Effectiveness and safety of tripterygium glycosides tablet (雷公藤多苷片) for lupus nephritis: a systematic review and Meta-analysis
2022-10-14ZHOUYingyanLIANGHuashengYANJingyaoHEXiaohongPANLiliLIXueCHENXianghongCHENXiuminYANGAichengHUANGQingchun
ZHOU Yingyan,LIANG Huasheng,YAN Jingyao,HE Xiaohong,PAN Lili,LI Xue,CHEN Xianghong,CHEN Xiumin,YANG Aicheng,HUANG Qingchun
ZHOU Yingyan,LIANG Huasheng,YAN Jingyao,HE Xiaohong,PAN Lili,LI Xue,CHEN Xianghong,CHEN Xiumin,HUANG Qingchun,Department of Rheumatology,the Second Affiliated Hospital,Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine),Guangzhou 510006,China
YANG Aicheng,Department of Nephrology,Jiangmen Hospital of Chinese Medicine Affiliated to Jinan University,Jiangmen 529000,China
Abstract OBJECTIVE: To investigate the effectiveness and safety of tripterygium glycosides (TG) tablet (雷公藤多苷片) for the treatment of Lupus nephritis (LN).METHODS: Several databases were systematically searched including PubMed,Embase,Cochrane,Wiley,China National Knowledge Infrastructure Database,SinoMed and Wanfang Library till June 20,2020.Revman5.3 was utilized to analyze the data according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement.RESULTS: In total,8 randomized controlled trials involving 583 participants were identified.Meta-analyses showed that,compared with glucocorticoids (GC) alone,the combination with TG tablet provided a statistically significant improvement in total remission (TR) (RR=1.27,95% CI: 1.08–1.50,P=0.004),complete remission(CR) (RR=1.61,95% CI: 1.05–2.47,P=0.03) and C3 levels (WMD=0.27,95% CI: 0.14–0.39,P < 0.000 1),C4 levels (WMD=0.12,95% CI: 0.07–0.17,P < 0.000 01).No significant differences were seen in TR,CR,proteinuria,serum creatinine,C3 and C4 (TR: RR=1.00,95% CI: 0.87–1.16,P=0.95;CR: RR=1.10,95% CI:0.78–1.56,P=0.58;proteinuria levels: WMD=-0.06,95%CI: -0.13 to 0.01,P=0.10;serum creatinine levels: WMD=-0.01,95%CI: -7.36 to 7.35,P=1.00;C3 levels: WMD=0.01,95%CI: -0.06 to 0.07,P=0.84;C4 levels: WMD=-0.01,95%CI: -0.03 to 0.01,P=0.49) between azathioprine (AZA)/ leflomit (LEF)+GC and TG tablet +GC.Adverse events (hepatic dysfunction,nausea,vomitting) showed no statistical differences between the TG tablet+GC group and the GC group.There were more new onset of irregular menstruation in the TG tablet+GC group than those in the AZA+GC (RR=3.57,95%CI: 1.40–9.11,P=0.008)/LEF+GC (RR=6.69,95% CI:2.42-18.46,P=0.000 2) group,but leucopenia lower than those in AZA+GC group (RR=0.38,95% CI: 0.17-0.85,P=0.02) and alopecia (RR=0.14,95% CI: 0.03-0.77,P=0.02) and rash (RR=0.09,95% CI: 0.01-0.69,P=0.02)lower than those in LEF+GC group.Conclusions:This review indicates that TG tablet maybe effective in LN treatment.Nevertheless,adverse events cannot be ignored.Large sample,multi-center,highquality clinical studies are needed to verify the exact effects and safety of TG tablet in treatment of LN.
