Three-dimensional printed carbon-based microbatteries:progress on technologies, materials and applications
2022-10-10HESujiaoZHANGKaiqiangZOUYajunTIANZhihong
HE Su-jiao, ZHANG Kai-qiang, ZOU Ya-jun, TIAN Zhi-hong,*
(1. Henan Vocational College of Applied Technology, Zhengzhou 450042, China;2. Engineering Research Center for Nanomaterials, Henan University, Kaifeng 475004, China;3. Global Energy Interconnection Group Co., Ltd., Beijing 100031, China)
Abstract: Next-generation wearable and portable devices require rechargeable microbatteries to provide energy storage. Threedimensional (3D) printing, with its ability to build geometrically complex 3D structures, enables the manufacture of microbatteries of different sizes and shapes, and with high energy and power densities. Lightweight carbon materials have a great advantage over other porous metals as electrode materials for rechargeable batteries, because of their large specific surface area, superior electrical conductivity and high chemical stability. In recent years, a variety of rechargeable microbatteries of different types have been successfully printed using carbon-based inks. To optimize their electrochemical performance and extend their potential applications, it is important to analyze the design principles with respect to the 3D printing technique, printable carbon materials and promising applications. This paper provides a perspective on recent progress in the four major 3D printing techniques, elaborates on conductive carbon materials in addressing the challenging issues of 3D printed microbatteries, and summarizes their applications in a number of energy storage devices that integrate with wearable electronics. Current challenges and future opportunities for carbon-based microbattery fabrication by 3D printing techniques are discussed.
Key words: 3D printing;Microbattery;Carbon materials;Graphene
1 Introduction
Upon the evolution of the Internet of Things(IoT) concept, the raging demand for miniaturized,customized, flexible, integrable, and lightweight electronics is booming the development of microscale electrochemical energy storage devices[1]. Currently,micro-power sources represented by microbatteries have been attracting attention, as they are endowed with features of micro-sized dimension and high energy density to be compatible with the next-generation electronic systems[2-5]. However, the need to reduce volume and weight while maximizing energy storage capacity challenges the elaboration of techniques to rapidly design and manufacture a battery with a desired structure.
Three-dimensional (3D) printing, also known as additive manufacturing (AM), offers an efficient, flexible and cost-effective approach to quickly fabricate electrodes with high-mass loadings for rechargeable microbatteries[6]. It takes a novel production principle of layered manufacturing, which simplifies the production procedure and makes it possible to realize the scale-up customized production of microbatteries[7].Compared with the batteries fabricated with conventional techniques, 3D printing has precious control over the thickness, porosity, rigidness, and geometrical shape of the electrodes, thus it is able to obtain a desired complex architecture[8]. Moreover, 3D printed batteries normally possess high areal/volumetric energy density due to the maximized mass loading enabled by the well-designed 3D-patterned architecture with optimized internal topologies, and can avoid decrease of specific surface of electrode caused by disorder accumulation at the same time, and deliver high power densities owing to short electron/ion diffusion pathways.
The last decade has witnessed an acceleration of interest in the development of microbatteries using 3D printing methods. Several of the electrode materials that are most common used in rechargeable batteries have been successfully printed: such as metal, metal sulfides[9]and oxides[10-13], polymer and carbon-based materials. In contrast to its metal counterparts, carbon features a versatile nanostructure, excellent chemical stability, as well as unique electrochemical, mechanical, thermal, and electrical characteristics[14-18]. Therefore, carbon-based materials have been widely utilized in 3D printed battery systems as active materials(i.e., functional fillers) or conductive additives[19].
To date, existing reviews have been focused on the advances in 3D printed batteries and other energy storage devices[20-22]. However, carbon-based microbatteries using 3D printing technologies for energy storage has not been well analyzed. To unveil the potentials of carbon materials within this context, this review firstly provides an overview of advanced 3D printing methods of fabricating carbon-based electrodes for microbatteries. The fabricated electrodes are then discussed with respect to the strategies of their architecture design and performance optimization. Furthermore, their applications in electrodes for different lithium ion batteries (LIBs), sodium ion batteries (SIBs), and other rechargeable microbatteries,as well as assembling integrated devices, are comprehensively summarized. The framework of this paper is displayed in Fig. 1.
