Design of Copper Oxide Nanosheets-Loaded Zeolite with Efficient Inhibition of Marine Bacteria
2022-10-24HOUJinandYEYongcheng
HOU Jin, and YE Yongcheng
Design of Copper Oxide Nanosheets-Loaded Zeolite with Efficient Inhibition of Marine Bacteria
HOU Jin1), 2),*, and YE Yongcheng1)
1)Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, College of Chemistry and Chemical Engineering, Ocean University of China,Qingdao 266100, China 2) Shandong Key Laboratory of Corrosion Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266100, China
Copper oxide-loaded zeolite 4A nanocomposites (CuO-zeolite NCs) were successfully prepared by modifying zeolite 4A with CuSO4aqueous solution as the copper source. Zeolite 4A showed an important role in hosting copper oxide, and CuO-zeolite NCs exhibited a controlled release of copper ions. XRD patterns confirmed the appearance of new diffraction peaks of copper oxide at 35.83°and 38.83°.CuO nanosheets formed on the surface of zeolite 4A were about 30–40nm thick over a width of 200–300nm. CuO-zeolite NCs were primarily composed ofCu, Si, Al and O, as measured by XPS. Cu 2p3/2and Cu 2p1/2peaks were fitted into two main peaks centered at 934.7eV and 954.6eV, which were attributed to Cu (II). Zeolite 4A and CuO-zeolite NCdisplayed reversibleLangmuir isotherm (type-I) characteristics and showed maximum adsorption quantities of almost 200cm3g−1at a relative pressure of 1.0 by CO2adsorption-desorption experiments. The antibacterial efficiencies of the CuO-zeolite NC were 99.4%, 96.7%, and 96.8% against(),(),(),respectively. These values showed that nano CuO-loaded zeolite 4A acted as a strong bactericide against marine bacteria. CuO-zeolite NCs can be further applied to marine antifouling coating.
antibacterial activities;marine bacteria; copper oxide nanosheets; zeolite
1 Introduction
Nanocomposites (NCs) are prospective materials, which have drawn a considerable amount of interest due to their ability to combine distinct properties that are not found in conventional composites (Ahmad, 2020). The distinctive properties of NCs vary from the bulk, which have led lots of researchers to research NCs in various fields (Sultana, 2013). Nanomaterials have attracted the interests of many scientists because of their excellent chemical stability, biocompatibility, antibacterial, antifungal, and antioxidant.
Copper oxide nanomaterials (CuO NMs) have been thoroughly researched in many fields based on their superior physical and chemical performances, such as catalysis (Rutkowska, 2020; Sorekine, 2021), gas sensors (Nayan, 2016; Thangamani and Pasha, 2021), bio-sensors (Show, 2020), supercapacitor (Majumdar and Ghosh, 2020; Yadav, 2021), solar cells (Vajda, 2020; El-Shafai, 2021), environmental remediation (Akintelu, 2020), and textile industries (Rezaie, 2018). In a report, the United States Environmental Protection Agency (US EPA) was reported to have approved copper and its alloys as a solid surface material to continuously kill 99.9% bacteria with- in 2h on high-touch surfaces (Ananth, 2015). The bactericidal efficiency of copper depends on the concentration, where a low concentration of copper does not provide significant inhibition and a high concentration can lead to toxicity. Therefore, in order to successfully apply copper-based bactericides, an active copper source is necessary and presents controlled copper ion release to avoid toxicity. Copper oxide can be merged with many polymers and other substrates, so much research has been performed in this area (Panda, 2020; Perla, 2020; Lv, 2021; Murugappan and Sreeram, 2021; Wang., 2021).
Among the various types of substrates, zeolites are regarded as hopeful and important materials due to their high ion exchange capacity, thermal stability, high surface area, and eco-friendly nature (Meng, 2015; Noroozi, 2018; Araki, 2019).The fabrication of copper oxide-loaded zeolites for various uses has been resear- ched. Mekatel and Nibou studied the preparation of CuO- supported zeolite nanocomposites. The nanocomposite was applied as a catalystfor the photodegradation of me- thyl orange (Mekatel, 2015). The synergistic effects of copper oxide-clinoptilolite nanoparticle composites as photocatalysts were studied with respect to the degradation of 2,6-dimethylphenol aqueous solution (Soori and Ejhieh, 2018). Cheng (2021) studied the preparation of CuO-modified CsX zeolites (Cesium-exchanged X zeolites) as catalysts. In this work, CuO was recognized as an efficient promoter to improve the performance of CsX catalyst with respect to reactivity and styrene selectivity. Dong (2015) reported the fabrication of CuO nanoparticles incorporated in hierarchical MFI zeolites, which was used as a highly active electrocatalyst for non-enzymatic glucose sensing. CuO-located MFI zeolites displayed good performance as an electrocatalyst for the oxidation of glucose in alkaline media. Alswat (2017) studied the preparation of copper oxide nanoparticle-loaded zeolite. Antibacterial activities were evaluated against Gram-ne- gative and Gram-positive bacteriainhibition zone testing. As far as we know, there are various preparation methods of copper oxide nanoparticle-loaded zeolite. Moreover, CuO NMs are commonly known for being an- tibacterial agents, which are effective at curbing bacterial growth (Menazea and Ahmed, 2020; Karuppannan, 2021). But their antibacterial properties against marine bacteria have not been extensively discussed.
One unique characteristic of zeolite is that the framework has negative charges, which needs cations to balance. Zeolite 4A is one of the most frequently used due to its high cation exchange capacity. The cations in zeolite 4A can be exchanged by different cations such as copper ions. Therefore, zeolite can have antibacterial activitycation exchange with copper ions. Antibacterial zeolite has generated more interest compared to conventional an- tibacterial agent. The incorporation of antimicrobial metallic ion within the zeolite framework allow their controlled release and prevent concentration-dependent toxicity (Ninan, 2014).
