Clearance of the liver remnant predicts short-term outcome in patients undergoing resection of hepatocellular carcinoma
2022-10-24AtsushiMikiYasunaruSakumaHideyukiOhzawaAkiraSaitoYoshiyukiMeguroJunWatanabeKazue
Atsushi Miki, Yasunaru Sakuma, Hideyuki Ohzawa, Akira Saito, Yoshiyuki Meguro, Jun Watanabe,Kazue
Morishima, Kazuhiro Endo, Hideki Sasanuma, Atsushi Shimizu, Alan Kawarai Lefor, Yoshikazu Yasuda,Naohiro Sata
Abstract
Key Words: Liver function; Hepatectomy; Cirrhosis; Fusion image; Complication; Mortality
lNTRODUCTlON
Advances in surgical technique and postoperative care have improved the outcomes of patients undergoing hepatectomy. However, posthepatectomy liver failure (PHLF) can lead to increased rates of morbidity and mortality in patients with hepatocellular carcinoma (HCC) especially in patients with chronic liver damage[1]. Major hepatectomy must be performed to preserve the maximal remnant liver function. However, adequate hepatectomy must be performed to ensure adequate surgical margins around the tumor[2]. Therefore, preoperative assessment of remnant liver function reserve is important to determine the appropriate surgical procedure.
An algorithm including the presence of ascites, serum bilirubin, serum albumin concentration,prothrombin time and encephalopathy is commonly used to determine the indications for resection of HCC. The indocyanine green (ICG) test is the most commonly used test and is considered relatively reliable for assessment of liver functional reserve[3]. However, the Child-Pugh score and ICG test do not accurately predict the development of PHLF[4]. A simple method using conventional data has been reported. The albumin-bilirubin (ALBI) score is an effective predictor of PHLF in patients with HCC compared to that of ICG test[5].
Computed tomography (CT) volumetry can accurately determine the regional liver volume, and has been used to estimate remnant liver function[6]. However, CT volumetry can never reflect the function of the remnant liver. The liver function of each lobe varies with progression of chronic liver disease or steatosis, which indicate that liver function is not distributed homogeneously[7]. Liver function is unevenly modified, resulting from impaired blood circulation[8], biliary stenosis, or induction by the tumor[9]. Changes in portal hemodynamics and a regional reduction in liver function must be considered to determine the optimal surgical procedure[7,10]. A novel method is needed to preoperatively plan for hepatic resection.
Taniguchiet al[11] described that99mTc-labelled galactosyl-human serum albumin (GSA) hepatic clearance strongly correlates with the degree of liver fibrosis and conventional liver function tests. GSA scintigraphy is widely used to evaluate liver function[10,12-16]. Asialoglycoprotein receptors exist predominantly in the liver on the surface of hepatocytes and are responsible for the metabolism of serum glycoproteins[17]. The receptor density in the liver is closely related to serum asialoglycoprotein level and hepatocyte function[18]. However, little is known about the clinical utility of hepatic clearance for the prediction of PHLF, morbidity and mortality. The aim of present study was to evaluate the effectiveness of measuring hepatic clearance of the remnant liver and to verify risk factors based on the standardized PHLF criteria and complications in patients undergoing hepatectomy.
MATERlALS AND METHODS
Patients
We included patients who underwent hepatectomy between July 2011 and March 2021 at Jichi Medical University (Shimotsuke, Tochigi, Japan). The protocol for this research project was approved by a suitably constituted Ethics Committee of the institution and it conformed to the provision of the Declaration of Helsinki (Committees of Jichi Medical University, Approval No. A21-029). Blood samples obtained preoperatively were analyzed for conventional liver tests.
The procedures for hepatectomy were categorized according to the Brisbane Nomenclature from the International Hepato-Pancreato-Biliary Association[19]. The anatomical resection was defined as resection of the tumor together with the related portal vein branches and the corresponding hepatic territory. The procedure was classified as a hemihepatectomy, an extended hemihepatectomy(hepatectomy plus removal of additional contiguous segments), a sectionectomy (resection of two Couinaud subsegments), or segmentectomy (resection of one Couinaud subsegment). All other nonanatomical procedures were classified as limited resections.
