Application of polypyrrole nanofibers in the selective extraction of methotrexate and its polyglutamate metabolites from hospital effluents
2022-10-18XieLiShuQingDengGuozheLiDaKangXuejunGeWeihong
Xie Li Shu Qing Deng Guozhe Li Da Kang Xuejun Ge Weihong
(1School of Biological Science and Medical Engineering, Southeast University, Nanjing 210018, China)(2College of Animal Science and Food Engineering, Jinling Institute of Technology, Nanjing 210038, China)(3Department of Pharmacy, Nanjing Drum Tower Hospital, Nanjing 210008, China)
Abstract:A novel solid-phase extraction (SPE) strategy based on polypyrrole (Ppy) nanofibers was developed for the determination of trace methotrexate and its polyglutamate metabolites (MTXs) in hospital effluents. Ppy was coated on the surface of electrospun polystyrene nanofibers by in situ oxidative polymerization to form Ppy electrospun nanofibers. The mechanism of adsorption on MTXs was explored through static adsorption studies. The MTX contents, after extraction, were determined by liquid chromatography-tandem mass spectrometry analysis. Results show that the physical/chemical adsorption of targets occurs on the surface of Ppy nanofibers, which is most likely dominated by multiple adsorptions and heterogeneous adsorption sites. Ppy nanofibers exhibit satisfying extraction performance. The content of targets detected in medical wastewater samples ranges from 21 to 2 908 ng/L. The novel strategy based on Ppy nanofiber SPE can extract trace MTXs effectively, guarantee analytical accuracy, and circumvent the storage and transportation of water samples during on-site sampling operations.
Key words:polypyrrole nanofibers; solid-phase extraction; liquid chromatography-tandem mass spectrometry (LC-MS/MS); hospital effluents; determination
Cyclophosphamide, tamoxifen, ifosfamide, and methotrexate are the most commonly used anticancer drugs in clinics and have been widely released into the aquatic environment[1], among which methotrexate is excreted 80% to 90% unchanged[2]. Methotrexate and its polyglutamate metabolites (MTXs) have the potential to be widely transported and dispersed in the aquatic environment because of their high polarity and acid-base character, and MTX concentrations between 1.6 and 4 756 ng/L have been detected[1]. Thus, a trace-level analytical method for the monitoring of MTXs in environmental matrices needs to be developed. Moreover, the method should be easy to use, sensitive, and selective enough.
Sample preparation is often the rate-limiting and labor-intensive step of the entire analytical procedure. Thus, the selection of the optimal sample preparation technique is the most significant step in setting up an analytical method for the measurement of MTX in different samples. Solid-phase extraction (SPE), which is based on the selective distribution of analytes between solid and liquid phases, has been widely applied in the extraction and preconcentration of MTXs based on the use of conventional commercial cartridges[3]; however, the extraction efficiencies of these cartridges suffer from several drawbacks, including slow adsorption kinetics, poor selectivity, and limited working capacity[4]. Meanwhile, nanoscale materials in SPE, i.e., adsorbents with large surface-area-to-volume ratios and easily chemically modified surfaces, show great potential to overcome the aforementioned problems. Several nanoscale materials, which exhibited a high adsorption capability because of unsaturated atoms and small average micropores occurring on their surfaces, have been utilized as sorbents to adsorb a variety of MTXs[3]. In 2019, Xie et al[5]developed a packed-fiber SPE method using polypyrrole (Ppy) nanofibers to extract the water-soluble vitamins B2, B9, and B12 in urine samples with satisfactory recovery, and the method was simple, rapid, and accurate and used inexpensive organic solvents.