Keywords: tripterygium glycosides;lupus nephritis;treatment outcome;safety;systematic review;Meta-analysis
1.INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disorder characterised by a wide spectrum of clinical manifestations.1Lupus nephritis(LN),one of the most serious and common complications of SLE,occurs in up to 60% of adults with SLE in China,2and up to 30% of LN patients progress to endstage renal disease at 5-year post-diagnosis.3The “gold standard” treatment for LN includes cyclophosphamide(CYC),mycophenolate mofetil (MMF) as well as azathioprine (AZA) and glucocorticoids (GC).4Leflomit(LEF) and other immunosuppressive agents are also applicated in LN patients.5However,frequent adverse events,such as infections and ovarian failure,and the lack of efficacy in some LN patients restrict the application of the current treatments,alternative treatments are still needed.
Leigongteng (Radix et Rhizoma Tripterygii) (RRT),a vine-like plant that grows in southern China,has been used as Chinese herbal Medicine for over 2 000 years.6There are approximately 380 metabolites identified from extracts of RRT.Triptolide,tripdiolide and triptonide are the principal ingredients for its immune regulatory and anti-inflammatory activities.7-9
Tripterygium glycosides (TG) in tablet form are extracted from RRT by column chromatography,with triptolide and tripdiolide as its main components,has been widely used to treat inflammatory and autoimmune diseases in Traditional Chinese Medicine,including rheumatoid arthritis,lupus erythematosus,ankylosing spondylitis and have aroused wide concern around the world since the end of the 20th century.10-13Trials in autoimmune and inflammatory diseases were carried out continuously,and rediscoveries of the anti-inflammatory and cytotoxic activities of TG were reported.14TG was found to inhibit antigen-and mitogen-stimulated proliferation of T cells and B cells,interleukin-2 production by T cells,and immunoglobulin by B cells,respectively.15,16
These results form a basis on which to plan future studies whether TG could have a more prominent role in the management of LN.This systematic review and Metaanalysis aims to evaluate the evidence for TG tablet (雷公藤多苷片) use in the management of LN.
2.METHODS
We conducted and reported this review according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).17
2.1.Data sources and search terms
The search strategy was designed to identify the full length of studies reporting outcomes of TG tablet treatment in LN patients.Two independent reviewers searched the following Chinese databases: China National Knowledge Infrastructure Database,China Science and Technology Journal Database,Chinese Biomedical Literature Database and Wanfang Database,and also the PubMed,Embase,and Cochrane Library databases.All of the databases were searched to identify all relevant human clinical studies published until June 20,2020.The Chinese databases were searched using the terms “lei gong teng” (which means RRT in Chinese) and“lang chuang xing shen yan” (which means lupus nephritis in Chinese) and “sui ji dui zhao shi yan” (which means RCT in Chinese).The English databases were searched using title/abstract “Tripterygium” and “Lupus Nephritis”.No language restrictions were applied.Reference list of retrieved articles and additional trials in review articles were sought.Authors of papers were contacted for unpublished reports or additional information from published reports.
2.2.Inclusion and exclusion criteria
2.2.1.Inclusion criteria
Types of studies: all prospective randomized controlled trials (RCTs) in which TG tablet was compared either to placebo or to another active therapy in participants with LN were eligible for inclusion.No language was limited.Open-labelled trials were also considered for inclusion.Types of participants: participants with a diagnosis of LN based on the American College of Rheumatology criteria4.
Types of interventions: experimental interventions included TG tablet alone or combined with GC.Control interventions included GC+placebo or another active therapy.
Types of outcome measures: primary outcomes:complete remission (CR),total remission [TR;total CR plus partial remission (PR)].
CR is defined as a normal serum creatinine and albumin and decrease in proteinuria to ≤ 0.3 g per 24 h;PR is defined as a ≤ 25% increase in baseline creatinine,serum albumin ≥ 30 g/L,and ≥ 50% reduction in baseline proteinuria to < 3 g per 24 h.
Secondary outcomes: proteinuria levels,serum complement levels (C3 levels,C4 levels),serum creatinine levels and adverse events (AEs).
2.2.2.Exclusion criteria
Exclusion criteria were: (a) abstracts,case reports,reviews,and editorials;(b) studies with insufficient details;and (c) duplicate reports from the same study.
