie,C.Zhang,H.C.Pan,Y.X.Wang,Y.P.Ren,and G.W.Qin,DOI: https://doi.org/10.1007/s12598-017-0945-2,Copyright 2017 [23].
In conventional deformation techniques,imposing large strains is a challenging task because of the change in dimensions of the workpiece and the formation of internal defects.So researchers used severe plastic deformation (SPD) techniques (such as groove pressing (GP),high-pressure torsion(HPT),equal-channel angular pressing (ECAP),and friction stir processing(FSP))to produce uniform UFG microstructure free of mechanical cracks or defects in metals by imposing extremely large strain on the bulk metal without a significant change in the overall dimensions [24].The grain refinement through SPD takes place by various recovery processes referred to as continuous dynamic recrystallization (cDRX)or geometric dynamic recrystallization (gDRX).In cDRX,the grains are divided into sub-grains which eventually develop into high angle grain boundaries,whereas in gDRX the serrated grain boundaries produced due to large deformation strains meet and pinch off resulting in finer grains [25].Li et al.found a part of the Mg2Ca phase along the grain boundaries in the deformed layer dissolved into the matrix after surface mechanical attrition treatment (SMAT) on Mg-1Ca alloy [26].Sahu et al.observed both the decreased grain size and the decreased amount of Mg2Ca precipitates in Mg-0.6Ca alloy after performing groove pressing at 330 °C [27].The grain size of the GP sample was 5 μm whereas in the annealed condition it was 64 μm.Li et al.performed ECAP on Mg-1Ca alloy where the preheated (400 °C,30 min) billet was subjected to ECAP pressing with 4 passes at 400 °C and with 2 passes at 300 °C [28].The ECAPed sample showed a more evenly dispersed Mg2Ca phase as compared to the as-cast one.The grain size decreased from 150 μm for ascast to 1 μm for the ECAPed sample.The ECAPed sample also showed substantial dislocations within the grain.Li et al.also observed decreased grain size with uniform distribution of Mg2Ca phase in Mg-1Ca alloy deformed by HPT [29].The Mg-1Ca alloy was deformed under a compressive pressure of 6 GPa at room temperature to 5 turns with a rotation speed of 1 rpm.The HPT processed sample had a grain size of 171 ± 52 nm whereas it was 183 ± 10 μm for the ascast Mg-1Ca alloy.In as-cast condition,the Mg2Ca had an average length of 134 ± 31 μm along the grain boundary and some Mg2Ca phase of average diameter of 13 ± 4 μm were precipitated within the grain.The HPT Mg-1Ca alloy has Mg2Ca phase of size 6 ± 2 μm.Parfenov et al.observed an average grain size of 100 ± 9 nm,1.1 ± 0.2 μm,and 42 ± 3 μm for HPT,HPT followed by annealing (250 ± 5°C,6 h),and as-cast heat treated (450 ± 5 °C,12 h) respectively for Mg-1Ca alloy [30].The Mg-1Ca alloy was deformed by placing the sample between two anvils and rotating the lower anvil (10 rotations) by applying a uniaxial pressure of 6 GPa at room temperature.Both the volume fraction and weight% of Mg2Ca phase follows the order of HPT Mg-1Ca alloy<HPT followed by annealed Mg-1Ca alloy<as-cast heat treated Mg-1Ca alloy.Shishir et al.reported UFG microstructure having 13 μm grain size without any secondary phase in FSPed Mg-0.66Ca alloy FSP [31].The grain sizes of various Mg-Ca alloys resulting from different processing methods are summarized in Table 1.

Table 1The summary of the grain sizes of various Mg-Ca alloy resulting from different processing methods.
2.3.Surface coating
Some researchers also focused on altering the surface morphology by coating the Mg-Ca alloy using different surfacecoating processes.Gu et al.performed alkaline treatment such as Na2HPO4,Na2CO3,NaHCO3treatment on Mg-1.4Ca alloy and observed a rough thick compact layer having some micro pits on the surface of the samples [32].A similar microstructure was also observed for fluoride-coated Mg-Ca alloy by Drynda et al.[33] and Rad et al.[34].Mohedano et al.[35] and Coquillat et al.[36] deposited plasma electrolyte oxidation (PEO) coating on Mg-0.8Ca alloy by using electrolytes with and without fluoride species and found that fluoride containing coatings are thicker.This indicates that fluoride-containing electrolytes enhance the growth rate of PEO films.At lower fluoride contents,a two-layer morphology was observed where the outer layer contains large sparse cavities and the inner layer contains numerous small pores.The coated sample surface with higher fluoride contents showed more compact and homogeneous coatings without a differential layer.
Gu et al.[37],Cui et al.[38],and Sedelnikova et al.[39] coated Mg-Ca alloy using micro-arc oxidation (MAO)and,as shown in Fig.7,found a deposition of a thick porous layer on the sample surface.The pore size and thickness of the deposited MAO layer increases with an increase in applied voltage due to the dielectric breakdown of the MAO layer.At a lower voltage,fine sparking was observed uniformly distributed on the substrate surface,resulting in fine micropores.With an increase in the applied voltage,the discharge intensity of single spark is enhanced and the melted product erupts and rapidly deposits around the discharge channels[40].As a result of this series of reactions,the pore size and the thickness of the layer increases with higher applied voltage.Similarly,plasma chemical oxidation (PCO) coating on Mg-1Ca alloy showed smaller number of larger pores as reported by Rosemann et al.[41,42].
Rau et al.[43] and Surmeneva et al.[44] coated hydroxyapatite on Mg-Ca alloy using pulse laser deposition (PLD)and radio frequency magnetron sputtering respectively and observed uniform coating without any patches on the surface.The PLD-coated layer showed granular morphology.Bita et al.also observed the rougher morphology of hydroxyapatite coated Mg-0.8Ca alloy using a magnetron sputtering system [45].Rad et al.observed that the fluorinedoped hydroxyapatite-coated Mg-Ca alloy surface showed needle shape crystals without any defects while the brushite coating surface has a flake-like structure with some defects(Fig.8) [46].
Istrate et al.coated ZrO2-CaO and ZrO2-Y2O3on Mg-Ca alloy by plasma spraying method and observed pores and splats on both the coated surface,however,ZrO2-CaO coating exhibited a more compacted layer [47,48].Li et al.coated the Mg-1Ca alloy with TiO2using the dip coating method followed by heat treatment at 500 °C for 2 h and observed uniformly distributed cracks on the coating surface due to the built-up of stresses resulting from the effect of thermal-expansion coefficient mismatch between the TiO2coating and the substrate after heat treatment [49].Rad et al.deposited nano-Si and nano-Si/TiO2composite coatings on Mg-1Ca alloy using physical vapor deposition (PVD) method[50].Micro pores and micro flaws were observed in the coating,but the microstructure of the Si/TiO2coating was denser than that of the Si coating indicating that the presence of TiO2reduces the occurrence of pores and cracks in the Si coating.
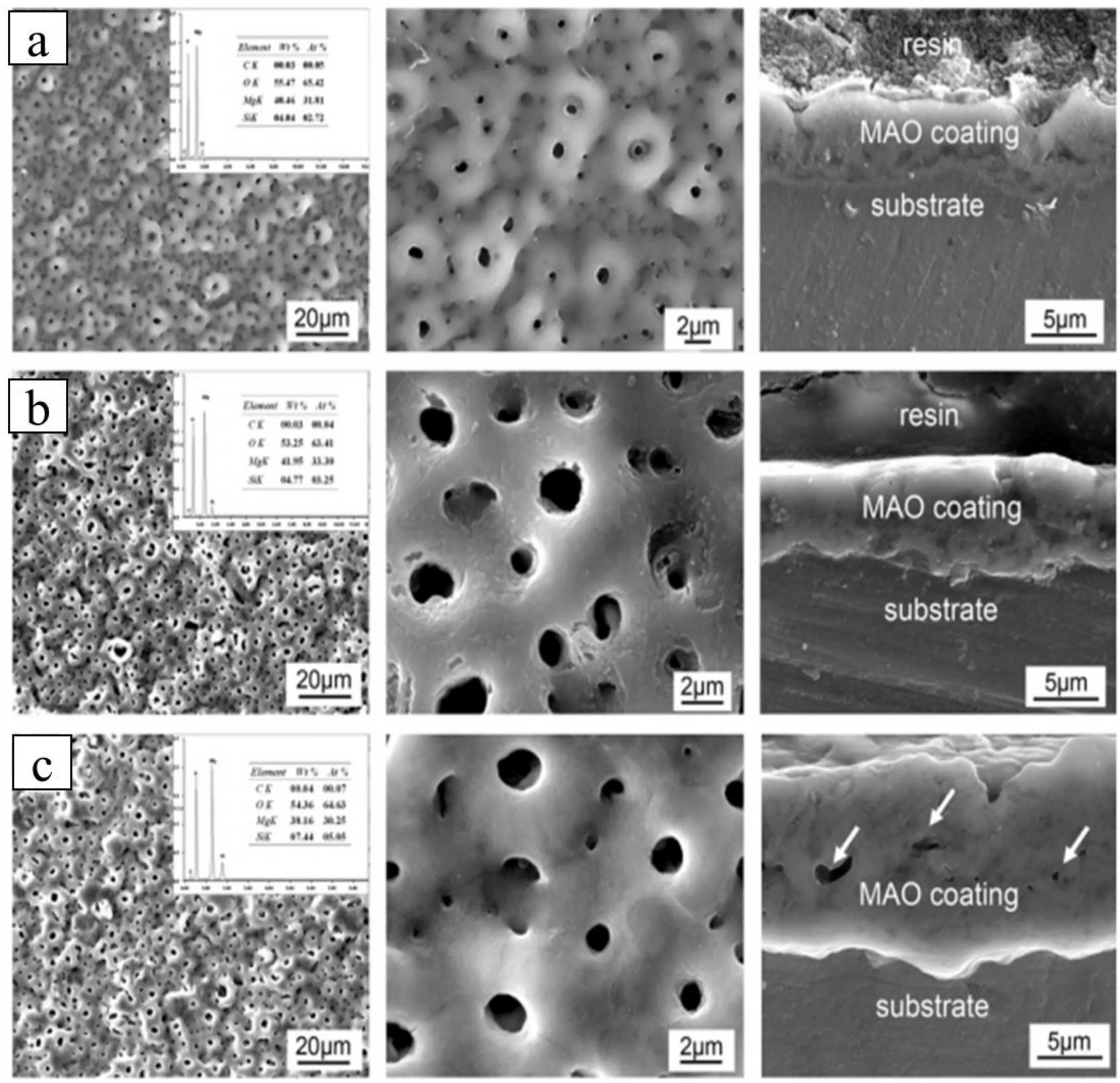
Fig.7.The surface and cross-sectional morphologies of (a) 300 V,(b) 360 V,and (c) 400 V MAO-treated Mg-Ca alloy samples.Reprinted from Acta Biomaterialia,vol 7,X.N.Gu,N.Li,W.R.Zhou,Y.F.Zheng,X.Zhao,Q.Z.Cai,Liquan Ruan,Corrosion resistance and surface biocompatibility of a microarc oxidation coating on a Mg-Ca alloy,pages no: 1080-1089,Copyright 2011,with permission from Elsevier [37].