In this work, a simple method was used to synthesize CuO nanosheets in the pores and on the surface of zeolite 4A to obtain CuO-zeolite NCs. The samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), and CO2adsorption-desorption experiments. The antibacterial properties of CuO-zeolite NC were separately assessed against(),(), and().andare Gram-negative bacteria, andis a Gram-positive bacterium, which are common marine bacteria.
2 Materials and Methods
2.1 Materials
Chemical reagents included sodium aluminate (NaAlO2, CP, Aladdin), sodium metasilicate (Na2SiO3·5H2O, 95%, Aladdin), sodium hydroxide (NaOH, AR, Shanghai Sinopharm Chemical Reagent Co., Ltd.), copper sulfate (CuSO4·5H2O, AR, Shanghai Sinopharm Chemical Reagent Co. Ltd.),2216E liquid medium and 2216E agar (BR, Qingdao Hope Bio-Technology Co., Ltd.), natural seawater (Qingdao offshore), marine bacteria (,and) were supplied by the College of Marine Life, Ocean University of China.
2.2 Methods
2.2.1 Preparation of zeolite 4A
Zeolite 4A was made from a synthetic solution by dissolving 10.33g of NaAlO2and 19.35g of Na2SiO3in 50 mL sodium hydroxide solution, respectively. The mixed solution was gained by rapidly dropping the Na2SiO3solution into the NaAlO2solution. The mole ratio of the re- sulting synthetic solution was as follows: 2.63Na2O: Al2O3:1.45SiO2:95.0H2O. This solution was transferred to a sealed polypropylene bottle after even stirring and then treated at 100℃ for 4h. After hydrothermal treatment, zeolite 4A (signed S0) was collected through centrifugation, washed with deionized water, and dried at 80℃ overnight.
2.2.2 Preparation of CuO-zeolite NCs
First, 1.0g of zeolite 4A was added to copper ion solutions of varying concentrations (0.005, 0.010, 0.014 and 0.018molL−1) respectively, and the final prepared CuO- zeolite NCs were accordingly labeled as S1, S2, S3, and S4. Copper ion exchanged zeolites were acquired after the ion exchange reaction was conducted at 80℃for 2h. The resulting suspension of copper ion-loaded zeolite was washed with deionized water. For the bonded Cu2+to oxidize, 2molL−1solution of sodium hydroxide was add- ed to the suspension until the pH reached 12, and then the suspension was stirred at 80℃ for 2h. The color of the mixture changed from blue to brown to black, and it could be preliminarily determined that copper oxide was formed. After that, CuO-zeolite NCs were acquired through filtration, thoroughly washed with deionized water, and dried at 80℃ overnight.
2.2.3 Characterization
Structural evaluation was done by X-ray diffraction (XRD, D8-Advance, German) using λ=1.5418Å Cu Kαradiation in the wide-angle range of 2(5˚–70˚) at a scan speed of 6˚min−1. The surface morphologies of zeolite 4A and CuO-zeolite NCs were observed by scanning electron microscopy (SEM, S-4800, Japan). Elemental analysis was performed by X-ray photoelectron spectroscopy (XPS, ESCALAB 250XI, USA). The pore structures of zeolite 4A and CuO-zeolite NCs were characterized by CO2adsorption-desorption isotherms using a static volumetric sorption analyzer (ASAP2020, USA) at −78.5℃.
2.2.4 Antibacterial activities test
2216E liquid medium (37.4g) was mixed with 1000mL of deionized water for marine bacteria cultivation. The liquid culture medium was obtained after sterilization of the solution at 121℃ for 15min and cooled to room temperature.
2216E agar (52.4g) was added to 1000mL of deionized water for marine bacteria cultivation. The solution was sterilized at 121℃ for 15min and cooled to about 45℃. About 30mL of the above disinfected solution was added to a sterile petri dish and solidified through cooling. In this way, solid culture media were obtained.
The bacteria strain activated at 37℃ for 24h, and 15mL of liquid culture medium were mixed in a sterile test tube, and the mouth of the tube was sealed. The bacteria culture solution was obtained after the mixture was incubated in a shaking incubator at 37℃ for 24h.
Liquid culture medium (15mL) and bacteria culture solution (200μL) were both added to the same sterile test tube, and then the tube mouth was sealed. The mixture was incubated in a shaking incubator at 37℃ for 24h and marked as a blank group.
Sterile CuO-loaded zeolite (20mg), liquid culture mediumm (15mL) and bacteria culture solution (200μL) were all mixed in a sterile test tube, and then the tube mouth was sealed. The mixture was incubated in a shaking incubator at 37℃ for 24h and labeled as an experimental group.
The solutions of the blank group and experimental group were diluted to their respective concentrations with sterile natural seawater and inoculated into varying solid culture media, respectively. The solutions were evenly coated on solid culture medium, respectively, and then cultured in an incubator at 37℃. The antibacterial efficiencywas calculated using the following equation:
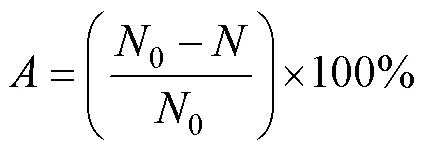
where0andrepresent the numbers of live bacteria corresponding to the blank group and experimental group, respectively.
3 Results and Discussion
3.1 Characterization
The effects of varying copper ion concentrations(0.0050, 0.010, 0.014 and 0.018molL−1) on CuO-zeolite NCs were discussed. The XRD patterns of zeolite 4Aand CuO-zeolite NCs are shown in Fig.1. The diffraction peaks, as shown in Fig.1 (S0), align with the characteris- tic peaks of zeolite 4A. The patterns of zeolite 4A (S0) and CuO-zeolite NCs (S1, S2) are similar as shown in Fig.1. This shows that CuO nanoparticles are so small that some of them can grow in the pores or on the surface of zeolite 4A. As the copper ion concentration increased, new diffraction peaks appeared at 35.83°and 38.83°, as shown in Fig.1 (S3, S4), which aligns with the characteristic peaks of CuO. It was reported that the diffraction peaks between 35°and 39°confirmed the formation of oxides and belonged to copper oxide (Brazlauskas and Kitrys, 2008). The diffraction peaks of CuO gradually strengthened with the increase of copper ion concentration.