2.3.Study selection
Two independent investigators were responsible for determining whether the reports were eligible for inclusion in the Meta-analysis.To resolve any inconsistencies,the investigators compared lists after reviewing the identified papers.A third investigator resolved any discrepancies to finalize the list of included studies.Based on the PRISMA requirements,a flow diagram of the study selection has been generated.
2.4.Data extraction and Management
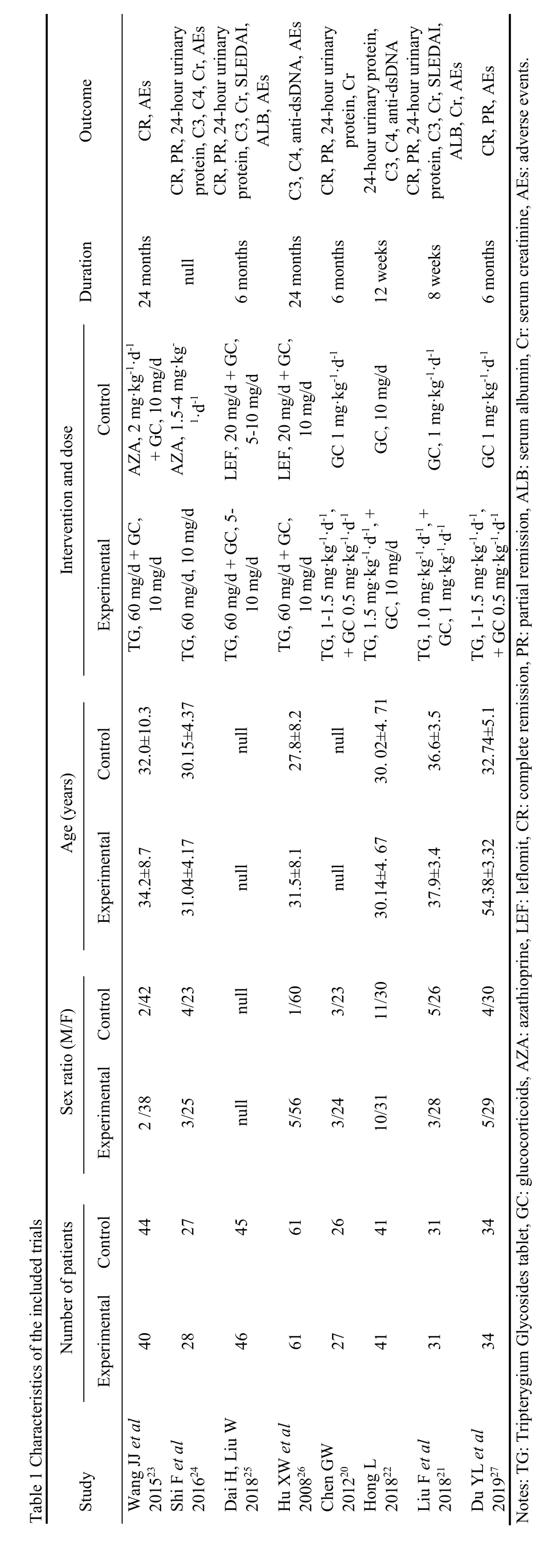
A custom Excel sheet was used to collect all the relevant data on the surname of first author,publication year,patient,intervention,and outcome characteristics.Two investigators extracted the data independently.The results were compared and discussed when there was disagreement.Essential information from each trial was collected: study design,demographic information given(age,gender,and ethnicity),intervention and dosing regimens,concomitant therapy,duration of therapy,and clinical outcomes.All studies were also scored by two independent reviewers in accordance to the Risk of Bias tool of Review Manager (RevMan) (Version 5.3.Copenhagen: The Nordic Cochrane Centre,The Cochrane Collaboration,2014).18
2.5.Statistical analysis
All analyses were conducted by using RevMan (Version 5.3.Copenhagen: The Nordic Cochrane Centre,The Cochrane Collaboration,2014).The heterogeneity among the included investigations was detected usingI 2.The Meta-analysis were carried out using a random effects model ifI 2> 50% or a fixed effects model ifI 2≤50%.19A significance level of 5% was used for all statistical tests.The pooled mean difference (MD) of proteinuria levels,serum complement (C3 and C4) levels,serum creatinine levels and the risk ratio (RR) of TR,CR,AEs were calculated.