3.Mechanical properties of Mg-Ca alloy
The implant should have adequate mechanical properties like yield strength,ultimate tensile strength,elongation,and elastic modulus to maintain its structural integrity during the degradation before the tissue is adequately healed.
Rad et al.reported the hardness value of as-cast Mg-Ca alloy increases with an increase in Ca content (Fig.9) due to the presence of the intermetallic compound of the Mg2Ca phase,which has a higher hardness value thanα-Mg matrix.[18].The pure Mg has a hardness value of 28.9 HV,which increases to 78.8 HV for Mg-10Ca alloy.Drynda et al.reported that the tensile strength of fluoride coated extruded Mg-Ca alloy increases rapidly with an increase in Ca content up to 2 wt.% and after that there is no significant increase and it is approximately 230 MPa at 2 wt.% and 240 MPa at 4 wt.% Ca [33].The 0.2 elastic limit also increases with Ca content and is approximately 200 MPa at 2 wt.%.Wan et al.reported that the bending and compressive modulus of heat treated (solutionized at 550 °C for 8 h followed by aging at 150 °C for 7 h) Mg-Ca alloy increases with an increase in Ca content [16].In Mg-xCa (x=0.6,1.2,1.6,2.0 wt.%)alloy,Mg with 0.6 wt.% Ca content showed higher bending and compressive strength because of lesser amounts of Mg2Ca phase compared to alloys with higher Ca content.Although the mechanical properties of Mg-Ca alloy are initially similar to that of bone,upon degradation it decreases which leads to early implant loosening and is a prime concern.So research has focused on improving the mechanical properties of Mg-Ca alloy using various thermo-mechanical deformation techniques.
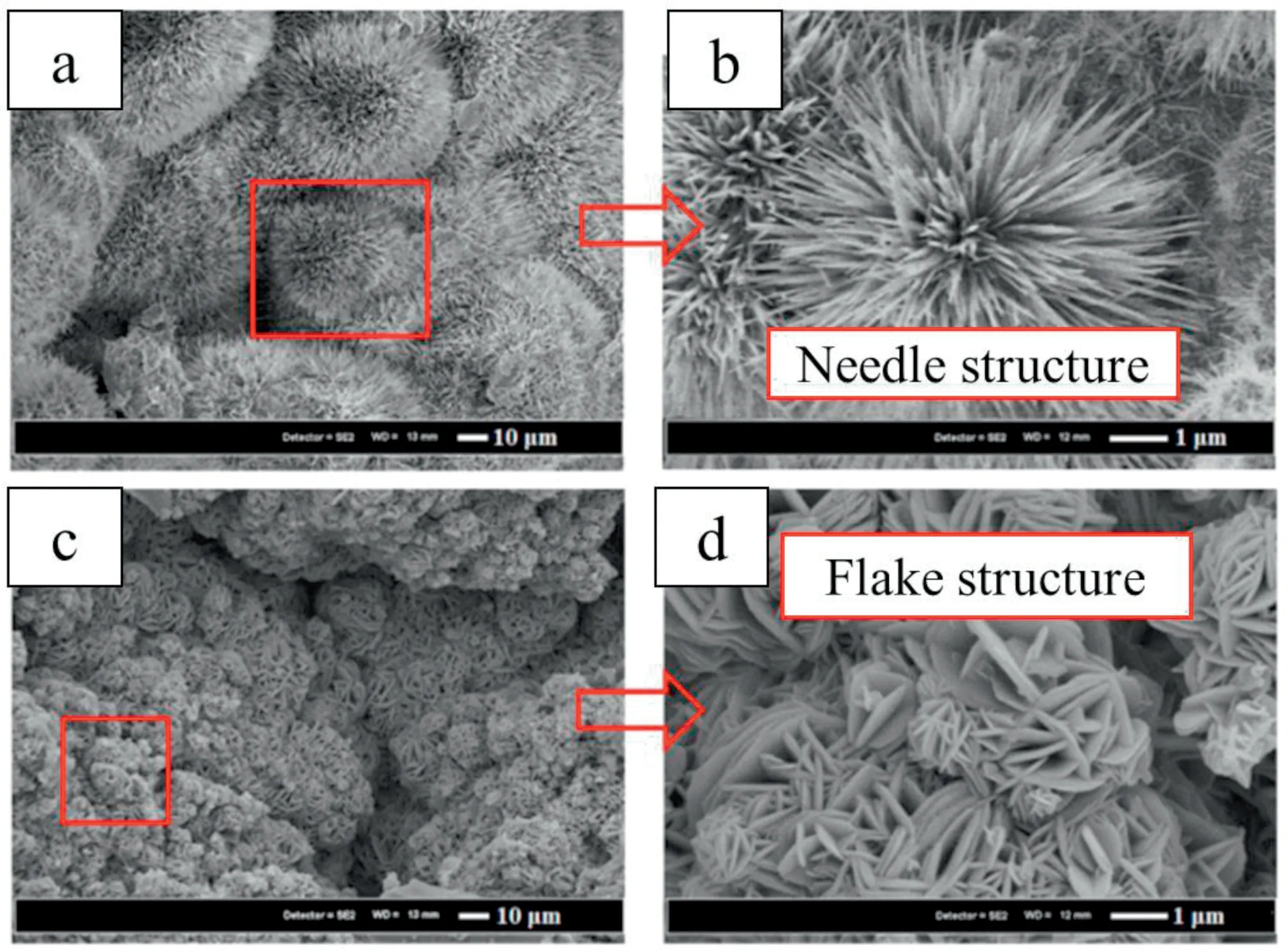
Fig.8.The scanning electron micrograph of the surface of (a and b) fluorine-doped hydroxyapatite-coated;(c and d) brushite-coated Mg-Ca alloy.Reprinted from Ceramics International,vol.40,H.R.Bakhsheshi-Rad,E.Hamzah,M.Daroonparvar,R.Ebrahimi-Kahrizsangi,and M.Medrajwith,In-vitro corrosion inhibition mechanism of fluorine-doped hydroxyapatite and brushite coated Mg-Ca alloys for biomedical applications,pages no: 7971-7982,Copyright 2014,with permission from Elsevier [46].
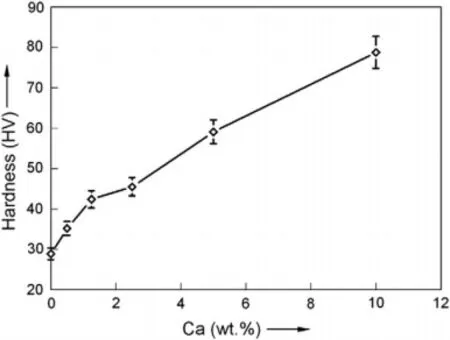
Fig.9.Effect of Ca content on the hardness of Mg-Ca alloy.Reprinted from Materials &Design,vol 33,H.R.B.Rad,M.H.Idris,M.R.A.Kadir,and S.Farahany,Microstructure analysis and corrosion behavior of biodegradable Mg-Ca implant alloys,page no.88-97,Copyright 2012,with permission from Elsevier [18].
Li et al.mentioned that mechanical properties (yield strength,ultimate tensile strength,and elongation) were improved by mechanical deformation processes like hot rolling and hot extrusion in Mg-1Ca alloy [17].Seong et al.showed that in Mg-Ca alloy subjected to the HRDSR process,the increased hardness compared to the as-cast and as-extruded Mg-Ca alloy was due to the increased dislocation density and increased particle strengthening effect because of finer Mg2Ca phase [12].After annealing,there was a decrease in the hardness in HRDSR processed Mg-Ca alloy due to the static recrystallization process which decreases the dislocation density.Seong et al.also reported that Mg-Ca alloy with higher Ca content (>1 wt.% Ca) leads to a higher volume fraction of the Mg2Ca phase which decreases the ductility even when the Mg2Ca phase is well refined and dispersed.This is because the interface between Mg and Mg2Ca is incoherent and weak,thereby easily nucleating cavities and micro-cracks during plastic deformation [20].There is a decrease in strength and an increase in ductility with annealing.
Harandi et al.mentioned that with an increase in temperature in the forging process,the hardness value of Mg-1Ca alloy increases and reaches a maximum at 350°C.The hardness then decreases even if the grain size decreases with increasing forging temperature [21].The decreased hardness value after 350 °C indicates the partial dissolution of the Mg2Ca phase.There was also an increase in the hardness value with an increase in the forging speed because of higher mechanical deformation.Hence an optimum temperature is also required for thermo-mechanical deformation and Mg-Ca alloy shows the highest hardness of 47 HV when forged at 350 °C.
Researchers also observed improvement in the mechanical properties using different severe plastic deformation techniques (GP,HPT,ECAP,and SMAT).Sahu et al.observed a 62% increase in the hardness and doubling of the yield strength and ultimate tensile strength due to grain refinement after GP performed at 330 °C on the Mg-0.6Ca alloy [27].Similarly,Li et al.reported improved tensile properties after HPT [29] and ECAP [28] in Mg-1Ca alloy.The grain refinement,increased dislocation density,and dispersion strengthening related to uniformly distributed Mg2Ca phases contributed to the improvement in the strength of the Mg-Ca alloy.The low ductility of HPT and ECAP processed Mg-Ca alloy is due to the loss of strain-hardening ability because of the presence of the high internal stresses in these materials.Hence it is suggested that additional annealing can lower the internal stresses and enhance ductility [51,52].Li et al.reported that there is a gradient in the hardness values after peening the surface of Mg-1Ca alloy with zirconia balls in the SMAT process.The hardness value decreases from the top layer to the matrix [26].The increased hardness value on the surface is due to the re-dissolution of the Mg2Ca phase and solution strengthening.However,it was observed that the peening time does not affect the hardness value.
Bian et al.performed fatigue test of hot extruded Mg and Mg-1Ca alloy in both air and simulated body fluid (SBF)solution [53].The fatigue life in the SBF solution was always lower than that performed in the air.In the air,both the alloys had a fatigue limit of 90 MPa.However,in the SBF solution,the hot extruded Mg-1Ca alloy had a fatigue strength of 70 MPa while the hot extruded Mg had a fatigue strength of 52 MPa at 4 × 106cycles.In air,the fatigue crack is initiated from the microstructural defects whereas for SBF solution it is initiated from the surface corrosion pits.Ikeo et al.also studied the fatigue behavior of hot extruded Mg-0.3Ca alloy in both air and Eagle-minimum essential medium (EMEM)+10%fetal bovine serum(FBS)solution and reported that in the air the alloy has a fatigue limit of 50 MPa whereas there was no fatigue limit in E-MEM+10% FBS solution[54].In E-MEM+10% FBS solution,at 30 MPa of stress amplitude,the number of cycles to failure was 2 × 105.The fatigue fractured surface was independent of the surrounding medium and it showed grain boundary fracture.