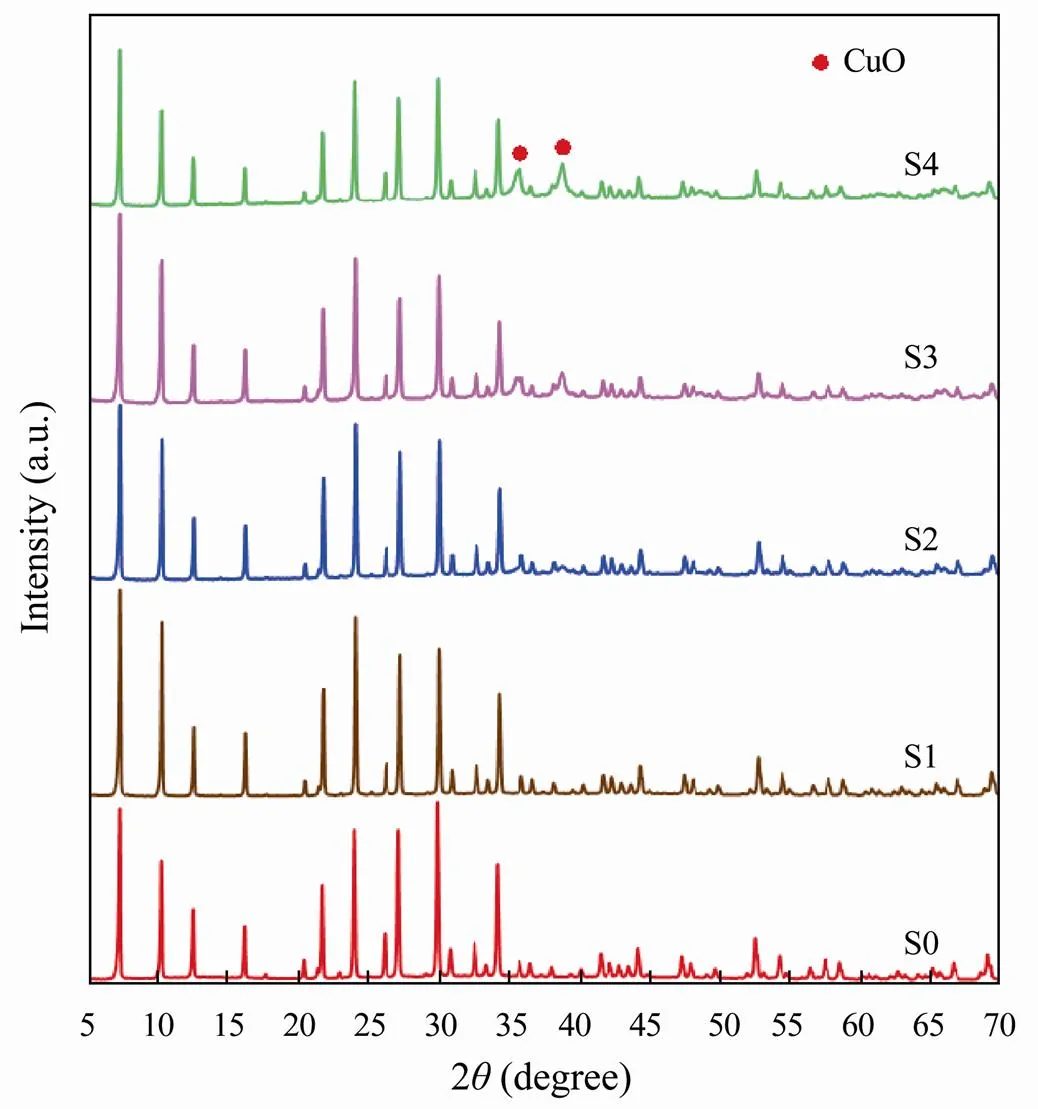
Fig.1 XRD patterns of samples: (S0) zeolite 4A; (S1–S4) CuO-zeolite NCs.

Fig.2 SEM images of prepared zeolite: (S0) zeolite 4A; (S2 –S4) CuO-zeolite NCs.
Fig.2 shows SEM images of the prepared zeolite 4Aand CuO-zeolite NCs. The images in Fig.2 (S0) show the prepared zeolite 4A has a cubic structure. The surfaces of CuO-zeolite NCs, as shown in Fig.2 (S2–S4), are different from zeolite 4A. As shown in Fig.2 (S2), a small amount of granular CuO nanoparticles appeared on the surface of zeolite when the concentration of copper ions was 0.010 molL−1. As seen fromFig.2 (S3), CuO nanosheets were formed when the concentration of copper ions was 0.014molL−1. InFig.2 (S4), CuO nanosheets with a clearer shape grew bigger when the concentration was 0.018molL−1. The thickness of the CuO nanosheets was about 30–40nm over a width of 200–300nm. This indicates the formation of CuO nanosheets. The SEM images in Fig.2 (S2–S4) agree with the patterns of XRD in Fig.1 (S2–S4). The outcome corroborates the correctness of the XRD analysis.
The elemental composition of the prepared zeolite 4A and CuO-zeolite NCs wereassessed by XPS, and the results are shown in Fig.3(a, b). The survey spectrum inFig.3(a) indicates thatzeolite 4A (S0) is mainly composed of Na, Si, Al, and O elements. The energy spectra of copper in CuO-zeolite NCs (S2 and S4) appeared and became clear gradually as the copper ion concentration increased. Meanwhile the energy spectrum of sodium gradually decreased with the increase of copper ion concentration. This indicates that sodium ions were partially replaced by copper ions. The high-resolution XPS spectrum of Cu 2p is shown in Fig.3(b). Cu 2p3/2and Cu 2p1/2peaks were fitted into two main peaks–the peaks centered at 934.7eV and 954.6eV were attributed to Cu (II) (Ninan, 2014; Alswat, 2017). The presence of only Cu (II) was verified by XPS results.