Ppy, as a conductive polymer, is equipped with many inherent and special functionalities, such as ion-exchange properties, π-π interactions, hydrogen bonding, acid-base properties, polar functional groups, and electroactivity[6], providing sufficient adsorption sites for polar compounds. Several reports on specially modified Ppy in the extraction of some polar compounds, such as phenolic compounds and polar vitamins, have been published[5,7]. However, researches on the use of electrospun nanofibers composed of Ppy for the extraction of multiple pterin compounds, such as MTX and its analogs, has not yet been conducted. In this work, an analytical method using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) combined with the packed-nanofiber-based solid-phase extraction (PFSPE) for trace determination of MTXs was validated. 4-amino-10-methylpteroylglutamic acid (MTX) and methotrexate polyglutamates 4-amino-10-methylpteroyldiglutamic acid (MTXPG2) and 4-amino-10-methylpteroyltriglutamic acid (MTXPG3) were selected, and their contents in hospital effluents were quantified successfully.
1 Methods
1.1 Chemicals and reagents
All chemicals and reagents were of analytical grade. Ammonium salts of MTX,MTXPG2, and MTXPG3 were purchased from Schircks Laboratories (Jona, Switzerland). Polystyrene (PS) and Ppy nanofibers were purchased from Dongqi Biotechnology Co., Ltd. (Suzhou, China). The Sep-Pak silica cartridge[8]used for sample treatment was purchased from Waters (Milford, MA, USA).
The standard stock solution composed of 0.1 mg/mL of MTXs in pure water was prepared and stored at 4 ℃ in a brown flask. Appropriate dilutions of the stock solutions were prepared and utilized as the working solutions.
1.2 Chromatography method
The adsorption and desorption studies were optimized using the high-performance liquid chromatography with ultraviolet-visible spectrophotometry (HPLC-UV) system equipped with the VP-ODS Shimadzu C18 (5 μm, 250 mm×4.6 mm) column to ensure a robust and tunable operation. The determination of MTXs in real samples was conducted using the HPLC-MS/MS system with an electrospray ionization (ESI) source to obtain the lowest limit of detection (LOD).
The binary mobile phase was 30∶70 methanol and 5mmol acetic acid solution. The injection volume for the samples was set at 20 μL, and the UV detection wavelength was 302 nm. For real sample determination, 5 μL of the extracted solution was injected into the LC-MS/MS system. The flow rate was 300 μL/min, and the column temperature was 30 ℃. Analyses were conducted in positive mode at an ion spray voltage of 5 000 V. Quantitative analysis was performed in the multiple reaction monitoring mode. Quantification and confirmation ions were 455.1 and 101.2 for MTX, 584.3 and 308.1 for MTXPG2, and 713.3 and 308.0 for MTXPG3, respectively.
1.3 Static adsorption studies
Three activated solid adsorbent Ppy nanofibers (10 mg) were placed in a cuvette containing 10 mL of the solution (i.e., 5 μg/mL of each analyte) under a constant temperature of 25 ℃. Then, the target compound in the liquid phase was determined at time intervals of 1, 3, 5, 10, 15, 20, 25, 30, and 60 min. The adsorption capacityqtof each compound was calculated using the following equation[9]:
whereC0andCtare the concentrations of each analyte at the initial time and at timet, respectively;Vis the volume of the solution; andMis the weight of Ppy.
The sorption isotherm experiments were conducted in 2mL working solutions with the initial concentrations of 0.5, 5, 7.5, 20, 35, 50, 62.5, 70, and 90 μg/mL of each analyte at 298, 318, and 338 K. The concentration of the solutions was measured after 2 h of sufficient adsorption equilibrium. The equilibrium adsorption capacityqeof each analyte was calculated using the following equation[9]:
whereCeis the equilibrium concentration.
The equilibrium data of the three temperatures were simulated using two well-known isotherm models, namely, the Langmuir and Freundlich models[10].
1.4 Sample collection and treatment
The SPE column was in situ prepared in an SPE cartridge[11]. The entire SPE procedure is schematically shown in Fig.1. If the concentration of targets in the sample is low, then the sample loading step can be done two or more times to treat a large volume of samples. The eluant, which was 30% methanol (pH adjusted to 1 to 3 with formic acid), was collected and stored at 4 ℃ for determination.