Table 2 shows the mechanical properties like tensile strength,ultimate tensile strength,compressive strength,bending strength,and elongation of different Mg-Ca alloy with and without several deformation techniques.

Table 2 Summary of the mechanical properties of various Mg-Ca alloy.
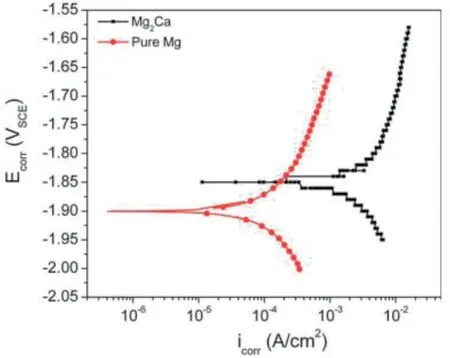
Fig.10.Potentiodynamic polarization curves of Mg2Ca phase and pure Mg.Reprinted with permission from John Wiley and Sons.Nicholas T.Kirkland,Nick Birbilis,Jemimah Walker,Tim Woodfield,George J.Dias,Mark P.Staiger,In-vitro dissolution of magnesium-calcium binary alloys: Clarifying the unique role of calcium additions in bioresorbable magnesium implant alloys,Journal of Biomedical Materials Research B: Applied Biomaterials,vol.95B,no.1,page no.91-100,John Wiley and Sons,© 2010 WILEY PERIODICALS,INC.[14].
Some of the researchers also tried to improve the mechanical properties by surface coating of the alloy.Rad et al.found that Mg-1Ca alloy with nano-Si and nano-Si/TiO2composite coatings showed higher compressive strength than the uncoated Mg-1Ca alloy after immersion in SBF,indicating a delay in the loss of mechanical properties [50].After immersion in SBF,the compressive strength decreases and it is around 188.1 MPa and 198.3 MPa for the alloy with nano-Si and nano-Si/TiO2coatings respectively.These values are comparable to the compressive strength of the human cortical bone(100-230 MPa [56]).The lower compressive strength of the alloy with nano-Si coating after immersion in SBF is due to its higher degradation rate because the mono-layered Si coating cracks easily and the barrier property was lost resulting in the SBF reaching the substrate through the cracks and pores leading to loss of mechanical integrity.The Si/TiO2composite coated sample demonstrated the highest compressive strength after immersion due to the compact and uniform structure with less pores and cracks in the coating.Rad et al.also observed a compressive strength of 203.6 MPa and 182.4 MPa for fluorine-doped hydroxyapatite and brushite coated Mg-3Ca alloy after immersion in SBF solution for 10 days [46].
4.Degradation studies of Mg-Ca alloy
The biodegradable implants are expected to degrade gradually in the human body,at a suitable rate that matches the recovery rate of the healing tissues.Therefore,it is necessary to understand the degradation behavior and its mechanisms and various techniques to control the degradation behavior.
4.1.Degradation mechanism of Mg-Ca alloy
The role of the Mg2Ca phase as anodic or cathodic in the corrosion process of Mg-Ca alloy was doubtful [57,58].However,recently researchers agreed with the anodic nature of the Mg2Ca phase in Mg-Ca alloy [59,60].The Mg2Ca phase has a higher corrosion potential than the Mg matrix(Fig.10)[14].Hence,the more electrochemically active Mg2Ca phase acts as an anode and a micro galvanic cell is established between the Mg2Ca phase and Mg matrix.The degradation of Mg-Ca alloy involves reactions (1) to (4) [7].
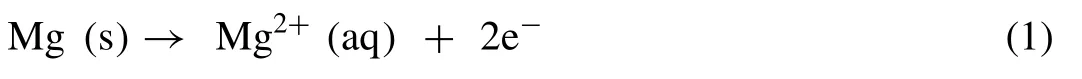
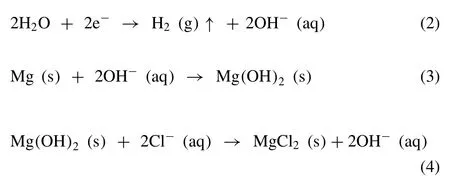
The Mg ions from the dissolution of the Mg matrix react with the hydroxyl ion to form Mg(OH)2on the surface of the Mg-Ca alloy.So the main degradation products are Mg(OH)2and H2.According to the potential -pH diagram for Mg -H2O system,Mg(OH)2acts as a stable protective layer over Mg surface in a high pH (pH>11.5) environment,whereas at a lower pH,the Mg(OH)2layer is not stable and accelerates the corrosion process.Witte et al.reported that after surgery,the pH is around 7.4 or lower at the bone-implant interface because of metabolic and resorptive processes [61].This lower pH leads to a partially protective unstable Mg(OH)2layer on the Mg-Ca alloy surface.The growth of this Mg(OH)2layer occurs by the dissolution -precipitation mechanism [62].The chloride ion (Cl-) present in the solution reacts with Mg(OH)2and forms soluble MgCl2by pitting corrosion as shown in reaction (4).So some pieces of the Mg(OH)2layer start to dissolve and fresh Mg-Ca alloy substrate is exposed to the solution.Then all the chemical reactions (1)-(4) would continue until the Mg-Ca alloy is completely exhausted.During the degradation process,the positively charged calcium ions from the surrounding fluid and from the corrosion of the Mg2Ca anodic phase interact with the negatively charged phosphate ions from the surrounding fluid resulting in the formation of a Ca-P layer on the surface of the alloy.This leads to surface passivation by which the degradation rate is lowered.The degradation behavior of Mg-Ca alloy is shown in Fig.11.

Fig.11.Schematic diagram of the alloy/solution biocorrosion interface: (a) the galvanic corrosion between Mg and Mg2Ca phase,(b) the partially protective film covering the surface of Mg-Ca alloys,(c) the adsorption of chloride ions to transform Mg(OH)2 into MgCl2,(d) the hydroxyapatite formation by consuming Ca2+ and PO43-,and (e) the disintegrated particle-shape residues falling out of the bulk substrate.Reprinted from Biomaterials,vol 29,Z.Li,X.Gu,S.Lou,and Y.Zheng,The development of binary Mg-Ca alloys for use as biodegradable materials within bone,page no.1329 -1344,Copyright 2008,with permission from Elsevier [17].
4.2.Effect of amount of Ca on the degradation of Mg-Ca alloy
Gao et al.reported that Mg-0.7Ca alloy has higher corrosion resistance than pure Mg by performing the immersion test using SBF solution [63].The lower corrosion rate of Mg-Ca alloy is due to higher surface passivation because of the presence of the Ca element.Wan et al.performed the electrochemical test using SBF as electrolyte on alloys with different Ca contents and reported that the corrosion resistance decreases with an increase in calcium content in Mg-xCa (0.6,1.2,1.6,and 2.0 wt.%) alloy because of the increase in amounts of Mg2Ca phase with increasing Ca contents [16].A similar trend was observed in the immersion test of Mg-xCa (x=0.5,1.25,2.5,5.0,and 10.0 wt.%) alloy in SBF solution for 84 h performed by Rad et al.[18].The Mg-0.5Ca alloy has the highest corrosion resistance and the Mg-10.0Ca alloy the least.This is because the presence of more Mg2Ca phase in the Mg-10Ca alloy promotes pitting corrosion.The pH variation results also showed an increase in pH value in all Mg-Ca alloys during the initial time due to the hydroxyl ion produced during the corrosion process.The pH becomes stabilized after longer immersion times in all samples except the Mg-10.0Ca alloy.The surface passivation due to the deposition of corrosion products and the formation of apatite in Mg-Ca alloy inhibits the corrosion process.The surface cracks that develop becomes deeper with an increase in Ca content due to the higher corrosion rate.Similar results was also observed in the potentiodynamic polarization tests,where the Mg-0.5Ca alloy shows the lowest corrosion current density and hence the lowest corrosion rate.Makkar et al.also performed corrosion test on Mg-xCa (x=0.5,1.6,3.8,and 5.0 wt.%) alloy by immersion test method using a different media called phosphate buffer saline (PBS) solution[64].As reported earlier a similar trend was observed in both pH and hydrogen evolution rate,where,with an increase in Ca content,both pH and hydrogen evolution rate increases.Harandi et al.showed that Mg-0.7Ca alloy with the lowest corrosion rate has the potential to be used in bio-implant application among Mg-xCa (x=0.7,1,2,3,4 wt.%) alloys[65].Blajan et al.agreed with previous results and reported that Mg-0.8Ca alloy showed higher corrosion resistance between Mg-0.8Ca and Mg-1.8Ca alloy by showing a lower hydrogen evolution rate and lower corrosion rate calculated from the electrochemical test in SBF solution [66].Mareci et al.reported that Mg-0.63Ca alloy showed more dynamic response under scanning electrochemical microscopy (SCEM)characterization with lower resistivity towards degradation in Ringer’s solution than Mg-0.89Ca alloy [67].
From the above literature,Mg-0.89Ca can be concluded that the Ca plays a dual role in the corrosion process of Mg-Ca alloy.Since,the Ca addition results in grain refinement in Mg-Ca alloy,the grain boundary provides more nucleation sites for surface passivation.The presence of Ca also helps in the early deposition of apatite on the sample surface by reacting with the P from the media and leading to surface passivation.At the same time,Ca leads to the formation of intermetallic Mg2Ca phase which accelerate the corrosion process.So the lower amount (0.6 -0.8 wt.%) of Ca addition in Mg-Ca alloy decreases the corrosion rate by surface passivation,whereas beyond this there is an increase in the corrosion rate because of the formation of more Mg2Ca phase.