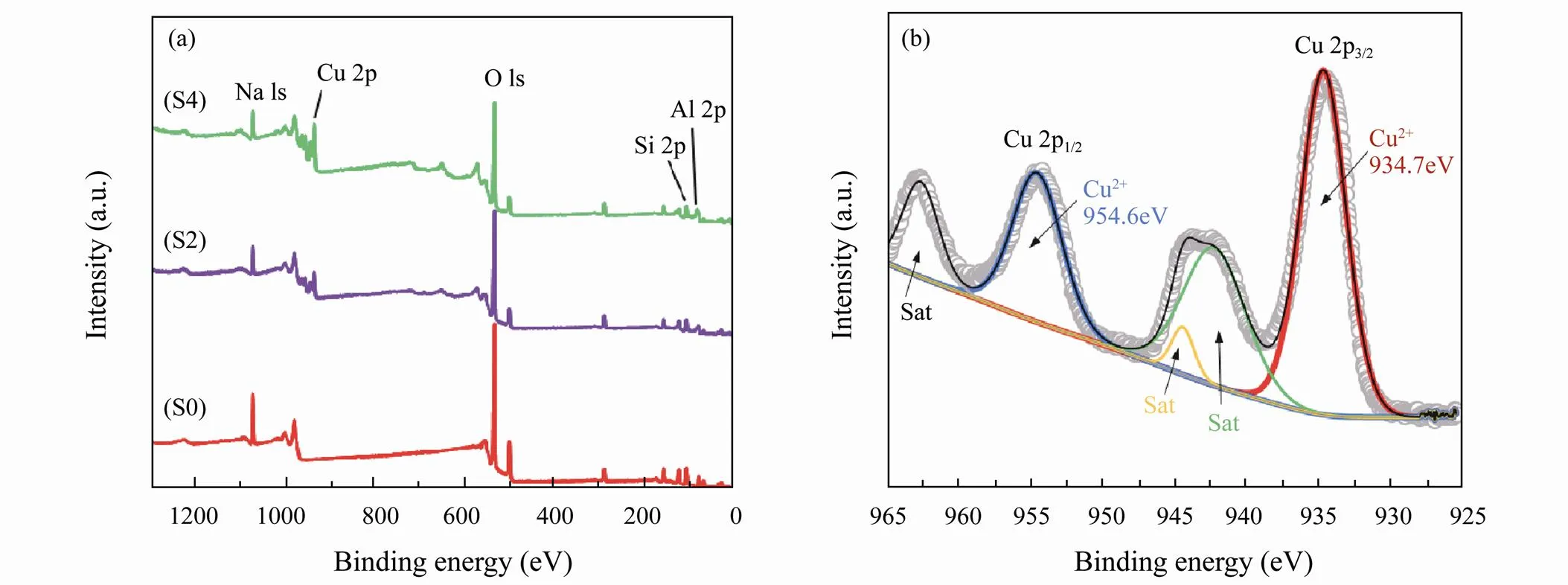
Fig.3 XPS spectra of S0, S2 and S4 samples: (a), survey; (b), Cu 2p.
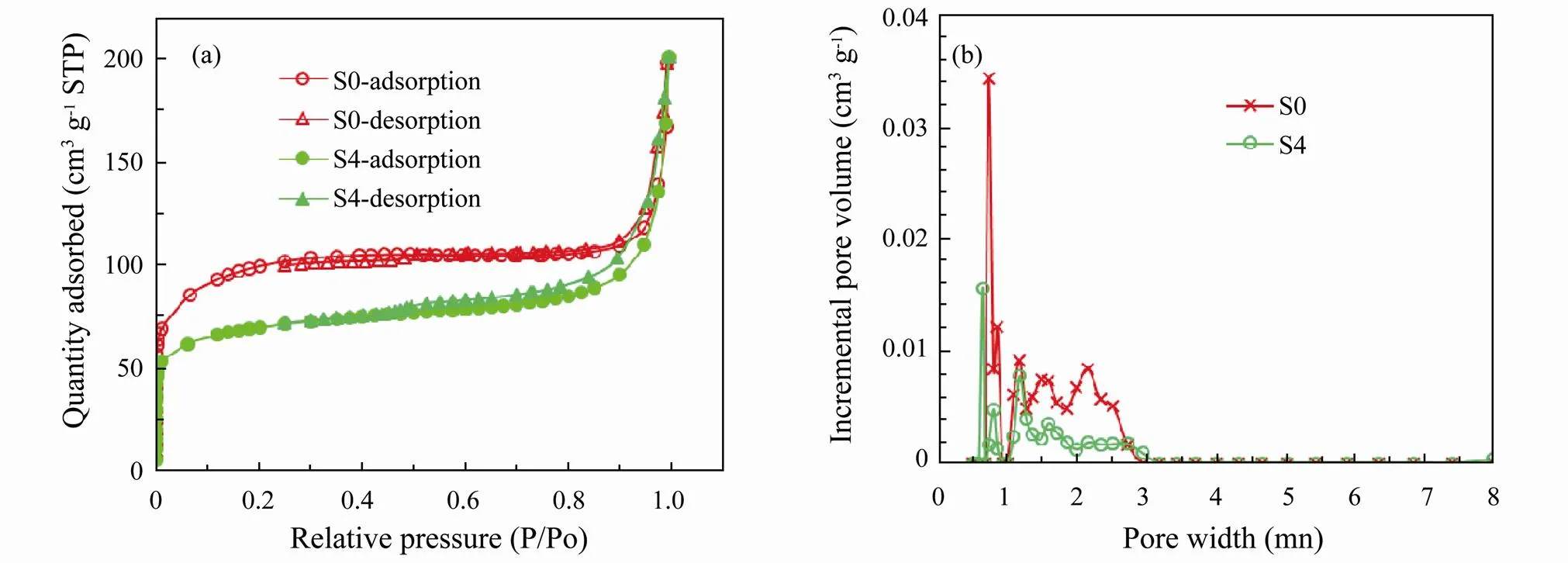
Fig.4 The CO2 quantity adsorbed (a) and pore size distribution curve (b) of samples.