3.RESULTS
3.1.Literature search results
Two hundred and fifteen records were identified through database searching and 1 additional record was identified through other sources.After duplicates removed,151 records were left,and 87 records were excluded for the no relevance to the overview.Thus,64 full-text articles were assessed for eligibility.Fifty-six full-text articles were excluded with reasons: non-RCTs (n=30),no date available (n=5),not humans (n=9),the intervention did not meet the inclusion criteria (n=12).According to the selection criteria defined in the methods section,eight RCTs with 583 participants were included for systematic review and Meta-analysis.The process of study selection is shown in Figure 1.The characteristics of the included trials are shown in Table 1.
3.2.Quality of Included Systematic Studies
50% (4/8) of the reports mentioned any specifics about how the randomization was carried out and were rated as low risk of bias.No trails described the allocation concealment,blinding of participants,personnel and outcome assessment.87.5% (7/8) described complete outcome data.One trial was judged being at high risk of attrition bias because of the imbalance in numbers and missing data across intervention groups.For the reporting bias,all trials were judged as unclear risk since the study protocols were not available and we did not have enough information in the study report to assess selective reporting (Figure 2).
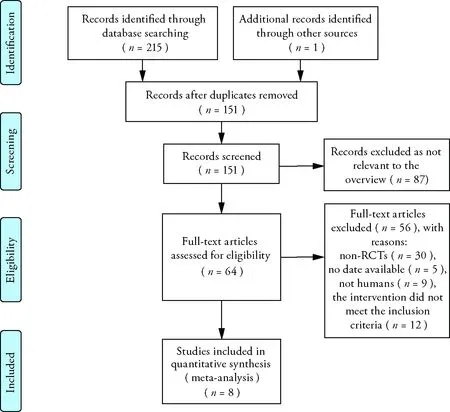
Figure 1 Process of searching for and screening studies
3.3.Effects of Interventions
3.3.1.Study of the therapeutic regimen of TG tablet +GCvsGC
Three studies were included into the Meta-analysis to assess the efficacy of TG tablet in patients with LN.20-22In the therapeutic regimen of TG tablet+GCvsGC,the TG tablet+GC group showed significantly better results in TR (RR=1.26,95%CI: 1.02–1.55,P=0.03),CR (RR=1.61,95%CI: 1.05-2.47,P=0.03)and C3 levels (WMD=0.27,95%CI: 0.14-0.39,P<0.0001),C4 levels (WMD=0.12,95%CI: 0.07-0.17,P< 0.000 01).However,the differences in CR rate,proteinuria and serum creatinine levels were not statistically significant (Figure 3).
3.3.2.Study of the therapeutic regimen of TG tablet +GCvsAZA/LEF+GC
Four studies were included into the Meta-analysis to assess the efficacy of TG tablet in patients with LN with the therapeutic regimen of TG tablet+GCvsAZA/LEF+GC,23-26and the results indicated that the differences in CR,TR,proteinuria levels,serum creatinine levels,C3 and C4 levels between TG tablet+GC and AZA/LEF+GC group were not statistically significant (CR:RR=1.10,95%CI: 0.78-1.56,P=0.58;TR:RR=1.00,95%CI: 0.87-1.16,P=0.95;proteinuria levels:WMD=-0.06,95%CI:-0.13 to 0.01,P=0.10;C3 levels:WMD=0.01,95%CI:-0.06 to 0.07,P=0.84;C4 levels:WMD=-0.01,95%CI:-0.03 to 0.01,P=0.49;serum creatinine levels:WMD=-0.01,95%CI:-7.36 to 7.35,P=1.00;Figure 4).