Contrast-enhanced CT
A three-phase enhanced helical CT scan of the liver was used to confirm tumor location and margins before surgery. A 16-row multi-detector CT scan was performed at 3 mm intervals with 100 mL iohexol(Omnipaque 300; Daiichi Sankyo, Tokyo, Japan) (3 mL/s) injected intravenously.
GSA single photon emission CT image
Patients underwent preoperative GSA scintigraphy using a dual-head rotating gamma camera system and a dedicated data processing unit (Prism Axis; Picker Prism International, Cleveland, OH, USA). A single bolus of 3 mg GSA (185 MBq; Nihon Medi-Physics, Tokyo, Japan) was injected intravenously.After confirmation that the detector covered the area in the liver and heart, acquisition of planar images was begun with an acquisition time of 15 s each for 16 min immediately after injection. After acquisition of planar images, dynamic single photon emission CT (SPECT) acquisition was started with an acquisition time of 20 s every 5 min. To generate a set of images equivalent to static SPECT images,projection data from dynamic SPECT were merged. Total liver function was calculated as the total liver GSA clearance, expressed as mL/min by the Patlak plot method.
Region of interest (ROI) was also generated over the entire liver on the tomographic images using isocount methods (25% cutoff of minimal count) to estimate the liver functional volume (mL). Functional liver volume does not include function parameters.
Estimation of function of the remnant liver
Hepatic clearance and functional volume of the remnant liver were estimated from the fusion with CT scan images (Figure 1). Images from the CT scan were aligned with the slice of the liver SPECT image with reference to the hepatic vein on every 3-mm liver cross-slice as a landmark on contrast-enhanced helical CT (Figure 1). After the transection line was set on the SPECT images based on the surgical procedure, the remnant liver with the resection line was determined manually. Remnant liver function was calculated from the proportional allocation of voxel count in static SPECT by the Patlak plot method and expressed by GSA clearance (mL/min). Regional functional liver volume (mL) was also calculated from the SPECT data by the outline extraction method[7].
Definition of major complication and PHLF
Postoperative complications were defined according to the Clavien-Dindo classification[20]. A major complication was defined as grade IIIa or higher. Postoperative mortality was defined as death within 30 d after surgery. PHLF was defined following the definition of the International Study Group of Liver Surgery[21]. Patients with increased prothrombin time-international normalized ratio (PT-INR) and hyperbilirubinemia (according to the normal cut-off levels defined by the local laboratory) on or after postoperative day 5 were considered to have PHLF. PHLF Grade A resulted in abnormal laboratory parameters and required no change in clinical management. Grade B was a deviation from the regular,postoperative clinical pathway, but patients could be managed without invasive treatment. Grade C resulted in deviation from the regular clinical management and required invasive treatment.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. All categorical data were analyzed by Pearson'sχ2test. Normally distributed values were analyzed by Student’sttest. Non-normally distributed values were analyzed using the Mann-WhitneyUtest. We analyzed the power for the prediction of PHLF, morbidity and mortality with the parameters of GSA scintigraphy with the area under the receiver-operating characteristic (ROC) curves, and the area under the ROC curve was calculated. In multivariate analysis, risk factors for PHLF were determined by logistic regression multivariate analysis with JMP statistical software (version 13; SAS, Inc. Cary, NC, USA). The level of statistical significance was set atP< 0.05.

Figure 1 Schematic model for analysis of regional hepatic clearance with computed tomography fusion images. The images of 99mTcgalactosyl serum albumin scintigraphy single photon emission computed tomography and computed tomography scans were merged using software. The cutting line was set based on tumor location and size on each fusion image. The liver function was calculated automatically as 99mTc-galactosyl serum albumin scintigraphy parameters. CT: Computed tomography; SPECT: Single photon emission computed tomography.