4.3.Effect of media on the degradation of Mg-Ca alloy
Kirkland et al.performed the electrochemical test in MgxCa (x=0.4,0.8,1.34,5,10,16.2,and 28 wt.%) alloy by using three different media such as Hank’s solution,minimum essential medium (MEM),and MEM+10% FBS[14].The corrosion rate of Mg-Ca alloy was the same or slightly decreased up to the solid solubility limit of Ca in Mg(1.34 wt.%) and then increased with an increase in Ca content in all three mediums.The MEM+FBS medium shows the lowest corrosion rates in all samples.The higher corrosion rate in alloys with higher Ca content was attributed to the presence of more Mg2Ca phase,which acts as anode and accelerates the corrosion process.The lowest corrosion rate in MEM+FBS was attributed to the presence of serum protein,which inhibits the anodic reaction.This protein helps in the formation of dense insoluble salt layer by incorporating Ca and P on the surface which hinder the diffusion of ions and alter the dissolution process and inhibit the corrosion process[68-70].However,Walker et al.reported an increased corrosion rate of Mg-0.8Ca alloy in MEM+40 g/L bovine serum albumin (Ab) media than in only MEM [71].Bita et al.studied the influence of different biological media like SBF and Dulbecco’s Modified Eagle Medium (DMEM) on the corrosive performance of Mg-0.8Ca alloy and reported that Mg-0.8Ca alloy showed better corrosion resistance in DMEM[72].Gnedenkov et al.observed a decreased corrosion rate in MEM than in NaCl solution because of surface passivation due to deposition of both hydroxyapatite and Mg(OH)2layer whereas there was only Mg(OH)2deposition layer on the sample surface in NaCl solution [59].Liu et al.also studied the corrosion behavior of Mg-1.5Ca alloy by performing both immersion and electrochemical test using various media such as distilled water (DW),Ab,and 0.9 wt.% NaCl in different combinations [73].The addition of Ab to DW increases the amount and density of hydrogen bubbles and leads to pitting corrosion during the immersion test (compared to when only DW is used as the medium).The immersion test using 0.9 wt.% NaCl solution resulted in severe corrosion attack with the evolution of large size hydrogen bubbles at the corroding sites leading to filiform corrosion.But,in 0.9 wt.% NaCl+10 g/l Ab solution,adsorption of proteins decreases the filiform corrosion and small size hydrogen evolution occurs uniformly over the sample surface.The hydrogen evolution test showed the volume of evolved hydrogen increases in the following order of DW+10 g/l Ab<0.9 wt.% NaCl+10 g/l Ab<0.9 wt.% NaCl+1 g/l Ab<0.9 wt.% NaCl.Similarly,the corrosion current density from the potentiodynamic polarization curve was higher in 0.9 wt.% NaCl solution than DW+10 g/l Ab and 0.9 wt.%NaCl+10 g/l Ab solutions.Ab mainly consists of amino acids and undergoes neutral-acidic transition at a pH of 7.4 and becomes negatively charged.The synergistic effect of the negatively charged Ab molecule and OH-ions due to the cathodic reaction occurring during the corrosion of Mg-1.5Ca alloy further retards the corrosive effect from the aggressive Cl-ions and decrease the corrosion rate of Mg-1.5Ca alloy.Hence,Ab act as a corrosion inhibitor and its inhibiting effect become more pronounced with an increase in its concentration.Hou et al.also performed the electrochemical test on Mg-1Ca alloy using 0.9 wt.% NaCl solution as electrolyte and reported that a localised corrosion leads to a ring shape corrosion product with a hole at the center on the surface of Mg-1Ca alloy and resulted in volcanic like pitting [74].
It has been observed that the presence of Cl-ions,one of the most prevalent inorganic ions in the physiological media,cause pitting corrosion by breaking down the passive film of corrosion products on the surface and accelerates the degradation process.Similarly,the presence of SO42-ions also accelerates the corrosion by a similar mechanism as caused by Cl-ions [8,75].However,the presence of inorganic ions such as PO43-,CO32-,Ca2+retard the corrosion process by surface passivation due to the precipitation of phosphate and carbonate salts.The influence of proteins on the corrosion rate of Mg-Ca alloys depends on the nature of the sample surface and the protein.It has been reported that the protein adsorption on surfaces depends on the hydrophilic/ hydrophobic character of the surface,as well as on the surface charges of the metal and the protein [76].Proteins can interact with metal cations,which might accelerate the corrosion process,but they can also retard the corrosion by adsorbing on metal surfaces and forming a protective layer [77-79].The presence of organic compounds such as amino acids,vitamins,glucose changed the micromorphology of corrosion products on the surface of the sample by reacting with the inorganic ions in the corrosive media and helps in more surface passivation by the formation of a dense insoluble salt layer which hinder the diffusion of ions and alter the dissolution process and inhibit the corrosion process.A similar trend was also explained for other bioabsorbable alloys [80-82].All media follow the same trend for the corrosion rate of Mg-Ca alloy with different Ca concentrations,where the corrosion rate decreases with Ca addition up to the solid solubility of Ca in Mg,and after that,it increases.
4.4.Effect of deformation techniques on the degradation of Mg-Ca alloy
Koleini et al.reported that the rolling process improve the corrosion resistance of Mg-1Ca alloy [19].The increase in rolling temperature from 330 °C to 410 °C leads to more weight loss and higher corrosion rate during the immersion test in SBF solution due to precipitation of more Mg2Ca phase at grain boundaries.The corrosion rate also decreases with an increase in thickness reduction by rolling from 20% to 60%at a constant temperature.Seong et al.studied the effect of HRDSR followed by annealing at 350 °C on the extruded sample of Mg-0.4Ca and Mg-1Ca alloy [20].The potentiodynamic polarization test showed that HRDSR Mg-0.4Ca has a decreased cathodic polarization current (related to hydrogen evolution reaction) while the anodic current remained almost unchanged and resulted in decreased corrosion current density when compared to HRDSR Mg.But,in HRDSR Mg-1Ca alloy,the cathodic current slightly decreased and anodic current increased resulting in increased corrosion current density when compared to HRDSR Mg-0.4Ca alloy.So the corrosion resistance can be expressed in the increasing order as HRDSR Mg-0.4Ca<HRDSR Mg-1Ca<HRDSR Mg.The evolved hydrogen volume and pH values for all samples followed the same trend as for the potentiodynamic polarization test.Denkena et al.investigated the correlation between the sub-surface properties and corrosion progression by performing deep rolling in Mg-3Ca alloy and found that high compressive residual stress generated due to deep rolling decreased the corrosion rate by a factor of approximately 100[83].Gu et al.studied the effect of different rotating speed of Mg-3Ca ribbons produced by a single roller melt-spinning method on the corrosion performance and found that as spun Mg-3Ca alloy has lower corrosion current density compared to as-cast Mg-3Ca alloy indicating corrosion resistance increases after spinning [84].The Mg-3Ca alloy melt spun at 45 m/s wheel rotating speeds showed higher corrosion resistance in SBF solution compared to that melt spun at 15 m/s and 30 m/s.Zheng et al.reported that among extruded MgxCa (x=0.54,0.79 and 1.35 wt.%) alloys,Mg-0.79 alloy has the lowest degradation rate because of the dual nature of Ca in Mg-Ca alloy [22].The addition of Ca to Mg leads to grain refinement and improves the corrosion resistance,but it also leads to an increase in the Mg2Ca phase which form galvanic cell with Mg matrix and decreases the corrosion resistance.Zheng et al.also showed that the anodic reaction was deaccelerated after extrusion resulting in enhanced corrosion resistance compared to the as-cast alloys[55].After extrusion,the continuous distribution of the Mg2Ca phase becomes discontinuous and thus the corrosion front does not penetrate the grain boundaries of the Mg matrix resulting in improved corrosion resistance.Xie et al.observed that Mg-2Ca alloy extruded at 300 °C has higher corrosion resistance than that extruded at 350 °C.The larger grain size of the alloy after higher extrusion temperature leads to a higher corrosion rate because of more solution contacting area and less surface passivity of the oxide film [23].The higher corrosion resistance of Mg-2Ca alloy compared to the Mg-1Ca alloy when extruded at the same temperature of 300 °C is due to the lower dislocation density in the former one.
Bian et al.studied the effect of cyclic loading in circulating SBF on hot extruded Mg and Mg-1Ca alloy and reported that all the material exhibited a higher corrosion rate under cyclic loading in circulating SBF compared to static immersion in SBF [53].In all the cases,Mg-1Ca alloy has higher corrosion resistance than Mg.Harandi et al.reported that with an increase in the forging temperature from 250 °C to 450 °C,the corrosion of Mg-1Ca alloy increases because the finer grain provides more site for the attack,whereas the corrosion rate of Mg-1Ca alloy decreases with an increase in forging speed[21].Idris et al.agreed with the above result for the forged Mg-1Ca alloy and also observed that the forging temperature has a more pronounced effect than the forging speed on the degradation of Mg-1Ca alloy [85].
Severe plastic deformation techniques also result in a decrease in the corrosion rate of Mg-Ca alloy.Sahu et al.observed a decreased corrosion rate of groove pressed Mg-0.6Ca alloy compared to the annealed condition after performing both the static immersion and electrochemical test using SBF as the corrosion media [27].The decreased corrosion rate is attributed to the grain refinement,which enhanced the biomineralization tendency of the alloy,which facilitates more deposition of calcium phosphate on the grain boundary.These calcium phosphate deposits passivate the surface leading to lower corrosion rates.A similar observation was also reported by Shishir et al.for friction stir processed Mg-0.6Ca alloy[31].Li et al.reported that the increased corrosion resistance of Mg-1Ca alloy after HPT is due to a more uniform distribution of secondary Mg2Ca phase which results in the formation of a more uniform surface corrosion layer,providing efficient protection of the matrix [29].However,Li et al.showed that the SMAT does not provide better corrosion resistance because the surface after SMAT has a high density of strain induced crystalline defects which enhance the corrosion process [26].
It can be concluded that both the decreased grain size and the decreased Mg2Ca phase after the deformation process increased the corrosion resistance.The decreased grain size provides a more preferential site for surface passivation whereas the lower amount of Mg2Ca phase leads to discontinuous distribution and thereby corrosion rate decreases.However,in some cases,the decreased grain size even after deformation processes does not decrease the corrosion rate because of the presence of high dislocation density.Hence,the dislocation density,grain size,and the distribution of the Mg2Ca phase after the deformation process has a tremendous effect on the corrosion process of Mg-Ca alloy.The Mg-Ca alloy with smaller grain size,lower amount of Mg2Ca phase,and lower dislocation density will show the lowest corrosion rate.The corrosion rate of Mg-Ca alloy increases with an increase in the deformation temperature.The ideal temperature for deformation is 250 to 330 °C,deformation above this temperature leads to a higher corrosion rate because of the larger grain size,and higher amount of Mg2Ca precipitates.
4.5.Effect of surface treatment on the degradation of Mg-Ca alloy
It has been reported that surface treatment plays a pivotal role in tailoring the degradation rate of Mg-Ca alloy.According to formation mechanism of coating layer,the surface treatment methods can be divided into three categories:chemical modification,physical modification,and combination of chemical and physical modification [86].
Chemical modifciation coating:In this technique a new phase is formed on the surface of the alloys by chemical or electrochemical treatment [87].It results in a tightly adherent layer to the substrate due to chemical bonding.The major chemical conversion coating techniques for Mg-Ca alloys includes acid treatment,alkali heat treatment,MAO coating,fluoride coating,electrodeposition,ion implantation,and hydrothermal treatment.
Acid treatment is usually carried out to remove the residual oxide film or surface contamination.The films formed on the sample surface create an equipotentialized surface and helps in surface passivation and reduce the corrosion rate.Rahim et al.observed an improved corrosion resistance by acid treatment (nitric acid and phosphoric acid) of Mg-Ca alloy [88].The acid treated surface provides more nucleation sites for the nucleation and growth of calcium-phosphate layer thereby improving biomineralization and decreasing the degradation rate.