3.4.Adverse events
Five trials reported adverse events (Table 2).Subgroup analysis was performed according to different therapeutic regimen.21,23-26
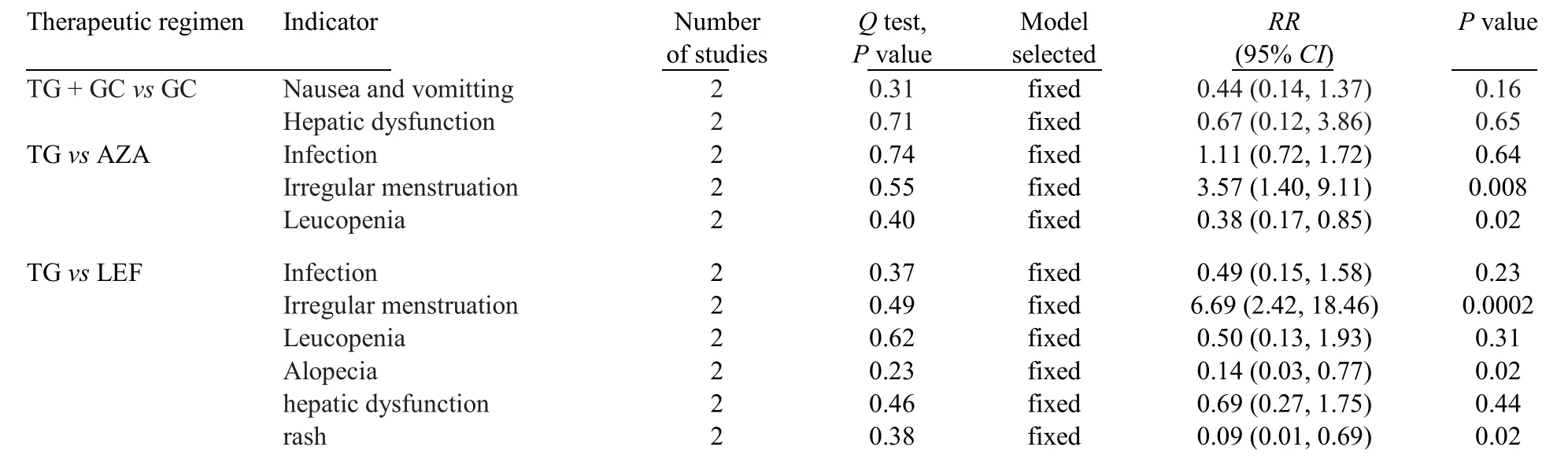
Table 2 Meta-analysis of the safety of TG tablet in treatment of patients with lupus nephritis
3.4.1.Study of the therapeutic regimen of TG tablet +GCvsGC
Two trials reported the safety of TG tablet in patients with LN with the therapeutic regimen of TG tablet +GC vs GC.21,27The incidence of nausea and vomitting(RR=0.44,95%CI: 0.14-1.37,P=0.16;Figure 5)and hepatic dysfunction (RR=0.67,95%CI: 0.12-3.86,P=0.65;Figure 5) showed no statistical differences.
3.4.2.Study of the therapeutic regimen of TG tablet +GCvsAZA+GC
Two trials were included into the Meta-analysis to assess the safety of TG tablet in patients with LN with the therapeutic regimen of TG tablet+GCvsAZA +GC.23,24In this Meta-analysis,the incidence of leucopenia (RR=0.38,95%CI: 0.17-0.85,P=0.02;Figure 6) in TG tablet+GC group were lower than those in AZA+GC group.However,the incidence of irregular menstruation (RR=3.57,95%CI: 1.40–9.11,P=0.008;Figure 6) in TG tablet+GC group were higher than that in AZA+GC group.The incidence of infection in TG tablet+GC group were higher than those in AZA+GC group,although there were no statistical differences (RR=1.11,95%CI: 0.72-1.72,P=0.64;Figure 6).