Fig.1 Schematic diagram of PFSPE column and sample processing
1.5 Method validation
The extraction recoveries were estimated by spiking each analyte with 5, 50, and 100 ng/mL tap water. The LOD was obtained as the signal-to-noise ratio of 3∶1. The limit of quantification (LOQ) was 10∶1. Five replicates at three concentrations (i.e., 50, 500, and 1 000 ng/L) were analyzed to evaluate the precisions and recoveries. Recoveries were determined as the percentages of the measured concentrations (calculated using the standard curve equation) against the spiked concentrations.
The matrix effect (ME) was computed as the ratio of the peak areas of analytes in spiked concentrations at 50, 500, and 1 000 ng/L in the samples to the peak areas of analytes in diluted standard solutions at 50, 500, and 1 000 ng/L.
2 Results
2.1 Comparison of the adsorption efficiencies of different adsorbents
MTXs were conventionally extracted using materials with high specific surface area and loading capacity[8, 12-13]. A previous study indicated that intraporosity and interporosity will enhance the functionality of adsorption, where the local properties down to the scale of individual molecules are critical for the performance of the material[14]. The textural properties, BET surface area, pore size, and pore volume of Ppy nanofibers were decreased and compared with PS[5]. The spiked hospital effluents (50 ng/mL) were extracted with three kinds of nanofibers and a conventional Sep-Pak silica cartridge[8](see Fig.2), and the Ppy nanofibers exhibited an adsorption efficiency for targets that is superior to that of PS nanofibers and commercial products, indicating that the surface characteristics of nanofibers were not key contributors to their adsorption capability.
(a)
(b)

(c)

(d)
2.2 Optimization of the extraction parameters
The extraction pH, elution pH, and methanol ratio in eluant were optimized (see Fig.3). The extraction pH was optimized to 5 to 7, the elution pH was optimized to 1 to 3, and the ratio of methanol in the eluant was optimized to 30%.