Fluoride treatment of Mg and its alloys leads to the replacement of original oxide film with a thin and more homogeneous layer of MgF2with higher polarization resistance[8].Drynda et al.also reported that the fluoride coating on Mg-Ca alloy retards the corrosion process by forming MgF2on the surface [33].The decreased electrical conductivity of the fluoride insulates the central magnesium/calcium bulk from the electrolyte and thereby decreases the corrosive attack of chloride ions.There was no hydrogen evolution occurring within the first 8 to 40 h in the fluoride coated alloy.Rad et al.performed hydrofluoric acid (HF) treatment on Mg-0.5Ca alloy with different concentrations of HF (5%,10%,20%,35%,40%,and 48% HF) and reported that HF coated sample showed approximately 35 times higher corrosion resistance than the uncoated sample in the electrochemical test using SBF as the electrolyte [34].The immersion test performed using the SBF solution also agreed with the above.The Mg-0.5Ca alloy with 40% HF treated for 24 h is a promising candidate for a biodegradable implant because of its low degradation rate and good biocompatibility.
Alkaline heat treatment on Mg and its alloys leads to the deposition of Mg(OH)2or MgO layer on the surface of the sample which improves the corrosion resistance [8].Gu et al.reported that alkaline heat treated (Na2HPO4heated,Na2CO3heated,and NaHCO3heated) Mg-Ca samples showed less pH variation and less hydrogen gas evolution than untreated Mg-Ca alloy in SBF solution [32].The corrosion rate of alkaline heat treated and untreated sample during the immersion test in SBF solution varied in the following order: NaHCO3heated<Na2HPO4heated<Na2CO3heated<untreated sample.However,the alkaline treated samples without heat treatment showed a higher corrosion rate because of the loosened alkaline layer and the presence of many defects.However,during the heat treatment,the loose surface layer was fused and consolidated and formed a compact MgO layer,which blocked the transport of species (such as H2O and Cl-) and lowered the corrosion rate in alkaline heat-treated sample.The thicker layer has higher passivation for ions.The NaHCO3heated sample has the thickest surface layer and hence showed the highest corrosion resistance.
The MAO coating is a high potential electrolytic process which generates plasma discharge that results in a dense coating on the substrate which will act as the anode [87].This technique is also called PEO process.Gu et al.also showed that MAO coating on Mg-1Ca alloy at 360 V showed higher corrosion resistance than at 300 V and 400 V in Hank’s solution [37].The coating thickness increased with increased applied voltage.So,the thinnest oxide layer on the surface of the Mg-1Ca alloy MAO coated at 300 V is the reason for its lowest corrosion resistance in Hank’s solution.However,at 400 V,even if the thickness of the oxide layer is more than at 360 V,it contains micro pores,which allow ion transport and decrease the corrosion resistance.A similar trend(360 V<400 V<300 V) in pH and hydrogen evolution in Hank’s solution was observed for Mg-1Ca alloy.Cui et al.studied the effect of MAO coating on the corrosion behavior of the extruded Mg-xCa (x=0.54,0.79 and 1.34 wt.%) alloy by electrochemical test using Hank’s solution as electrolyte and reported that Mg-0.79Ca alloy has higher corrosion resistance than the other two alloys [38].In Mg-1.34Ca alloy the higher porosity and larger micro crack in the MAO coating leads to lower corrosion resistance.Pan et al.agreed with the above results and found that MAO coated Mg-0.82Ca alloy showed better corrosion resistance among MAO coated Mg-xCa (x=0.06,0.58,0.82,and 1.56 wt.%) alloys [89].The number and distribution of the Mg2Ca phase can control the corrosion behavior of MAO-coated Mg-xCa alloy and Mg with 0.5 -0.8 wt.% Ca has better corrosion resistance.Sedelnikova et al.evaluated the corrosion rate of both uncoated andβ-TCP particles containing MAO coated Mg-0.8Ca alloy by hydrogen evolution rate and electrochemical studies using 0.9%NaCl solution[39].The volume of the evolved hydrogen after 8 days of immersion for the uncoated and MAO coated samples were 26.0 mL and 3.2 mL respectively,indicating improved corrosion resistance in MAO coated sample.The mass of released Mg into 0.9% NaCl solution agreed with the previous trend with 23.8 mg and 2.9 mg for uncoated and MAO coated samples respectively.The potentiodynamic polarization test showed that there was a reduction in the corrosion current density in MAO coated samples compared to the uncoated samples.There was a phase change fromβ-TCP toα-TCP with an increase in voltage from 350 V to 450 V for MAO coatings containingβ-TCP particles.This results in an increase in the thickness of the coated layer,which increases the corrosion resistance in Mg-0.8Ca alloy.
Mohedano et al.reported that PEO coated Mg-0.8Ca alloy with fluoride species as the electrolyte showed a shift in the corrosion potential and reduction in current densities compared to the uncoated sample indicating an improvement in the corrosion resistance[35].The hydrogen evolution rate calculated for 10 weeks for both the uncoated and PEO coated sample by immersing in SBF solution showed that the rate initially decreases and then remains steady for the uncoated sample,whereas the PEO coated sample showed an initial lower hydrogen evolution rate but which increased with time.The accumulated corrosion products at the coating/ substrate interface cause stress and weaken the PEO coating and this leads to the loss of the protective nature of the PEO layer and electrochemical activities start at the coating/substrate interface.
Electrochemical deposition is mainly used to deposit inorganic phases under the action of an electric field.Rad et al.coated Mg-3Ca alloy with nano fluorine-doped hydroxyapatite (FHA) and brushite (DCPD) using the electrochemical deposition method [46].The corrosion current density from the potentiodynamic polarization curve and the pH value from the immersion test in SBF solution were found to be in the increasing order of FHA<DCPD<uncoated Mg-3Ca alloy.So the more dense and uniform FHA coating has higher corrosion resistance.Zhang et al.studied the corrosion behavior of electrodeposited calcium phosphate coating on Mg-1Ca alloy in Hank’s solution [90].The electrochemical test showed that the coated sample has higher corrosion potential and lower current density than the uncoated sample indicating higher corrosion resistance of the coated sample.The coated sample also showed lower hydrogen evolution compared to the uncoated sample indicating that the calcium phosphate coating can inhibit the degradation process.Zhang et al.also performed calcium phosphate coating on Mg-1Ca alloy by electrodeposition method for a various time period of 20,60,120,and 240 min and studied the corrosion performance by electrochemical test using Hank’s solution as the electrolyte[91].The sample in which calcium phosphate coating was done for 240 min showed the highest electropositive corrosion potential and the lowest current density.This indicates that with an increase in the coating duration,a denser fully covered coating of lamellar crystallites of brushite (DCPD,CaHPO4·2H2O) is formed which increases the corrosion resistance of the alloy.
Ion implantation is an effective technique to form protective films with tight adhesion via ion mixing [86].Liu et al.performed both electrochemical and immersion test using Hank’s solution to evaluate the influence of surface implanted biocompatible metal ions (Ag,Fe,and Y) on the corrosion behavior of Mg-1Ca alloy [92].The electrochemical test revealed that Ag and Fe ion implantations lower the corrosion resistance whereas the corrosion resistance was enhanced by a factor of three by Y ion implantation as compared to untreated Mg-1Ca alloy.The pH value showed a contradictory result where the pH value of the Hank’s solution for the Y ion implanted sample increases more rapidly than untreated Mg-1Ca alloy after 6 h of immersion but it was always less than that of the pH value of Ag and Fe ion implantation.Therefore,Y ion implantation indicates a short period of protection.
Physical modifciation techniques:If there is no chemical bond formed between the coated layer and the substrate,then the treatment is called physical modification [86].The major physical modification techniques reported on Mg-Ca alloy includes plasma spraying,dip-coating,physical vapor deposition (PVD),and pulse laser deposition.
Plasma spraying involves the injection of powders into a direct current plasma jet,where they are melted and the stream of molten particles is accelerated onto a substrate,where they form a coating as they spread and solidify[93].Istrate et al.coated Mg-0.4Ca alloy with 95% ZrO2-5% CaO coating by plasma spraying and performed the electrochemical testing using Ringer’s solution as electrolyte [47].The thicker,compact,and dense layered coated sample (75 μm thick) showed better corrosion resistance than the thin layer coated sample (60 μm thick).Istrate et al.also coated the Mg-1Ca and Mg-0.7Ca alloy with ZrO2-CaO (95% ZrO2and 5%CaO,in wt.%) and ZrO2-Y2O3(92% ZrO2and 8% Y2O3,wt.%) by atmospheric plasma jet spraying method and reported that ZrO2-Y2O3coating has higher polarization resistance than ZrO2-CaO coating but ZrO2-CaO coated sample showed better adhesion to the substrate [48].Daroonparvar et al.performed potentiodynamic polarization test on air plasma sprayed nanostructured coating on Mg-1Ca alloy where commercial NiCoCrAlYTa alloy powders were used as inner layer,nanostructure Al2O3-13% TiO2powders as an intermediate layer,and nanostructured TiO2powder as the top layer [94].The dense top layer of TiO2provides more protection to the corrosion attack and the intermediate Al2O3-13% TiO2layer further provides protection to the corrosive medium when it passes through the top layer.
Dip coating allows the formation of functional polymeric or ceramic layers on the sample surface to prevent corrosion.Li et al.coated the Mg-1Ca alloy by TiO2using dip-coating method and found that TiO2coating enhanced the corrosion resistance [49].The immersion test in SBF solution showed that TiO2coated Mg-1Ca alloy remained almost intact after 168 h whereas uncoated Mg-1Ca alloy corroded heavily after 48 h.
The PVD process results in uniform distribution and good adhesion of the coating layer.At present magnetron sputtering is the main processing technology for PVD [95].Rad et al.coated Mg-1Ca alloy with nano-Si and nano-Si/TiO2composite coatings using the PVD method [50].The electrochemical test performed using SBF as the electrolyte showed that nano-Si/TiO2coating offers a higher corrosion resistance with a significant reduction in the corrosion rate (0.57 mm/year) compared to the nano-Si coated (0.91 mm/ year) and the uncoated alloys (6.21 mm/ year).A similar trend was observed for the pH and hydrogen evolution in all the samples.The lowest corrosion rate in nano-Si/TiO2composite coating is due to its highly dense layer and the nano TiO2particles,which acts as barrier to corrosion.Surmeneva et al.also performed the electrochemical test in SBF solution to evaluate the corrosion performance of RF magnetron sputtered hydroxyapatite coating on Mg-1Ca alloy and reported that the coated sample has higher polarization resistance and lower corrosion current density than the uncoated sample indicating improved corrosion resistance [44].