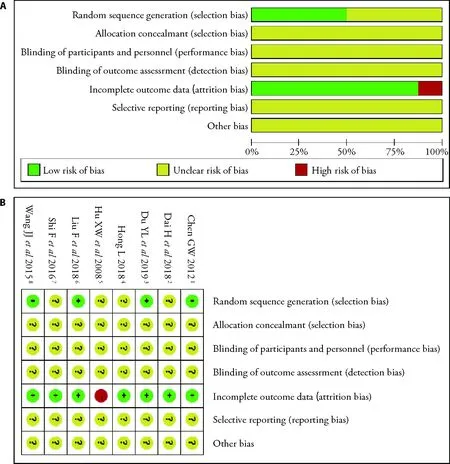
Figure 2 Risk of bias graph and summary
3.5.Study of the therapeutic regimen of TG tablet+GC vs LEF+GC
Two trials were included into the Meta-analysis to assess the safety of TG tablet in patients with LN with the therapeutic regimen of TG tablet+GCvsLEF+GC.25,26
The incidence of alopecia (RR=0.14,95%CI: 0.03-0.77,P=0.02;Figure 7) and rash (RR=0.09,95%CI: 0.01-0.69,P=0.02;Figure 7) in TG tablet+GC group were lower than those in LEF+GC group.However,the incidence of irregular menstruation (RR=6.69,95%CI:2.42-18.46,P=0.0002;Figure 7) in TG tablet+GC group were higher than that in LEF+GC group.The incidence of infection (RR=0.49,95%CI: 0.15-1.58,P=0.23;Figure 7),leucopenia (RR=0.50,95%CI: 0.13-1.93,P=0.31;Figure 7) and hepatic dysfunction (RR=0.69,95%CI: 0.27-1.75,P=0.44;Figure 7) in TG tablet+GC group were lower than those in LEF+GC group,although there were no statistical differences.
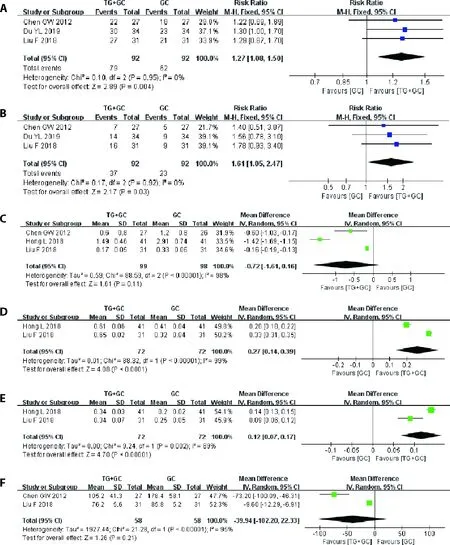
Figure 3 Results of the Meta-analysis of the efficacy of TG tablet combined with GC versus GC

Figure 4 Results of the Meta-analysis of the efficacy of TG tablet combined with GC versus AZA/LEF combined with GC

Figure 5 Results of the Meta-analysis of the safety of TG tablet combined with GC versus GC