RESULTS
Clinicopathological characteristics
A total of consecutive 199 patients with HCC were included, including 156 men and 43 women, with a median age of 70 (range, 24-87 years) (Table 1). Among the 199 patients, 94 (47%) had hepatitis C virus infection and 22 (11%) had hepatitis B virus infection. Most patients were Child-Pugh class A (197/199,99%) and the remaining patients were class B (2/199, 1%). According to ALBI grade, 68% (135/199) of patients were stratified into Grade 1, 32% (64/199) Grade 2, and 1% (2/199) Grade 3. There were 6% of ALBI Grade 1 patients who developed major complications and 18% ALBI Grade 2 patients had major complications (P= 0.04).
Postoperative morbidity, PHLF and mortality
Among the 199 patients, 41 (21%) developed postoperative complications (Table 2). The most common complication was PHLF (12%, 23/199), followed by wound infection (5.0%, 10/199). Thirty-three (17%)patients developed minor complications, including Grade I complications in 25 (13%) patients and Grade II complications in eight (4.0%) patients. Major complications occurred in 27 (14%) patients,including Grade IIIa (11%, 21/199), Grade IIIb (1.5%, 3/199), Grade IVa (0.5%, 1/199) and Grade V (1%,2/199). Eleven patients (5%) had PHLF Grade A, eight (4%) had PHLF Grade B, and four (2%) had PHLF Grade C. Two patients died of PHLF within 30 d after surgery, for a postoperative mortality rate of 1% (Table 2).
Correlations between hepatic clearance of the remnant liver and PHLF
ROC curve analysis of hepatic clearance of the remnant liver, liver to heart-plus-liver radioactivity at 15 min (LHL15), and ALBI score were used to predict the risk of developing PHLF (Figure 2A). The area under the ROC curve for hepatic clearance of the remnant liver, LHL15, and ALBI score for predictingthe development of PHLF were 0.868, 0.629, and 0.655, respectively. Hepatic clearance of the remnant liver had the highest area under the curve for predicting the development of PHLF. The cutoff values for predicting PHLF with highest sensitivity and specificity were 192 mL/min (sensitivity, 87.0%;specificity, 76.1%) for hepatic clearance of the remnant liver, 0.91 (sensitivity, 47.8%; specificity, 73.3%)for LHL15, 2.96 (sensitivity, 34.9%; specificity, 95.7%) for ALBI score. The relationship between hepatic clearance of the remnant liver and PHLF grade was evaluated (Figure 2B). The median hepatic clearances of the remnant liver were 239, 153, 150.5 and 119.5 mL/min for no PHLF, Grades A, B and C,respectively. The differences were significant for no PHLF and Grade A (P= 0.002), no PHLF and Grade B (P= 0.003), and no PHLF and Grade C (P= 0.02).