2 Fundamentals of microbatteries
Small-scale batteries with sizes less than 1 cm3,often called microbatteries, will be the key in the system powering the miniature wearable sensors and medical electronics. Based on the same physical processes as classical LIBs, microbattries are normally composed of two electrodes (positive and negative)separated by an electrolyte layer. Today’s most common rechargeable microbatteries are in the appearance of coin and button cells (thick metallic discs),and pouch cells (core battery material encased by a metallic film). However, the low areal energy density and limited miniaturization of the commercialized microbatteries has become bottlenecks in various electronic applications. In order to store and deliver enough energy for such a small battery, and at the same time being safe under a broad range of conditions, both the materials and the battery design need to be rationally reconsidered.
3D printing has been widely applied to fabricate microbatteries as it provides feasible solutions to the construction of 3D structures with high loading and precision. In general, the assembled microbatteries using 3D printed electrodes can form several configurations, such as interdigitated architecture[19], sandwiched structure[33], or even flexible fibers[34]. The interdigitated electrodes are microelectrodes deposited on a substrate where the working and counter electrodes are in the form of interlaced fingers separated by a small distance on the submillimeter scale as can be seen in the comparison of conventional battery and one example of the 3D printed interdigitated battery in Fig. 2[35]. Rocha et al.[36]printed interdigitated graphene-based electrodes for LIBs using the direct ink writing (DIW) technique. Such configuration could provide high stability and short ion migration distance, and hold promise for electronic applications such as small energy storage devices and all-solidstate batteries[36,37]. The sandwich-type structure is a classic design for cost-effective production of microelectrodes, where each module is placed on a different planes and stacked in a layer-by-layer pattern. The printed microbatteries are normally designed with arbitrary geometries (round, triangle or square)[2,38].However, this architecture in large dimension may limit some miniature applications. The fiber-shape architecture is a unique microbattery configuration,where a cable-like anode core is coated by a tubular cathode, or two electrodes in a wire shape are twisted.For instance, in the all-fiber LIBs fabricated by Wang et al.[39], cathode and anode fibers were intertwisted and coated with a polymer gel electrolyte, enabling good electrolyte penetration. The fiber configuration is promising towards flexible and wearable energy storage devices.
3 3D carbon printing techniques for battery manufacturing
According to the American Society for Testing and Materials (ASTM), the additive manufacturing(3D printing) is normally classified into seven categories based on its technical features: (1) material extrusion (e.g., DIW and fused deposition modeling(FDM)), (2) material jetting (e.g., inkjet printing(IJP)), (3) powder bed fusion (e.g., selective laser sintering (SLS), direct metal laser sintering (DMLS)),(4) vat photopolymerization (e.g., stereolithography(SLA)), (5) binder jetting (BJ), (6) directed energy deposition (DED) and (7) sheet laminating (e.g., laminated object manufacturing (LOM)). Despite the progress on 3D printing for batteries, due to the especial fabrication demand of carbon materials, not all of the additive manufacturing methods are directly applicable to carbon. Therefore, we restrict our discussion to technologies that are suitable to print the currently carbon-based batteries so far, mainly including DIW,FDM, SLS and SLA. Their schematic diagrams and comparison in properties are presented in Fig. 2[40]and Table 1[6,9,19,23,30,34,36,41-51], respectively.