In pulse laser deposition (PLD) process,a high power pulsed laser beam is directed toward a target material with the aim of vaporizing the target material and depositing it as a thin film on a substrate facing the target [96].Rau et al.studied the effect of using pulse laser deposited glass-ceramic coating on the corrosion behavior of Mg-1.4Ca alloy by performing the electrochemical test using SBF solution as electrolyte and reported that glass-ceramic coating showed more electropositive corrosion potential and lower corrosion current density than the uncoated sample.This indicates that glassceramic coating offers protection to the alloy by improving the corrosion resistance [97].Rau et al.also deposited hydroxyapatite using pulse laser deposition coating technique at 100 °C,200 °C,300 °C,and 400 °C on Mg-Ca alloy and showed that the coating deposited at 200 °C showed better corrosion resistance in SBF solution [43].
Chemical and physical modifciation technique:Nowadays researchers have also investigated composite coatings with chemical and physical treatments.The two layers on the surface of substrates result in improved degradation resistance of the alloy.Chemical treatments are usually carried out before using physical deposition processes to improve adhesion of coating to the substrate.
Rad et al.performed a double layer coating (MAO coating with sodium aluminate based electrolyte followed by dipcoating of poly-lactic acid) on Mg-1.34Ca alloy and reported that the double coating layer has higher corrosion resistance than the uncoated Mg-1.34Ca alloy [98].The poly-lactic acid as a top layer with a thickness of 37-40 μm effectively sealed the porosities of the MAO layer and provide better corrosion resistance to the Mg-1.4Ca alloy in SBF solution.
Rosemann et al.mentioned that the sample coated with both PCO and biodegradable polymer shows better corrosion resistance than only PCO coated sample as the polymer seals the pores [41].Rosemann et al.performed hydrogen evolution test in both cast and extruded Mg-1Ca alloy and reported that the extruded sample always shows a lower amount of hydrogen evolution compared to the cast sample in SBF solution[42].The PCO+poly L-lactide-co-caprolactone(PLLC) coated samples showed lower evolved hydrogen volumes compared to the only PCO coated sample and it further decreases when PCO coating is applied at higher voltage.The PLLC coating delayed the start of substrate corrosion,while the PCO coating decreased the substrate corrosion rate.
Dilshad et al.performed electrochemical testing on combined hydrothermal treated followed by PVA/Mg-P composite coated Mg-Ca alloy and reported that the protective Mg(OH)2layer formed on the surface of the coated sample during hydrothermal treatment leads to anodic shift in the potentiodynamic polarization curve indicating higher corrosion resistance [99].
From the literature of different surface treatment techniques,it can be concluded that the corrosion rate of Mg-Ca alloy can also be altered using different coating techniques.The coated Mg-Ca alloy showed a lower corrosion rate as compared to the uncoated one because the coating surface hinders the transport of ions.The corrosion resistance increases with an increase in coating time and thickness of the coating layer.In some cases,the decreased corrosion resistance with thicker coatings as compared to the thinner coatings is due to the presence of defects such as pores and microcracks on the coating surface.The heat treatment performed after coating consolidates the coating layer and thereby further increases the corrosion resistance.The adherence of chemically modified coated layers are usually more than physically deposited layers.However,the physical deposited layers provide better control of the degradation rate and bioactivity.Therefore,a combination of both chemical and physical composite coatings will result in better control of corrosion rates.
Table 3 shows the corrosion rate of various as-cast Mg-Ca alloy and also for different processing techniques employed to alter the corrosion rate in different media.

Table 3 List of corrosion data of various Mg-Ca alloys.
5.In vitro cell culture studies of Mg-Ca alloy
The metal ions released during the degradation of the metallic implants in the human body impact the cells.The cytocompatibility of the materials can be evaluated in terms of the cytotoxicity test of biodegradable metals.Li et al.performed cytotoxicity test by culturing L929 cell line in the Dulbecco’s Modified Eagle’s Medium (DMEM),10% fetal bovine serum,100 U/ml penicillin,and 100 mg/ml streptomycin at 37 °C in a humidified atmosphere of 5% CO2and reported that Mg-1Ca alloy showed better cell viability with flattened spindle shaped cells because of its early osteoblast response than the control [17].Gu et al.performed cytotoxicity test using the L929 cell line in both as-spun and as-cast Mg-3Ca alloy and reported that the as spun Mg-3Ca alloy did not show any toxicity whereas as-cast Mg-3Ca alloy did [84].The poor corrosion resistance of as-cast Mg-3Ca alloy is the reason for its toxicity.The higher corrosion rate of as-cast alloy increases the pH of DMEM,which is undesirable for cell adhesion and proliferation,whereas the less corroded asspun alloy showed spindle shape cell attachment to the sample surface (Fig.12).Li et al.observed that cytocompatibility of Mg-1Ca alloy with MC3T3-E1 and human mesenchymal stem cells (hMSC) improved after ECAP [28] and HPT[29] process because of the ultra-fine grained microstructures produced which enhanced cell adhesion.
Gu et al.showed that alkaline treated (Na2HPO4heated,Na2CO3heated,and NaHCO3heated) and untreated sample of Mg-Ca alloy showed no toxicity to the L929 cell line and there was no difference in cell adhesion and proliferation between these [32].However,Gu et al.also observed enhanced cell adhesion,proliferation,and differentiation using MG63 cell line for MAO treated Mg-1Ca alloy than untreated one indicating that MAO treated sample provide more favorable biological environment.This is because the MAO coating is mainly composed of MgO which is biocompatible and does not introduce any toxic elements [37].Rosemann et al.performed cell colonization studies using fibroblastic MC3T3-E1 cells and reported that after 96 h no survivingcells were found on the uncoated sample because of the increase in pH value of the cell media due to its significant degradation,whereas PCO coated sample showed active cell culture due to the lower pH of cell media [41].Mohedano et al.studied the cytocompatibility via live/dead cell viability assay using human bone-derived cells (HBDC) in both uncoated and electrolytic PEO coated (with and without fluoride species) Mg-0.8Ca alloy and found that PEO coated sample with fluoride species in electrolyte showed a higher number of flat shaped cells adhesion with well improved bioactivity[35].The uncoated and PEO coated without fluoride samples showed round-shaped cells with high gas evolution indicating low cell adhesion and low proliferation.
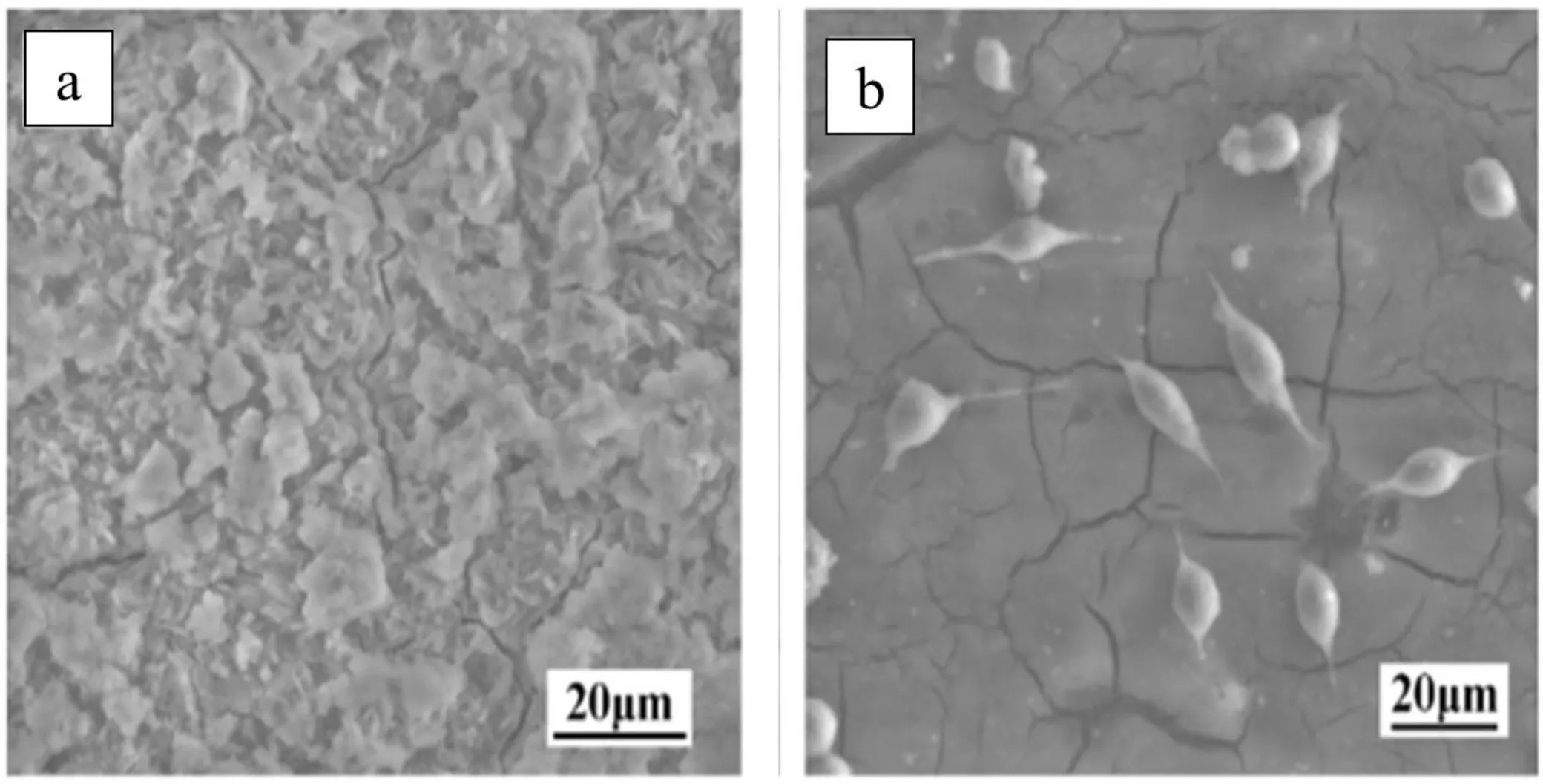
Fig.12.The scanning electron micrographs of L-929 cells cultured on the (a) as-cast and (b) as-spun Mg-3Ca alloy for 2 days.X.N.Gu,X.L.Li,W.R.Zhou,Y.Cheng,and Y.F.Zheng,Microstructure,biocorrosion and cytotoxicity evaluations of rapid solidified Mg-3Ca alloy ribbons as a biodegradable material,Biomedical Materials,vol.5,no.3,page.035,013,Jun.2010,DOI: 10.1016/j.msec.2015.05.042,© IOP Publishing.Reproduced with permission.All rights reserved [84].