Theadsorbed CO2quantity and pore size distributionsof zeolite 4A (S0) and CuO-zeolite NC (S4) were measured by conducting CO2adsorption-desorption isotherm experiments at −78.5℃ as shown in Figs.4(a, b). Before the gas adsorption measurements, samples were degassed at 200℃ for 600min under vacuum. This outcome in Fig. 4(a) indicated that the CO2adsorption isotherms were similar. Both materials displayed features of areversibleLangmuir isotherm (type-I), and the type of adsorption isotherm remained constant when sodium ions were replaced by CuO. The adsorbed quantities were close to 200cm3g−1when the relative pressure reached 1.0. The incremental pore volume of sample S4 is smaller than that of sample S0 in the main pore size range as shown in Fig. 4(b). The BET surface areas of zeolite 4A and CuO-zeolite NC were calculated as 310m2g−1and 216m2g−1, respectively. The BET surface area and pore volume of CuO-zeolite NC decreased because sodium ions were re- placed by copper oxide, which is larger in size.
3.2 Antibacterial Activities of CuO-Zeolite NC
The antibacterial efficiencies of CuO-zeolite NC (S4) were tested using,,andas shown in Figs.5–7 and Table 1After several hours of incubation, the number of bacteria in the experimental groups were notably reduced compared to those in the blank groups as shown in Table 1.
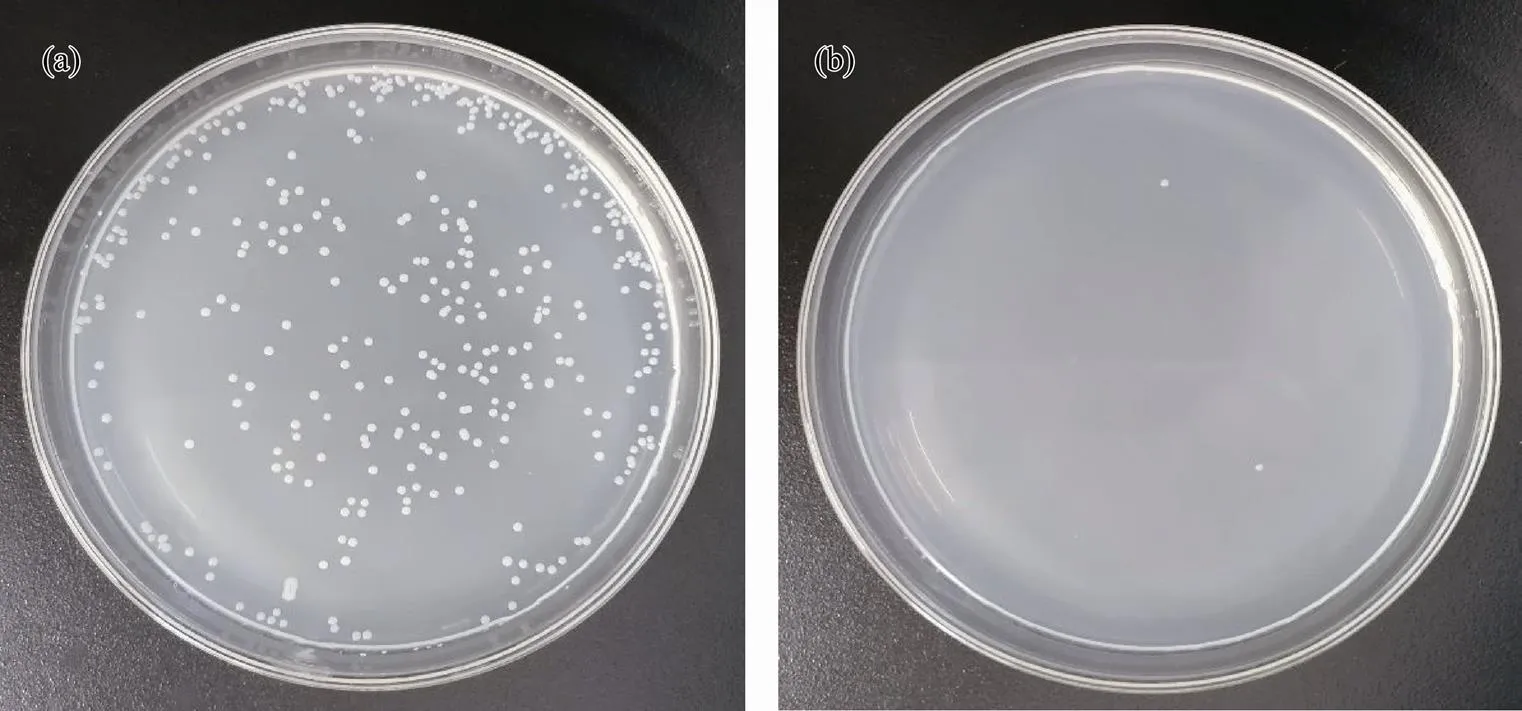
Fig.5 Antibacterial testing against P. tetraodonis: (a), blank group; (b) experimental group.