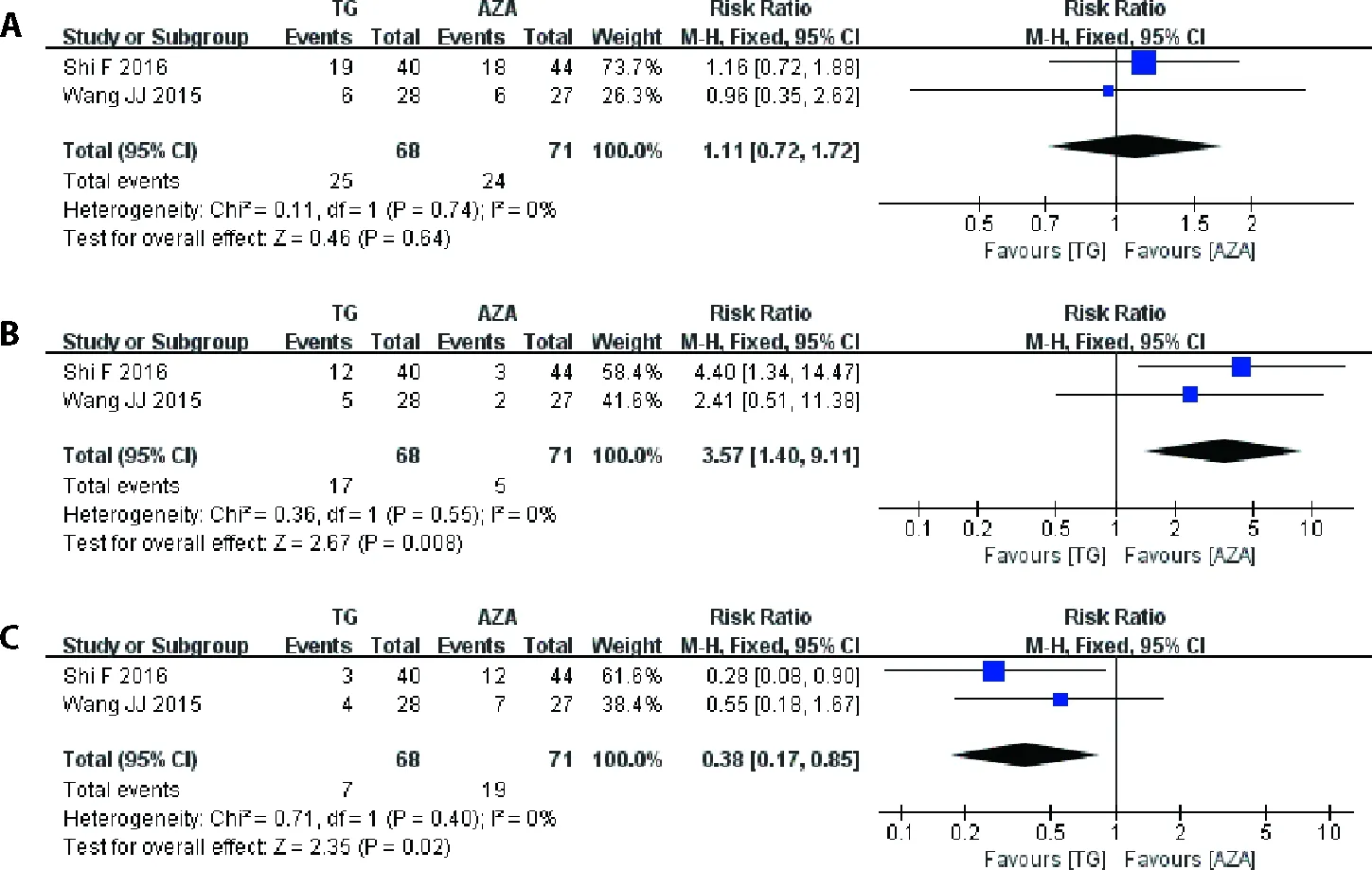
Figure 6 Results of the Meta-analysis of the safety of TG tablet combined with GC versus AZA combined with GC

Figure 7 Results of the Meta-analysis of the safety of TG tablet combined with GC versus LEF combined with GC
4.DISCUSSION
Although some new drugs have been developed in LN treatment,RRT agents has the merits of positive curative effect,abundant resource and relatively lower price in treating LN.Unfortunately,high-quality evidences are still lacking.Meanwhile,there are some concerns about its toxicity.
As we mentioned above,RRT agents are a huge family,TG tablet is a representative drug of RRT agents.This Meta-analysis including 8 trials,evaluated the efficacy and safety of TG tablet in the treatment of LN.Although the quality of these studies were not highly satisfactory,for small sample size,lack of blinding,variable inclusion and remission criteria,short follow up periods and so on,the results showed that,compared to treatment with GC alone,the combination with TG tablet improved both TR,CR and C3,C4;compared to treatment with AZA/LEF +GC,TG tablet+GC showed an equivalent efficacy in TR,CR,proteinuria,serum creatinine,C3 and C4.