Santos-Coquillat et al.also performed cytotoxicity test using three different cell lines such as endothelial (C166-GFP),premyoblastic (C2C12-GFP),and preosteoblastic (MC3T3)murine cell lines on PEO coated Mg-0.8Ca alloy where NaF or Na2SiO3along with Ca-P was used as the base electrolyte for coating [36].The premyoblastic cell line exhibited good performance in all the coatings.The coating containing silicon showed a good monolayer of the preosteoblastic cells formed on the smoother surface (Ra<1 μm).The coating containing fluorides showed a reduced monolayer formation of the preosteoblastic cells due to the presence of fluoride as well as the greater roughness (Ra=4 μm).The endothelial cell showed differential behavior over the PEOcoated materials.The coating having fluorides did not show a monolayer of endothelial cells whereas the silicon-containing coating showed an organized monolayer of these cells.Coquillat et al.performed cytotoxicity test using a premyoblastic(C2C12-GFP) cell line on Mg-0.8Ca alloy and Cp Ti sample coated with porous three dimensional polymeric PCL layer over a PEO ceramic layer [100].The higher cell adhesion and proliferation was observed on coated Mg-0.8Ca alloy with cell colonization to the inner pores and cells anchored to the underlying ceramic coating compared to the Cp Ti sample.Liu et al.implanted biocompatible metal ions (Ag,Fe,and Y) on the surface of Mg-1Ca alloy by metal vapor vacuum arc technique (MEVVA) and performed cytotoxicity test using human osteosarcoma cells (MG63) [92].The Y-implanted Mg-1Ca alloy showed good biocompatibility compared to the Ag-and Y -implanted alloy because of its lower change in pH value during the degradation process.Yu et al.reported that nano-hydroxyapatite collagen (NHAC)/Mg-Ca scaffolds shows enhanced cell adhesion and proliferation than NHAC for MC3T3-E1 cell line [101].
From the above literature,it can be concluded that Mg-Ca alloy with lower Ca content (<1 wt.%) is nontoxic to different cell lines because of its lower corrosion rate.However,the Mg-Ca alloy with higher Ca content (>1 wt.%) shows some toxicity due to its rapid degradation rate because of the presence of more Mg2Ca phase,which increases the pH of the cell culture medium and make the medium undesirable for cell growth.The Mg-Ca alloy processed by different techniques such as deformation and surface treatment showed better cell adhesion and proliferation compared to the as-cast condition.
6.In vivo studies of Mg-Ca alloy
In vivo testing is the most reliable method to estimate the biological performance of a biomaterial.Li et al.implanted Mg-1Ca and CP-Ti pins into the femoral shaft of adult male New Zealand rabbits and reported that Mg-1Ca alloy showed active bone formation with a large number of osteoblasts and osteocytes and some lymphocytes at the implant site.However,no new bone formation was seen in CP-Ti pins (Fig.13)[17].The bone formation near the Mg-1Ca pin is due to the release of Mg ions during the degradation process.It has been reported that Mg ions promote osteogenesis by enhancing the adhesion of osteoblast cells [102].Fig.14 shows that white color corrosion products covered the Mg-Ca alloy pin.The volume of the pin decreases with implantation time and after three months it is completely absorbed leaving a small hole at the implant position.
Thomann et al.implanted extruded Mg-0.8Ca and LAE442 pins into the tibia of New Zealand white adult female rabbits of 3.5 kg.for 12 months and reported that although the Mg-0.8Ca alloy showed more volume loss,the bone-implant contact was more stronger because of the presence of Ca which enhanced the formation of precipitates of Ca and P at the implant surface [103].Thomann et al.also implanted magnesium-fluoride (MgF2) coated Mg-0.8Ca pins into the marrow cavity of both tibiae of New Zealand white adult female rabbits [104].The MgF2coated implants showed enhanced bone formation with a controlled degradation compared to the uncoated implants after 6 months of implantation.The fluorine peaks were observed at the outer region of the degradation layer,but no fluorine peaks were detected in the implant,the degradation layer,or in the marrow cavity.
Razvan et al.implanted Mg-0.8Ca alloy pins into bone,proximal femur and intramedullary tibia area,and thigh muscle of giant German rabbits [105].There were some gas bubbles around the muscle implant at 4 weeks of postimplantation and partially degraded Mg-0.8Ca implants along with some bubbles around the soft tissues at 6 weeks of postimplantation(Fig.15).The histopathology results also did not show toxicity to cell around gas bubbles.In the proximity of gas bubbles,viable muscle fibers with minimal muscle tissue fibrosis were observed.Similarly,in the proximity of the implant,viable bone cells with minimal fibrosis and reactive bone tissue with newly formed osteoblasts was also observed.
Jung et al.explained the mechanism of in vivo degradation of Mg-Ca alloy by implanting Mg-10Ca cylindrical specimens into the rat femoral condyle [106].The interdiffusion of Ca and O led to rapid corrosion of the lamellar network of the Mg2Ca phase and resulted in nanocrystalline MgO.In addition to this,the surface corrosion of Mg by O also leads to the formation of nanocrystalline MgO.It has been also observed that there was an interfacial reaction layer between the implant and the bone tissue.The rapid interdiffusion of Ca into the interface leads to the formation of CaO phase,which acts as a selective leaching path for Ca,where Ca reacts with P from body fluid and forms calcium phosphate.Hence,the in vivo corrosion mechanism of Mg-Ca alloys is governed by the interdiffusion of Ca and O and by the surface diffusion of the primary Mg phase.Makkar et al.implanted Mg-xCa (x=0.5,and 5.0 wt.%) alloy plate into the femur of New Zealand white rabbits [64].After 4 weeks of post-implantation,good biocompatibility,controlled degradation,and enhanced bone formation has been observed for Mg-0.5Ca alloy compared to the Mg-5.0Ca alloy (Fig.16).As reported in the degradation mechanism of Mg-Ca alloy,a partial protective layer of Mg(OH)2was observed in the Mg-0.5Ca alloy after 2 weeks and 4 weeks of implantation.However,Mg-5.0Ca alloy almost completely degraded in all the observation periods because of the presence of larger amounts of Mg2Ca phase which accelerate the degradation process.
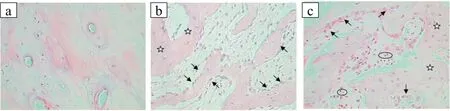
Fig.13.Histological photographs of hematoxylin and eosin stained sections of the tissues at 40x magnification (↑-osteoblast,∗-osteocyte,° -lymphocyte)(a) CP-Ti pins after 1 month of operation,(b) Mg-1Ca alloy pin after 1 month of operation,(c) Mg-1Ca alloy pin after 2 months of operation.Reprinted from Biomaterials,vol 29,Z.Li,X.Gu,S.Lou,and Y.Zheng,The development of binary Mg-Ca alloys for use as biodegradable materials within bone,page no.1329 -1344,Copyright 2008,with permission from Elsevier [17].

Fig.14.The macroscopic observation of the femoral shaft with Mg-1Ca alloy pins (a) 1 month,(b) 2 months,(c) 3 months post-implantation (↑-irregular shape hole in the femoral shaft).Reprinted from Biomaterials,vol 29,Z.Li,X.Gu,S.Lou,and Y.Zheng,The development of binary Mg-Ca alloys for use as biodegradable materials within bone,page no.1329 -1344,Copyright 2008,with permission from Elsevier [17].
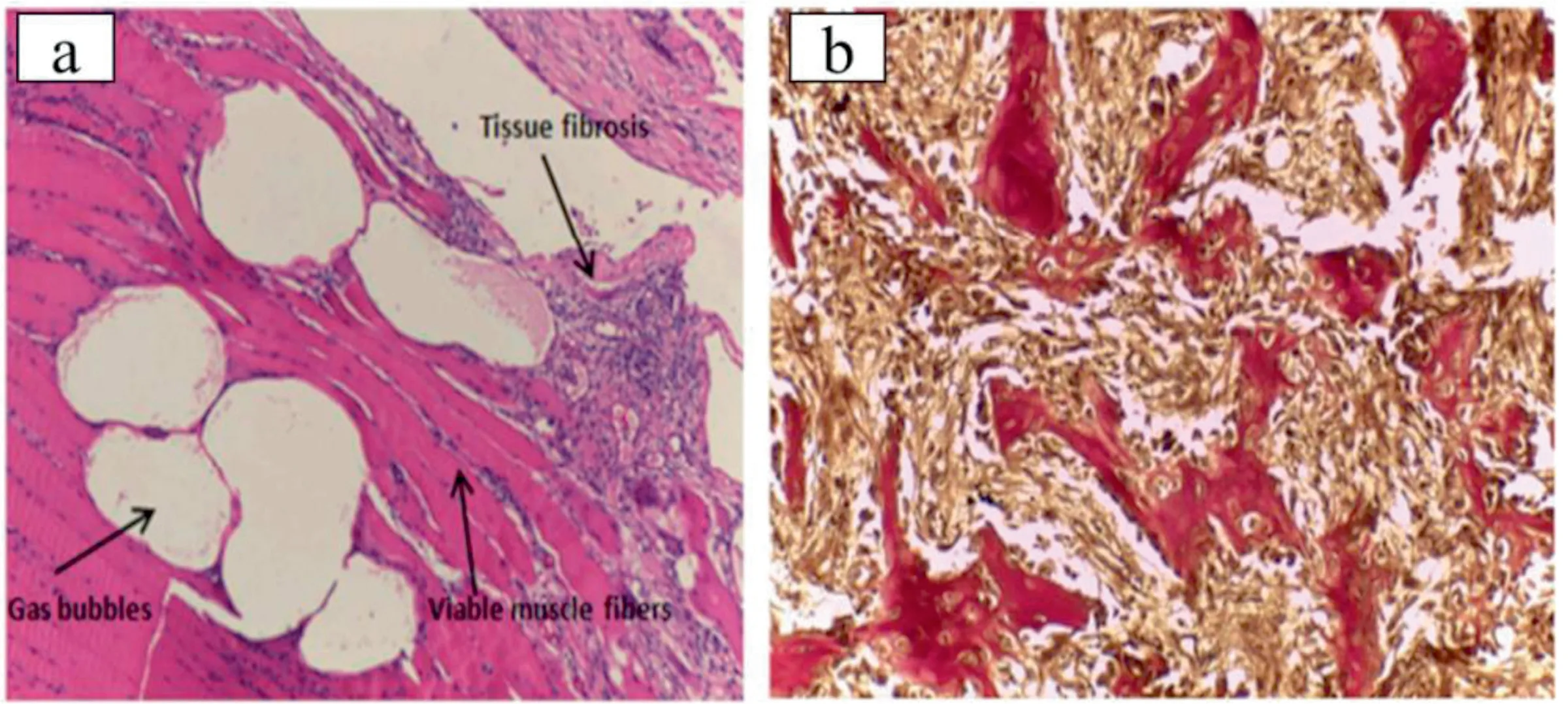
Fig.15.The histological results-optical microscopy images: a) viable muscle fibers in the proximity of the gas bubbles;b) viable bone cells and reactive bone tissue with newly formed osteoblasts.Republished with kind permission of Trans Tech Publications,from https://doi.org/10.4028/www.scientific.net/KEM.745.50,Key Engineering Materials,vol.745,2017,page no.50 -61,Results of in vivo biological tests performed on a Mg-0,8Ca alloy,A.Razvan,O.Horia,P.Elisa,V.Dan,and B.Adrian,Fig.6,© 2017 Trans Tech Publications,Switzerland [105].