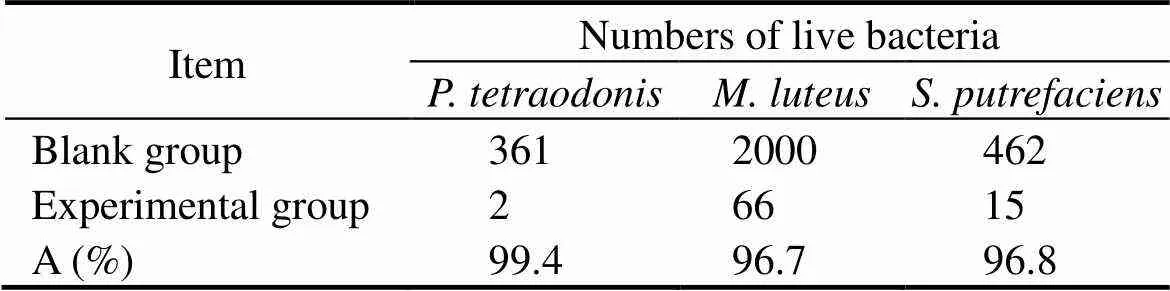
Table 1 Antibacterial activities of CuO-zeolite NC (S4)
Seen in Fig.5, the numbers of live bacteria are 361 and 2 in the blank group and experimental group, respectively, which shows an antibacterial efficiency of 99.4% against. As shown in Fig.6, the numbers of live bacteria are 2000 in the blank group and 66 in the experimental group, where the antibacterial efficiency is calculated to be 96.7% against. From Fig.7, the numbers of live bacteria are 462 and 15 in the blank group and experimental group, respectively, showing that the antibacterial efficiency reaches 96.8% against.
The antibacterial efficiencies are all greater than 96%, and these values show that copper oxide really acted as a strong bactericide against bacteria. The test results prove that CuO NPs loaded zeolite 4A is highly effective agai- nst marine bacteria. A proposed mechanism is that Cu2+ions released from nanosheets or nanoparticles may adhere to the negatively charged bacterial cell wall and break it, resulting in protein denaturation and cell death (Pandiyarajan, 2013). Another proposed mechanism is that Cu2+ions are small enough to destroy the bacterial cell membrane, and then enter the cell and destroy the enzyme functionality. The indirect effect caused by the change in the surrounding charge environment will also affect the effectiveness of nanoparticle metals against bacteria (Stohs and Bagchi, 1995; Pandiyarajan, 2013).
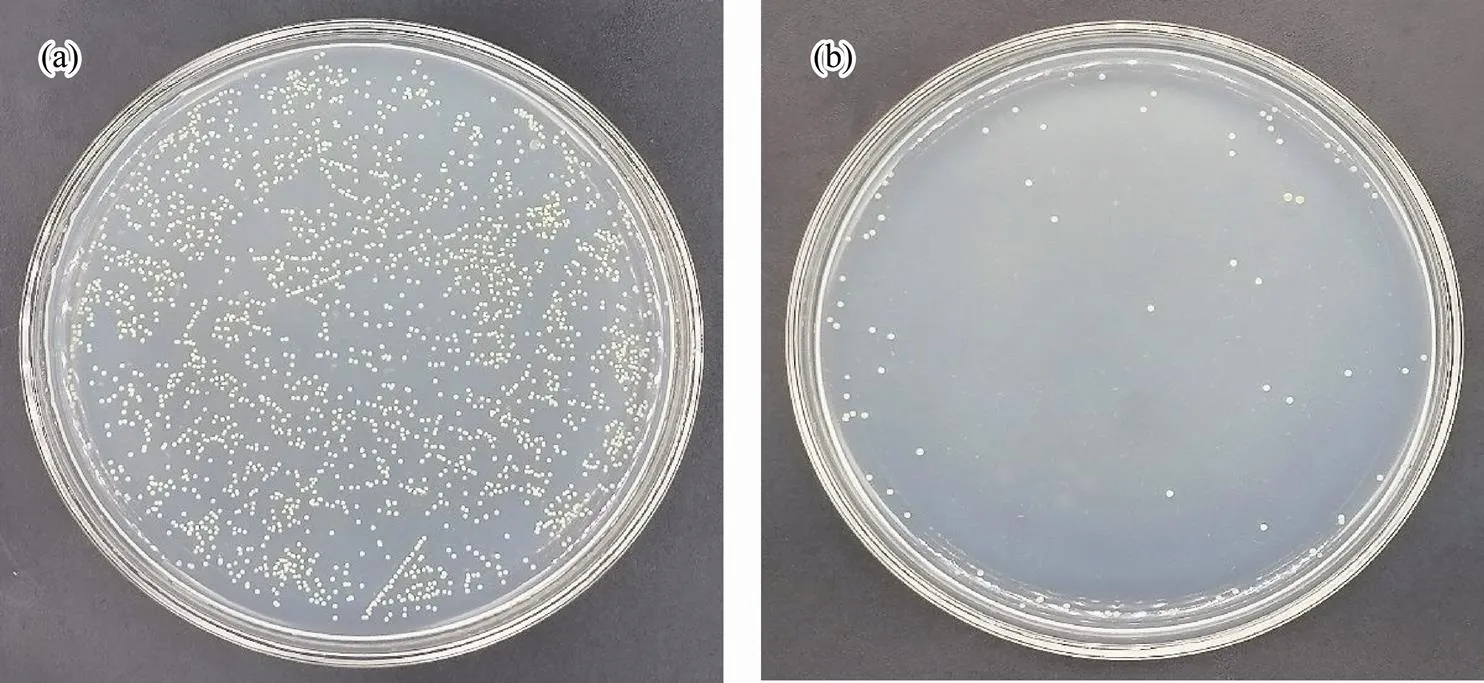
Fig.6 Antibacterial testing against M. luteus: (a), blank group; (b) experimental group.