Nevertheless,adverse events could not be ignored.In the Meta-analysis for adverse events,the TG tablet+GC treatment showed lower incidence of alopecia and rash or leucopenia,when compared with the LEF+GC treatment or the AZA+GC treatment,respectively.However,it showed higher incidence of irregular menstruation than the AZA+GC and LEF+GC treatment.
The reproductive toxicity of TG tablet is the main reason for the limitation of clinical application.28In this Metaanalysis,the menstrual disorder occurred in as high as 25.1% (44/175).It is even higher than that reported in the previous Meta-analysis of the safety profiles of TG tablet,which showed that the incidence of adverse reproductive outcomes was 11.7% (95%CI10.3%,13.3%).29
Efficiency and side-effects of TG tablet are dosedependent,and the conventional therapeutic dose is 60 mg/d.30The doses of TG tablet in the included trials varies from 1.0 to 1.5 mg·kg-1·d-1.However,subgroup analysis with different doses of TG tablet is not conducted in this study,as number of trails included is insufficient.As known,CYC,in conjunction with GC,has conventionally been used for the initial treatment of LN.31The reproductive toxicity is also a major concern of CYC.32However,no trials included in this study compared the adverse events between TG tablet and CYC.
In addition,some included trials reported infection and hepatic dysfunction after administration of TG tablet.Although these results were not statistically significant,damage to liver function and risk of infection cannot be ignored.Four trials reported a total of 29 patients’infections,whereas most showed slightly infections.Three trials reported eight patients with elevated transaminases.Lipid production and peroxidation in the liver,induced by TG,may be related to this type of adverse event.33The toxicity of TG tablet needs further investigations.There are also some limitations of this systematic review:(a) the quality of the included trials was not very high for inadequate randomization,double-blinding,and allocation concealment and so on;(b) only 7 trials met the inclusion criteria and all of them were conducted in China;(c) the sample size was insufficient to reach a robust conclusion and the very low number of events(several subgroup analysis was included in only one study) on which the results were based was another limitation that can affect the interpretation of results;(d)some dosage of TG tablet was not uniform;(e)definitions of CR/TR/PR were not clearly described in the enrolled studies and might differ across different studies;(f) it is not clear if TG tablet was considered as induction or maintenance therapy in the retrieved studies.Considering the above problems and limitations,more rigorous clinical RCTs are needed to further verify the role of TG tablet in LN patients in the future.
In conclusion,TG tablet plus GC compared with GC alone has additional benefits for LN and shows effects comparable to those of AZA/LEF,which have a good clinical application prospect.However,adverse events should always be noted.Furthermore,the sample size of the included studies is small and significant statistical heterogeneity still existed in the included studies,so it should be further explored and confirmed.Large sample,multi-center,high-quality clinical studies are needed to verify the exact effects and safety of TG tablet in treatment of LN.
猜你喜欢
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Shugan Jieyu capsule (舒肝解郁胶囊) improve sleep and emotional disorder in coronavirus disease 2019 convalescence patients: a randomized,double-blind,placebo-controlled trial
- Application value of Qisexingtai hand diagnostic method in diagnosis of coronary artery disease
- An infrared thermographic analysis of the sensitization acupoints of women with primary dysmenorrhea
- Preliminary single-arm study of brain effects during transcutaneous auricular vagus nerve stimulation treatment of recurrent depression by resting-state functional magnetic resonance imaging
- Effect of treatment with Fufang Huangqi decoction (复方黄杞汤剂)on dose reductions and discontinuation of pyridostigmine bromide tablets,prednisone,and tacrolimus in patients with type I or II myasthenia gravis
- Wenshen Jianpi recipe (温肾健脾方) induced immune reconstruction and redistribution of natural killer cell subsets in immunological nonresponders of human immunodeficiency virus/acquired immune deficiency syndrome: a randomized controlled trial