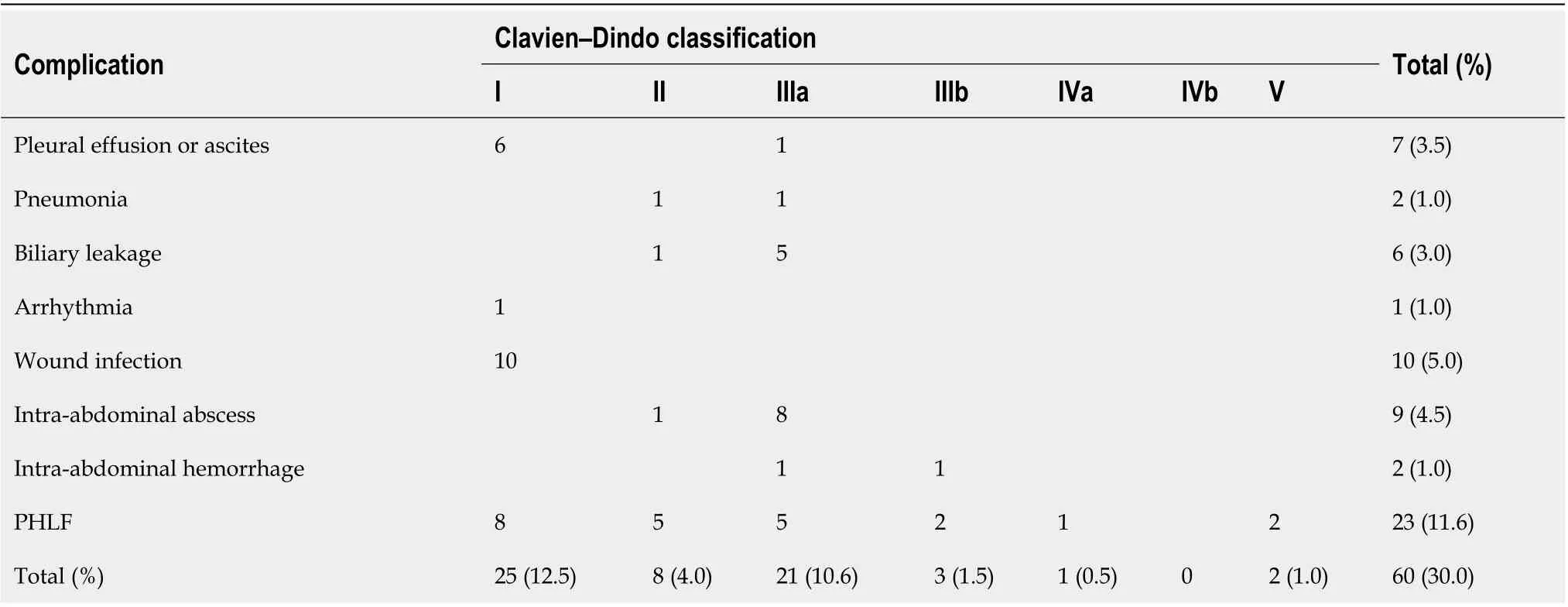
Table 2 Postoperative morbidity in patients with hepatocellular carcinoma

Figure 2 Analysis of hepatic clearance for post hepatectomy liver failure. A: Receiver-operating characteristic curve analysis of hepatic clearance of the remnant liver, LHL15, and albumin-bilirubin (ALBI) score in predicting PHLF. The area under the receiver-operating characteristic curve values for analysis of hepatic clearance of the remnant liver, LHL15, and ALBI score in predicting PHLF were 0.868, 0.629, and 0.655, respectively; B: Hepatic clearance of the remnant liver for each PHLF. The median hepatic clearances of the remnant liver were 239, 153, 150.5, and 119.5 mL/min for normal, Grades A, B, and C, respectively.LHL15: liver to heart-plus-liver radioactivity at 15 min; ALBI score: Albumin-bilirubin score; PHLF: Post hepatectomy liver failure.
Correlation between hepatic clearance of remnant liver and morbidity and mortality
ROC curve analysis of hepatic clearance of the remnant liver, LHL15, and ALBI score were used to predict the risk of developing major complications (Figure 3A). The area under ROC curves for hepatic clearance of the remnant liver, LHL15, and ALBI score for predicting major complications were 0.758,0.594, and 0.647, respectively. Hepatic clearance of the remnant liver had the highest area under the curve for predicting the development of major complications. The cutoff values for predicting PHLF with highest sensitivity and specificity were 237 mL/min (sensitivity, 100%; specificity, 51.9%) for hepatic clearance of the remnant liver, 0.94 (sensitivity, 84.2%; specificity, 36.2%) for LHL15, 2.63(sensitivity, 69.3%; specificity, 63.2%) for ALBI score. The relationship between hepatic clearance of the remnant liver and Clavien-Dindo classification was evaluated (Figure 3B). The median hepatic clearances of the remnant liver were 238 and 179 mL/min for Clavien-Dindo < IIIa and Clavien-Dindo≥ IIIa. The differences were significant for Clavien-Dindo < IIIa and Clavien-Dindo ≥ IIIa (P= 0.0004).