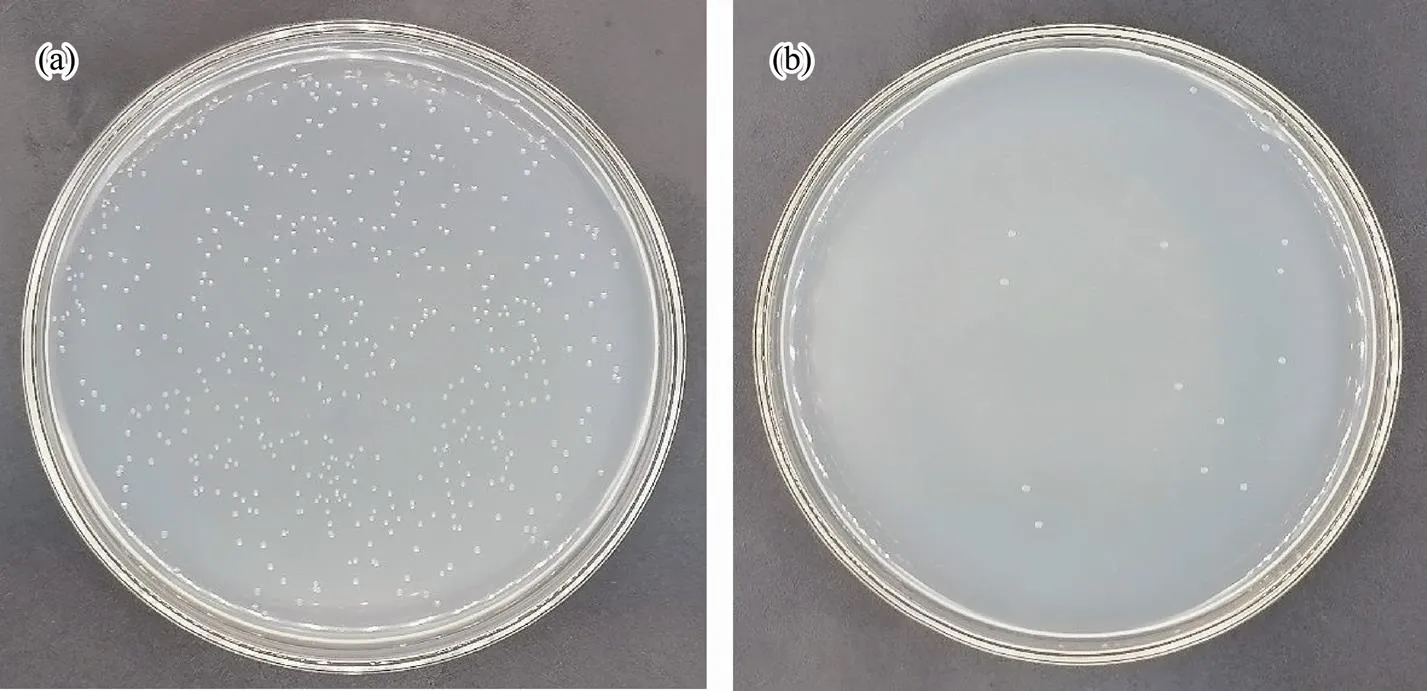
Fig.7 Antibacterial testing against S. putrefaciens: (a), blank group; (b) experimental group.
4 Conclusions
CuO-zeolite NCs were successfully prepared by modifying zeolite 4A, and a simple method was applied to synthesize CuO nanosheets on the surface of zeolite 4A. Zeolite 4A showed an important role in hosting copper oxide, and CuO-zeolite NCs exhibited a controlled release of copper ions. The XRD and SEM results proved the formation of CuO nanosheets. The XPS results confirmed the existence of only Cu (II). When the concentration of copper ions was 0.018molL−1, CuO nanosheets displayed a definite sheet structure. Zeolite 4A and CuO- zeolite NC were both reversible type-Iisotherms as exhibited by CO2adsorption-desorption experiments. Nano CuO-loaded zeolite 4A had great antibacterial properties against,. The antibacterial test showed that nano CuO-loaded zeolite 4A was highly effective against marine bacteria, which can be further applied to marine antifouling coating.
Acknowledgements
The authors thank the support of the National Key Research and the Development Project (No. 2019YFC0312 103), and the Open Fund of Shandong Key Laboratory of Corrosion Science (No. KLCS201905).
Ahmad, I., Islam, M., Habis, N. A., and Parvez, S., 2020. Hot-pressed graphene nanoplatelets or/and zirconia reinfor- ced hybrid alumina nanocomposites with improved toughness and mechanical characteristics.,40: 135-145.
Akintelu, S. A., Folorunso, A. S., Folorunso, F. A., and Oyeba- miji, A. K., 2020. Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation., 6 (7): e04508.
Alswat, A. A., Ahmad, M. B., and Saleh, T. A., 2017. Preparation and characterization of zeolitezinc oxide-copper oxide nanocomposite: Antibacterial activities., 16: 19-24.
Ananth, A., Dharaneedharan, S., Heo, M. S., and Mok, Y. S., 2015. Copper oxide nanomaterials: Synthesis, characterization and structure-specific antibacterial performance., 262: 179-188.
Araki, S., Li, T., Li, K., and Yamamoto, H., 2019. Preparation of zeolite hollow fibers for high-efficiency cadmium removal from waste water., 221: 393-398.
Brazlauskas, M., and Kitrys, S., 2008. Synthesis and properties of CuO/zeolite sandwich type adsorbent-catalysts., 29 (1): 25-30.
Cheng, M., Wang, Y., Wang, W. H., Wang, G. W., Zhu, X. L., and Li, C. Y., 2021. Promoting effect of copper oxide on CsX zeolite catalyst for side-chain alkylation of toluene with me- thanol., 311: 110732.
Dong, J. P., Tian, T. L., Ren, L. X., Zhang, Y., Xu, J. Q., and Cheng, X. W., 2015. CuO nanoparticles incorporated in hierarchical MFI zeolite as highly active electrocatalyst for non- enzymatic glucose sensing., 125: 206-212.
El-Shafai, N. M., Abdelfatah, M., El-Mehasseb, I. M., Ramadan, M. S., Ibrahim, M. M., El-Shaer, A.,., 2021. Enhancement of electrochemical properties and photocurrent of copper oxide by heterojunction process as a novel hybrid nanocomposite for photocatalytic anti-fouling and solar cell applications., 267: 118 631.
Karuppannan, S. K., Ramalingam, R., Khalith, M. S. B., Musthafa, S. A., Dowlath, M. J. H., Ramanujam, G. M.,., 2021. Copper oxide nanoparticles infused electrospun polycaprolactone/gelatin scaffold as an antibacterial wound dress- ing., 294: 129787.