(a)

(b)

(c)
2.3 HPLC-MS/MS analysis
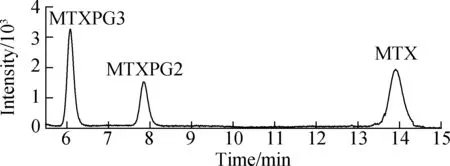
Effective chromatography resulted in the separation of MTXs to produce clean, discrete peaks. Moreover, no significant interference was observed during the entire analysis (see Fig.4). The peaks of MTX, MTXPG2, and MTXPG3 in real samples appeared at 13.95, 7.89, and 6.31 min, respectively.
2.4 Static adsorption studies
2.4.1 Adsorption kinetic models
The adsorption capacity-time curves of Ppy on MTXs are shown in Fig.5(a). Notably, the adsorption capacity of MTXs increased with the adsorption time. All adsorption processes reached equilibrium in a short time, and for MTX, the adsorption equilibrium could be achieved within 12 min, whereas for MTXPG2 and MTXPG3, the adsorption equilibrium could be achieved within 10 min.

(a)
(b)

(c)
The linear form of the pseudo-first-order and pseudo-second-order models can be expressed as follows:
whereqeandqtare the adsorption capacities at equilibrium and at timet, respectively[15].
The correlation coefficient (R2) indicated that the pseudo-second-order kinetic model can better describe the MTX adsorption process than the pseudo-first-order kinetic model.
2.4.2 Adsorption isotherm
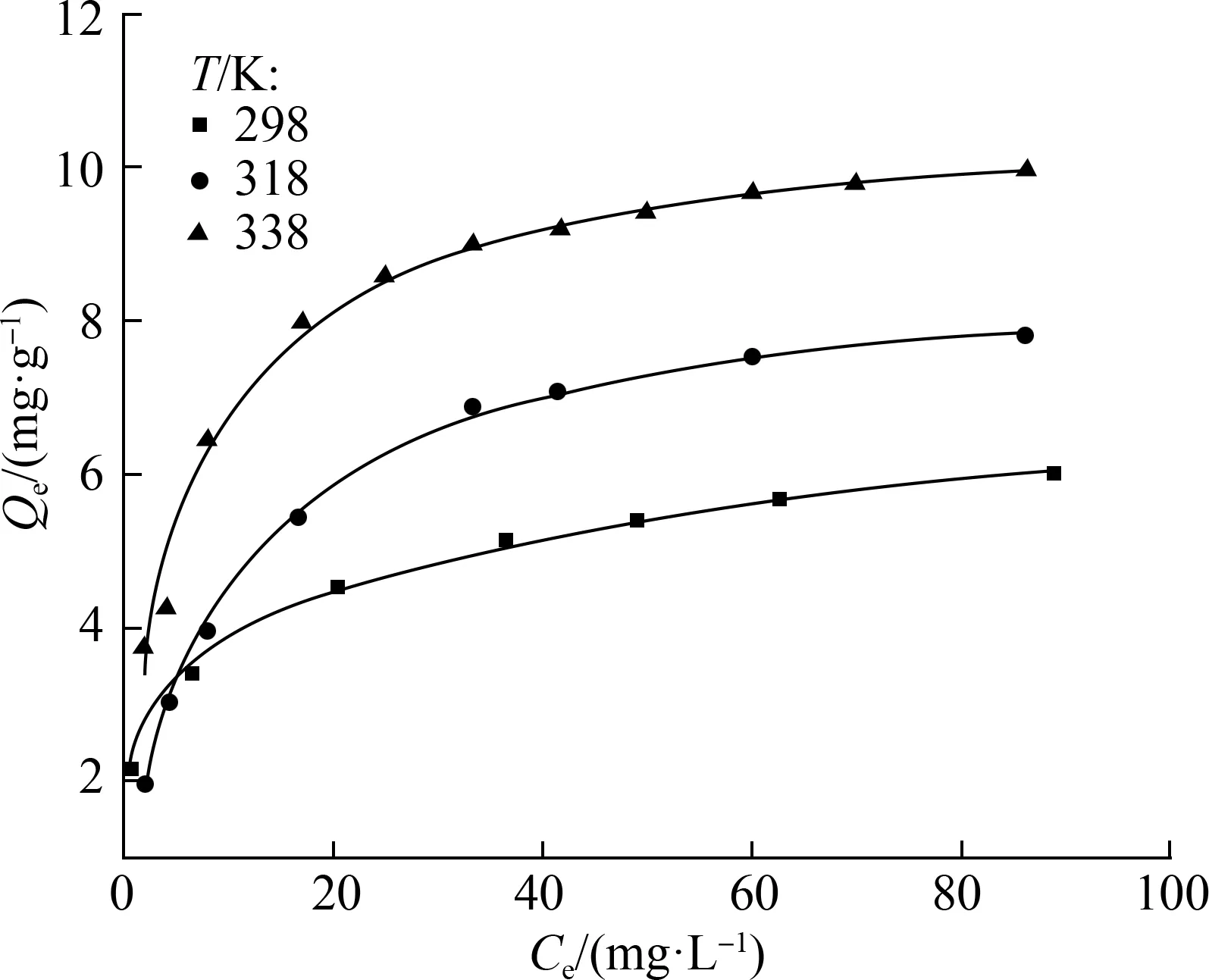
As shown in Fig.6,R2of the fitting curves of the Langmuir and Freundlich models indicates that the two tested isotherm models fit the equilibrium data well with high correlation coefficients (R2>0.9). Meanwhile, for all of the compounds, the Freundlich model matched the experimental data better than the Langmuir model with a higher correlation coefficient (R2>0.99), which indicated that the adsorption process was most likely dominated by multiple adsorptions and heterogeneous adsorption sites.