Li et al.implanted ECAP Mg and ECAP Mg-1Ca pin into the femoral shaft of female Sprague-Dawley (SD) rat and found that after 24 weeks of implantation,50% implant volume remained for ECAP Mg-1Ca alloy whereas it was only 25%for ECAP Mg[28].There was no severe localized corrosion in the material and the pins degraded gradually during the whole implantation period.The interface between the implant and the newly formed bone was analysed and observed that after 24 weeks of post-implantation,a thick two layer degradation product formed between the implant and the bone.The layer adjacent to the implant consists of Mg and O whereas the layer adjacent to bone mainly consists of Ca and P.Similar to ECAPed samples.Li et al.also showed enhanced bone formation in HPT processed Mg-1Ca alloy [29].
From the above literature,Mg-0.89Ca can be observed that the biodegradable Mg-Ca alloy showed better bone formation than non-degradable CP-Ti.The released Mg ion during the degradation of Mg-Ca alloy helps in the formation of new bone near the implant site.The presence of Ca in the Mg-Ca alloy makes the bone-implant contact stronger by the deposition of calcium phosphate,which result from the interaction of Ca ions from the Mg-Ca alloy and P ions from the body fluid.The Mg-Ca alloy with lower Ca content showed controlled degradation with better bone formation.However,Mg-Ca alloy with higher Ca content degraded completely because of the presence of larger amount of Mg2Ca phase which accelerate the degradation process.The Mg-Ca alloys subjected to different deformation and coating techniques further showed better biocompatibility compared to the as-cast Mg-Ca alloy.
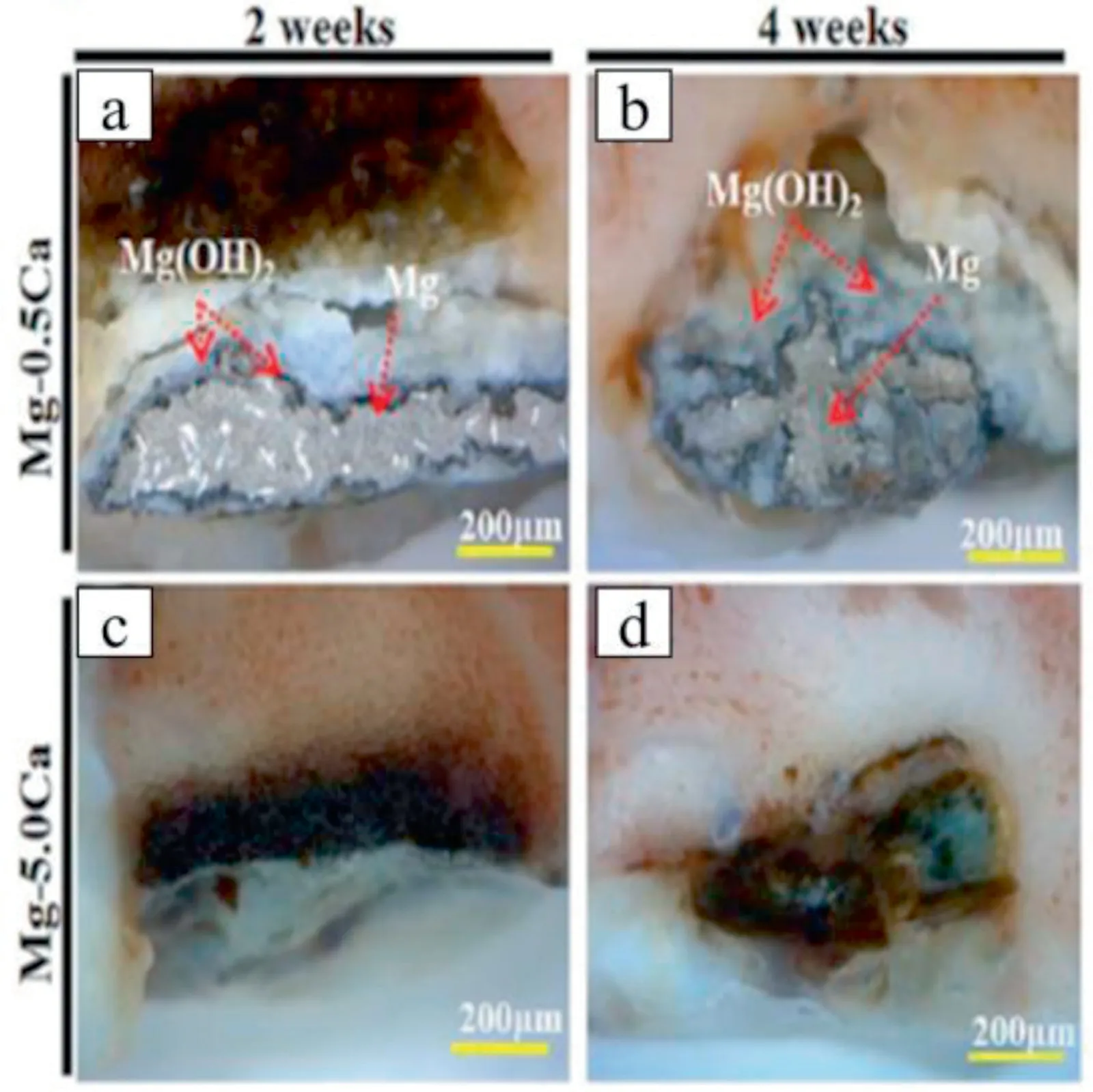
Fig.16.The optical micrographs of Mg-Ca alloy 0.5 wt% Ca (a,b) 5.0 wt%Ca(c,d)after 2 and 4 weeks of implantation.Reprinted with permission from SAGE Publications.P.Makkar,S.K.Sarkar,A.R.Padalhin,B.-G.Moon,Y.S.Lee,and B.T.Lee,In vitro and in vivo assessment of biomedical Mg-Ca alloys for bone implant applications,J.Appl.Biomater.Funct.Mater.,vol.16,no.3,pp.126-136,Copyright 2018,SAGE Publications [64].
7.Future areas and challenges
This section discusses the challenges faced by the researchers and the areas which require more attention near future.The combination of thermo-mechanical deformation and surface modification techniques may draw attention in the future,where the mechanical properties are mainly improved by deformation techniques while surface modification will be used to improve corrosion resistance.The optimization of parameters for the heat treatment after the coating process also needs more attention because the final heat treatment results in more compact and consolidated layer which hinders the corrosion reaction by blocking the transport of ions.The physiological environment contains organic and inorganic ions,and proteins.So the analysis of both mechanical and corrosion properties of Mg-Ca alloy in complex human physiological environment still requires further studies.The combination of both chemical and physical composite coating layer also needs further research where the outer layer delays the start of the corrosion process and the inner layer decreases the substrate corrosion rate.A detailed study of the effect of various combination of ceramic-polymer coating on the corrosion process is also required.Although a few in vivo studies have been carried out on Mg-Ca alloys,more extensive in vivo studies are required before clinical trials can be carried out.
Highly corrosion resistant Mg alloys with incredibly low corrosion rates of less than 0.1 mm/y have been recently created by alloying with tiny amounts of calcium (0.05,0.1 and 0.15 wt.%),paving a new way to ‘‘stainless Mg’’[107].The outstanding corrosion resistance of the Mg-Ca lean alloys is attributed to the inhibition of cathodic water reduction kinetics and the formation of a protective surface film within the first few minutes of encountering the corrosive electrolyte.The alloy seems to reduce significantly the formation of hydrogen bubbles,which are common in existing magnesium implant materials,and the formed corrosion products are not expected to be toxic and hence has immense potential for further exploration towards biomedical applications,such as scaffolds and implants.This also needs to be investigated further.
8.Conclusions
An ideal biodegradable material should have adequate mechanical properties,degradation rate that matches the tissue healing process,and good biocompatibility.Although Mg-Ca alloy possess good mechanical properties and biocompatibility,the high degradation rate compared to the growth of the healing tissue limits its application.The degradation rate decreases with the addition of calcium in the range of 0.6 to 0.8 wt.% in Mg-Ca alloy by surface passivation and further addition increases the degradation rate because of the formation of more continuous Mg2Ca phase.Similarly,the mechanical properties improved significantly up to 2 wt.% Ca in Mg-Ca alloy and after that no significant improvement was observed due to higher number of coarse Mg2Ca precipitates.Hence the optimum composition required for implant applications is Mg-Ca alloy with 0.6 -0.8 wt.% Ca.
Different thermo-mechanical processing techniques can be used to enhance the mechanical properties and improve the corrosion resistance.The thermo-mechanical processing parameters have to be optimized to achieve desired properties.Various surface coating techniques can also be used to improve the mechanical properties after exposure to degrading medium and the corrosion resistance in Mg-Ca alloys.The lower grain size and reduction in Mg2Ca phase after suitable deformation processes improved the mechanical properties and increased corrosion resistance.This is because the decreased grain size provides more preferential sites for surface passivation,whereas the lower amount of Mg2Ca phase leads to a discontinuous distribution and thereby corrosion rate decreases.However,in some cases,the lower grain size after deformation process does not decreases the corrosion rate because of the presence of high dislocation densities.The corrosion rate of the Mg-Ca alloy decreases with increase in coating thickness which hinder the transport of ions.However,the presence of defects such as pores,and micro-cracks on the coating surface increases the corrosion rate.Mg-Ca alloys processed by suitable techniques such as deformation and proper surface treatment showed better cell adhesion and proliferation,and excellent biocompatibility compared to the as-cast alloy.The Mg ions produced as a result of in vivo degradation help in the formation of new bone near the implant site.The presence of Ca in the Mg-Ca alloy makes the bone-implant contact region stronger by the deposition of calcium phosphate.
To conclude,biodegradable Mg-Ca alloys are promising potential candidates for temporary implant application.With further development,they are expected to partially replace traditional metallic implants and be used as next-generation implants.
Declarations of interest
None.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Improving the Young’s modulus of Mg via alloying and compositing -A short review
- Surface modifciation of magnesium alloys using thermal and solid-state cold spray processes: Challenges and latest progresses
- Preparation,structure and properties of Mg/Al laminated metal composites fabricated by roll-bonding,a review
- Analysis of element loss,densification,and defects in laser-based powder-bed fusion of magnesium alloy WE43
- Pore structure of porous Mg-1Mn-xZn alloy fabricated by metal-gas eutectic unidirectional solidification✩
- Role of bimodal-grained structure with random texture on mechanical and corrosion properties of a Mg-Zn-Nd alloy