Lv, Y. Y., Liu, J., Zhang, Z. Y., Zhang, W. H., Wang, A. Y., Tian, F.,., 2021. Green synthesis of CuO nanoparticles-loaded ZnO nanowires arrays with enhanced photocatalytic activity., 267: 124703.
Majumdar, D., and Ghosh, S., 2020. Recent advancements of copper oxide based nanomaterials for supercapacitor applications., 34: 101995.
Mekatel, E. H., Amokrane, S., Aid, A., Nibou, D., and Trari, M., 2015. Adsorption of methyl orange on nanoparticles of a syn- thetic zeolite NaA/CuO., 18 (3): 336-344.
Menazea, A. A., and Ahmed, M. K., 2020. Nanosecond laser ablation assisted the enhancement of antibacterial activity of copper oxide nano particles embedded though Polyethylene Oxide/Polyvinyl pyrrolidone blend matrix., 174: 108911.
Meng, T., Ren, N., and Ma, Z., 2015. Silicalite-1@Cu-ZSM-5 core-shell catalyst for N2O decomposition., 404-405: 233-239.
Murugappan, G., and Sreeram, K. J., 2021. Nano-biocatalyst: Bi-functionalization of protease and amylase on copper oxide nanoparticles., 197: 111386.
Nayan, N., Sahdan, M. Z., Wei, L. J., Ahmad, M. K., Lias, J., Fhong, S. C.,., 2016. Correlation between microstructure of copper oxide thin films and its gas sensing performance at room temperature., 20: 45-51.
Ninan, N., Muthiah, M., Yahaya, N. A. B., Park, I. K., Elain, A., Wong, T. W.,., 2014. Antibacterial and wound healing analysis of gelatin/zeolite scaffolds., 115: 244-252.
Noroozi, R., Musawi, T. J. A., Kazemian, H., Kalhori, E. M., and Zarrabi, M., 2018. Removal of cyanide using surface- modified Linde type–A zeolite nanoparticles as an efficient and eco-friendly material., 21: 44-51.
Panda, A. P., Jha, U., and Swain, S. K., 2020. Synthesis of nanostructured copper oxide loaded boehmite (CuO_Boeh- mite) for adsorptive removal of As(III/V) from aqueous solution., 37: 101506.
Pandiyarajan, T., Udayabhaskar, R., Vignesh, S., James, R. A., and Karthikeyan, B., 2013. Synthesis and concentration dependent antibacterial activities of CuO nanoflakes., 33 (4): 2020-2024.
Perla, V. K., Ghosh, S. K., and Mallick, K., 2020. Bipolar resistive switching behavior of carbon nitride supported copper oxide nanoparticles., 754: 137650.
Rezaie, A. B., Montazer, M., and Rad, M. M., 2018. Environmentally friendly low cost approach for nano copper oxide functionalization of cotton designed for antibacterial and pho- tocatalytic applications., 204: 425-436.
Rutkowska, I. A., Wadas, A., Szaniawska, E., Chmielnicka, A., Zlotorowicz, A., and Kulesza, P. J., 2020. Elucidation of activity of copper and copper oxide nanomaterials for electro-catalytic and photoelectrochemical reduction of carbon dioxide., 23: 131-138.
Show, B., Ahmed, S. F., Mondal, A., and Mukherjee, N., 2020. Hierarchical copper oxide as efficient enzymeless ampero- metric biosensor and promising photocatalyst., 9 (2): 104748.
Soori, F., and Ejhieh, A. N., 2018. Synergistic effects of copper oxide-zeolite nanoparticles composite on photocatalytic degradation of 2,6-dimethylphenol aqueous solution., 255: 250-256.
Sorekine, G., Anduwan, G., Waimbo, M. N., Osora, H., Velusamy, S., Kim, S.,., 2021. Photocatalytic studies of copper oxide nanostructures for the degradation of methylene blue under visible light., 1248: 131487.
Stohs, S. J., and Bagchi, D., 1995. Oxidative mechanisms in the toxicity of metal ions., 18 (2): 321-336.
Sultana, S., Rafiuddin, K. M. Z., Umar, K., and Muneer, M., 2013. Electrical, thermal, photocatalytic and antibacterial studies of metallic oxide nanocomposite doped polyaniline., 29 (9): 795-800.
Thangamani, J. G., and Pasha, S. K. K., 2021. Hydrothermal sy- nthesis of copper (II) oxide-nanoparticles with highly enhanced BTEX gas sensing performance using chemiresistive sensor., 277: 130237.
Vajda, M., Ursu, D., Duteanu, N., and Miclau, M., 2020. Low lying valence band edge materials based on copper oxide for tandem dye-sensitized solar cells., 275: 128151.
Wang, H., Luo, Y. Y., Liu, B., Gao, L., and Duan, G. T., 2021. CuO nanoparticle loaded ZnO hierarchical heterostructure to boost H2S sensing with fast recovery., 338: 129806.
Yadav, M. S., Sinha, A. K., Singh, M. N., and Kumar, A., 2021. Electrochemical study of copper oxide and activated charcoal based nanocomposite electrode for supercapacitor., 46: 5722-5729.
October 22, 2021;
April 17, 2022;
May 25, 2022
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
. E-mail: houjinqd@163.com
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- Stress Analysis of Wire Strands by Mesoscale Mechanics
- Effect of Temperature on the Carbon, Nitrogen, and Phosphorus Nutrient Budgets of Steelhead Trout (Oncorhynchus mykiss) with Different Sizes
- Impact of Evaporation Duct on Electromagnetic Wave Propagation During a Typhoon
- A New α-Cyclopiazonic Acid Alkaloid Identified from the Weizhou Island Coral-Derived Fungus Aspergillus flavus GXIMD 02503
- 3-D Marine CSEM Modeling in General Anisotropic Media by Using an Adaptive Finite Element Approach Based on the Vector-Scalar Potential
- Protective Effects of Sepia Ink Melanin on Hepatic Tissue in Streptozotocin-Induced Diabetic Mice