(a)

(b)

(c)
The maximum adsorption capacityqmwas calculated to be 6.0, 7.8, and 10.0 mg/g at 298, 318, and 338 K, respectively. These values were used to analyze trace MTXs in environmental water.
2.5 Method validation
A linear response between 5 and 5 000 ng/L was observed for MTX withR2=0.999 1, between 5 and 5 000 ng/L for MTXPG2 withR2=0.999 4, and between 10 and 5 000 ng/L for MTXPG3 withR2=0.999 0. The LOD for MTX, MTXPG2, and MTXPG3 were 2, 15, and 30 ng/L, respectively, and the LOQ for MTX, MTXPG2, and MTXPG3 were 6.7, 50, and 100 ng/L, respectively. The ME was 90.7% to 96.2%, which indicated that the matrix interference with the analyte signals from effluent samples was not significant (see Tab.1).
(a)

(b)
2.6 Application
MTXs were detected in the raw river water and hospital effluent samples in concentrations ranging from 21 to 2 908 ng/L (see Tab.2). MTX was the predominant
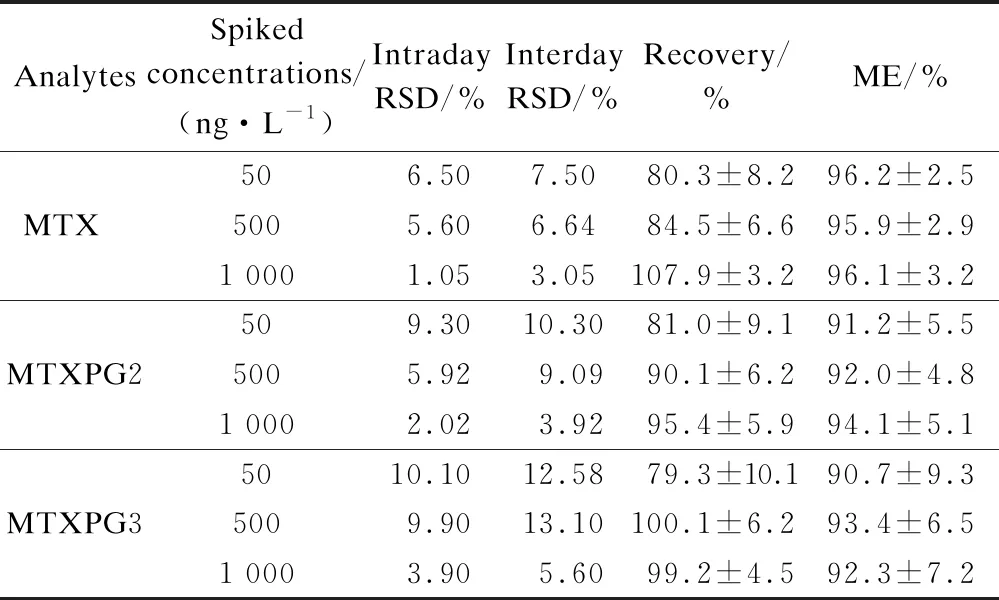
Tab.1 Imprecisions, recoveries, and MEs of the method
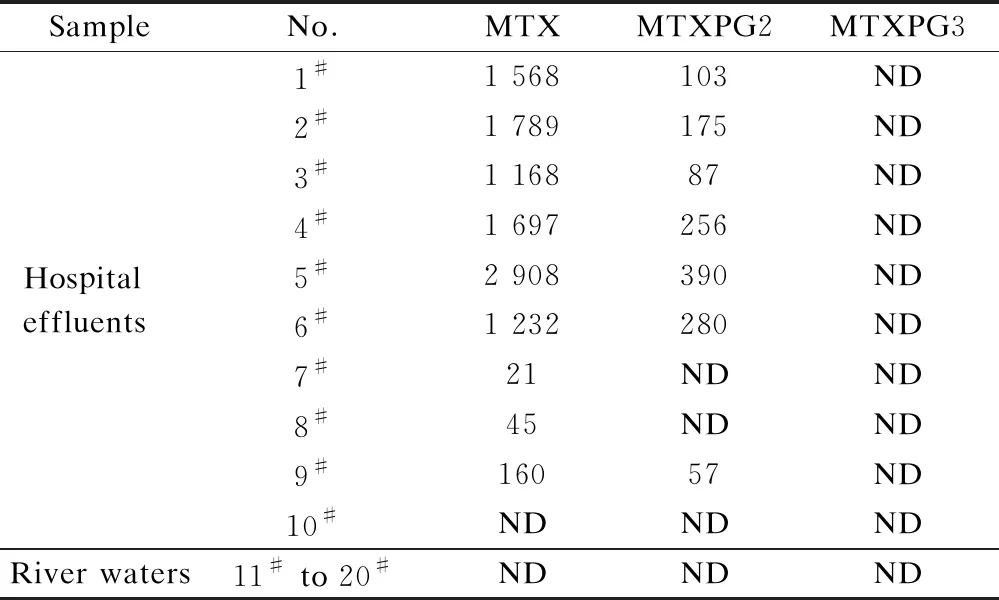
Tab.2 Pharmaceutical concentrations measured in hospital effluents and river waters in urban areas ng/L
pharmaceutical observed in the wastewater samples ((1 176.4±964.9) ng/L). MTXPG2 was also detected in most of the hospital effluents ((192.6±121.5) ng/L). However, in the river water samples, nearly no MTXs were detected.
2.7 Comparison with published methods
The PFSPE method proposed in this study was compared with those reported in the literature (see Tab.3). Notably, PFSPE was a sensitive technique for the determination of analytes with more convenient operation, smaller volume of samples and solvents, reduced sample loss throughout the manipulation, and reduced impurity contamination.

Tab.3 Comparative information from studies on the pretreatment and detection of MTX and its metabolites in various samples
3 Conclusions
1) A novel strategy applying Ppy electrospun nanofibers as the sorbent to extract and analyze MTXs simultaneously from wastewaters was developed. To our knowledge, this is the first time that the metabolites of MTX in hospital effluents were detected.
2) Compared with the other conventional pretreatment methods, the PFSPE method reduced the analysis time without the need for nitrogen drying, thereby reducing the loss of analytes. With the use of an in-tip microextraction device, the sampling volume can also be easily amplified to improve the extraction efficiency according to the contents of targets in the samples.
3) The Ppy nanofiber shows good molecular recognition capability and selectivity for the adsorption of MTXs. The extraction efficiency of Ppy from MTXs can be explained by the interaction mechanism between Ppy and the analytes. As a conjugated π structure can be found in the Ppy backbone, which has a strong interaction with the analytes, a hydrogen bonding interaction can also exist between the polymer and the analytes. The data confirmed that Ppy coupled with MTXs in the pH range of 5 to 7. When the pH becomes alkaline or acidic, the complex dissociates, so that the pterin molecules can be eluted.
4) The in-tip microextraction device is convenient and portable for site sampling. This study showed that Ppy can be used in the monitoring of antitumor drugs in environmental waters.
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Improved PBFT protocol based on phase voting and threshold signature
- Predictive current control system of PMSM based on LADRC
- Temperature prediction model for a high-speed motorized spindle based on back-propagation neural network optimized by adaptive particle swarm optimization
- Analysis on resonant shake table with novel variable stiffness mechanism
- BIM-based construction technologies for precast foamed lightweight concrete wallboards
- Analysis on degradation behaviors of sisal fibers at various pH conditions