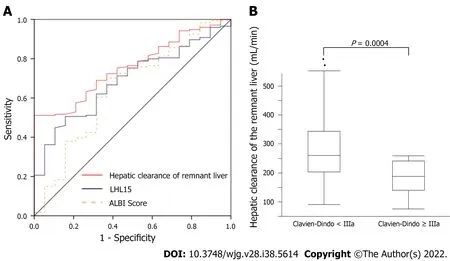
Figure 3 Analysis of hepatic clearance for morbidity and mortality. A: Receiver operating characteristic curve analysis of hepatic clearance of the remnant liver, LHL15, and albumin-bilirubin (ALBI) score in predicting major morbidity. The area under the receiver operating characteristic curve analysis of hepatic clearance of the remnant liver, LHL15, and ALBI score in predicting major morbidity were 0.758, 0.594, and 0.647, respectively; B: Hepatic clearance of the remnant liver for Clavien-Dindo classification. The median hepatic clearances of the remnant liver were 238 and 179 mL/min for Clavien-Dindo < III and Clavien-Dindo ≥ III.LHL15: liver to heart-plus-liver radioactivity at 15 min; ALBI score: Albumin-bilirubin score.
Multivariate analysis for PHLF Grade B or C
Multivariate regression analysis was performed between variables, with statistically significant differences following the univariate analysis regarding PHLF Grade B or C (Table 3). Hepatic clearance of the remnant liver [P= 0.001, odds ratio (OR): 0.973, 95% confidence interval (CI): 0.952-0.995] and intraoperative blood loss (P= 0.006, OR: 1.001, 95%CI: 1.0002-1.002) were independent risk factors for PHLF Grade B or C.
Multivariate analysis for major complication and mortality
Multivariate regression analysis was performed between variables, with significant differences following the univariate analysis regarding major complications (Table 4). Hepatic clearance of the remnant liver (P= 0.004, OR: 0.988, 95%CI: 0.979-0.999) and intraoperative blood loss (P= 0.005, OR:1.0005, 95%CI: 1.0002-1.0014) were independent risk factors for developing major complications.
DlSCUSSlON
Hepatic clearance was associated with PHLF and major complications. The independent risk factors for developing PHLF and major complications were the hepatic clearance of the remnant liver, and intraoperative blood loss. The results of this study show that the measurement of hepatic clearance of the remnant liver is reliable for predicting the development of PHLF and major complications.
The results of this study support the use of hepatic clearance of the remnant liver, LHL15, and ALBI score for predicting the development of PHLF and postoperative major complications in patient with HCC. LHL15 and HH15, which are hepatic uptake and blood clearance ratios in GSA scintigraphy, are the most popular and widely used in many institutions. However, they may be insufficient for accurately estimating the degree of liver function because these indices are calculated from planar scintigraphic images, which do not correctly reflect hepatocyte volume[11]. In contrast, hepatic clearance measured by SPECT analysis contains volumetric information and may correctly estimate the hepatocyte volume[11]. LHL15 reflects the function of the whole liver, but the hepatic clearance of remnant liver shows the liver function of remnant liver, therefore, hepatic clearance may reflect functional reserve and short term outcome.
Many studies have investigated the relationship between GSA scintigraphy and PHLF. However, this is the first report comparing residual liver function and major complications using GSA scintigraphy.Patients with lower remnant liver function are at higher risk for PHLF, morbidity and mortality. Thequality and volume of the postoperative remnant liver have been shown to be associated with postoperative outcomes[22]. Surgeons should emphasize the remnant liver functional reserve rather than the resected liver volume[12,23]. Elevation of serum bilirubin and PT-INR are associated with morbidity and mortality, regardless of the extent of resection[24]. Liver failure after limited resection can develop. Patients with reduced reserve of the remnant liver are at higher risk for the development of PHLF and major complications[22,25,26]. The extent of surgery should be considered to preserve as much liver function as possible. Moreover PHLF grade C is most severe types of liver failure that may lead to in-hospital death[24]. In ROC curve analysis, the cutoff line of PHLF grade C was 151 mL/min(sensitivity 87.5%, specificity 100%). Patients below the cutoff line should be given special consideration by surgeons before surgery and may not be ideal candidates for hepatic resection. Hepatic clearance of the remnant liver below 100 mL/min is associated with a high mortality rate. Therefore, PTPE should be performed when hepatic clearance of the remnant liver is below 100 mL/min, and surgery should be considered when the clearance is above 100 mL/min. In addition, if hepatic clearance of the remnant liver is greater than 100 mL/min preoperatively, unnecessary PTPE can be avoided.
The risk for developing PHLF and major complications is determined by patient and surgical factors.Intraoperative blood loss is a well-known risk factor for morbidity and mortality after hepatic resection[25,27-30]. Hemorrhage can lead to the development of metabolic acidosis as a consequence of intracellular derangements in oxygen and substrate utilization[29]. Reduced levels of cytokines and humoral factors, such as interleukin-6, hepatocyte growth factors, and growth hormone after extensive blood loss may result in decreased liver regeneration because of loss of growth factors needed for regeneration[28].
The present study had some limitations, including a retrospective design, and being a single center study. Preoperative GSA scintigraphy was routinely performed to estimate total liver function in this hospital. Total liver function, remnant liver function, laboratory data, and liver failure were objectively assessed in advance, which limited the risk of observation bias. Prospective multicenter trials are needed to validate the results of this study.
CONCLUSlON
Lower functional reserve of the remnant liver results in a higher risk of developing PHLF and major complications in patients undergoing resection of HCC. The estimation of hepatic clearance of the remnant liver may provide guidance for determining the extent of resection in a patient-specific manner.

Table 4 Predictive factors for morbidity (Clavien-Dindo classification ≥ llla)
ARTlCLE HlGHLlGHTS

ACKNOWLEDGEMENTS
I especially thank Michio Ashizaki for helping patient data acquisition.
FOOTNOTES
Author contributions:Miki A, Sakuma Y, Shimizu A and Yasuda Y designated the overall concept and outline the manuscript; Ohzawa H, Saito A, Meguro Y, Watanabe J, Morishima K, Endo K, Sasanuma H, and Sata N contributed to the discussion and design of the manuscript; Miki A and Lefor AK contributed to the writing, editing the manuscript, illustrations, and review of literature.
lnstitutional review board statement:The study was reviewed and approved by the Institutional Review Board of Jichi Medical University, Approval No. A21-029.
lnformed consent statement:Written informed consent from any patient for data collection in a prospectively collected data base is available. However, the need for written informed consent for this study was waived by the Institutional Review Board of Jichi Medical University in view of the retrospective design of the study, based on national and local guidelines such as the fact that all clinical/ laboratory measurements and procedures were part of routine care.
Conflict-of-interest statement:The authors declare no conflicts of interest for this study.
Data sharing statement:The database contains highly confidential data which may provide insight in clinical and personnel information about patients and lead to their identification. Therefore, according to organizational restrictions and regulations these data cannot be made publicly available. However, the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Japan
ORClD number:Atsushi Miki 0000-0002-2908-0177; Yasunaru Sakuma 0000-0003-3633-3221; Hideyuki Ohzawa 0000-0001-9422-2840; Akira Saito 0000-0002-3247-7845; Yoshiyuki Meguro 0000-0003-4328-1909; Jun Watanabe 0000-0003-4477-4238; Kazue Morishima 0000-0002-7837-3742; Kazuhiro Endo 0000-0002-2845-3533; Hideki Sasanuma 0000-0002-9758-7295; Atsushi Shimizu 0000-0001-6249-4489; Alan Kawarai Lefor 0000-0001-6673-5630; Yoshikazu Yasuda 0000-0002-4101-6629; Naohiro Sata 0000-0002-6689-5623.
S-Editor:Chang KL
L-Editor:Kerr C
P-Editor:Chang KL
杂志排行
World Journal of Gastroenterology的其它文章
- Ultrasound-based artificial intelligence in gastroenterology and hepatology
- Comparison of evaluation indexes for Gastroenterology and Hepatology journals in different databases
- A new scoring system to evaluate adjuvant chemotherapy for patients with T2N0M0 gastric cancer after D2 gastrectomy
- Timing of endoscopic retrograde cholangiopancreatography in the treatment of acute cholangitis of different severity
- Effect of low-dose radiation on thyroid function and the gut microbiota
- Oxidative stress bridges the gut microbiota and the occurrence of frailty syndrome