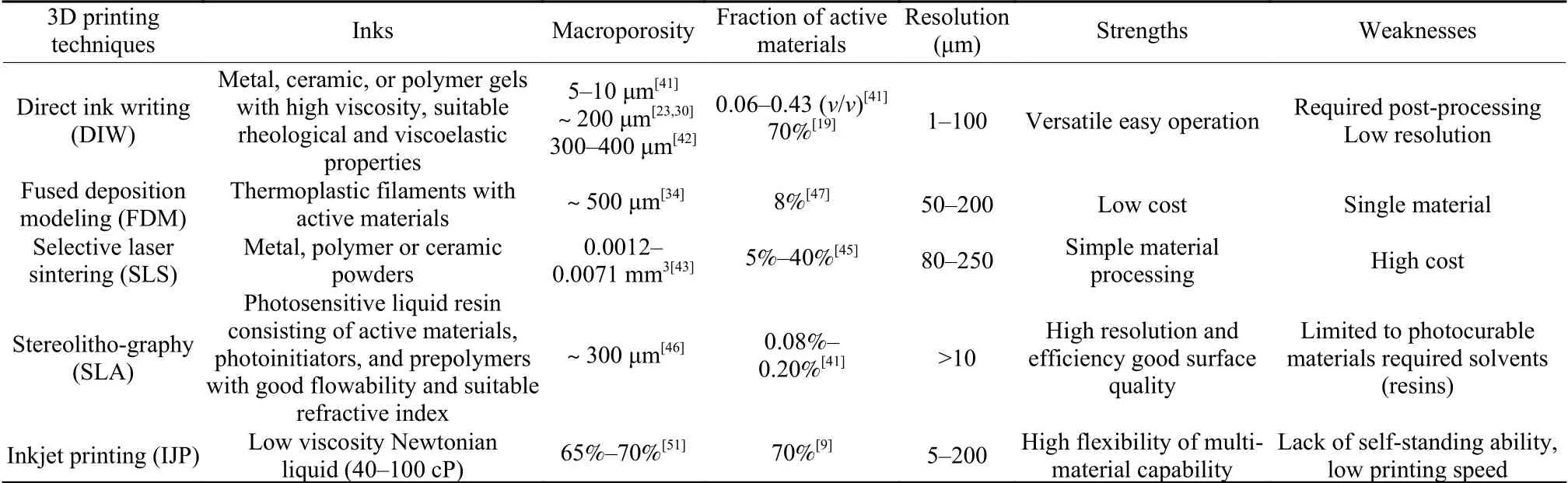
Table 1 A comparison of different 3D-printing techniques.
3.1 DIW
DIW is a typical printing method based on extrusion process, which can develop arbitrary 3D architecture with a layer-by-layer step by spraying the inks through a nozzle. Inks for DIW are usually composed of functional fillers, binders, solvents, and additives[6].Functional materials fundamentally affect the functionality, formability and the rheological properties of the inks. Binders can help to form uniformly dispersed inks. Solvents are usually used to modify viscosity and drying rate by being tuned to a suitable ratio. Additives like surfactants or humectants are effective to modulate the rheological properties or improve the surface tension and printability of the inks[52]. In a DIW printing process (Fig. 3a), the syringe filled with inks can move in 3D to print pre-designed 3D objects layer by layer through extruding filaments from the nozzle. After extruding, physical process like gelation and liquid evaporation or chemical treatments such as surface oxidation are performed to solidify the inks. In some cases, additive needs to be removed after the DIW process, as some insulated additives would inevitably deteriorate electrical conductivity[53]. This 3D printing technique possesses advantages of low cost, excellent versatility and easy availability, but also suffers from flaws like low resolution and unsatisfactory mechanical properties.
DIW technique has been widely used in printing carbon materials such as graphene or (reduced)graphene oxide (GO) into a 3D hierarchical structure.For instance, using an additive-free and aqueousbased DIW method, Lacey et al.[54]fabricated 3D printed holey graphene oxide (hGO) stacked meshes with inherent porosity, lyophilization-induced microscale pores and open mesh macroscale pores, which were favorable to prepare high-performance energy storage devices (Fig. 4a). DIW technique could also be supplemented with post-treatment such as phase conversion to enhance the porosity of printed materials.More recently, Gao et al.[31]reported a self-standing 3D-printed sulfur/carbon composite with a high sulfur loading as a cathode for Li-S battery based on commercial carbon black by DIW (Fig. 4b), where the Li+/e-transport was further improved by the continuous transport channels enabled by the nanopores formed by phase inversion.
3.2 Fused deposition modeling
FDM is one of the most commonly and widely used filament-extrusion 3D printing method[55]. During the printing process (Fig. 3b), thermoplastic material filaments are transferred to an extrusion and heated to a molten state by a resistive heater, which would solidify after being extruded from the extrusion head onto the substrate. Sometimes 2 nozzles are needed to extrude materials by one step or multiple steps to create architectures with both functional and support materials[40]. Therefore, FDM can be used to print individual components or a full microscale electrochemical storage device. The FDM technology is easy to perform, cost-effective, and has a high utilization upon raw materials[56]. Nevertheless, compared with DIW, FDM has a relatively narrow range of printable materials, since conductive active materials have to be incorporated with thermoplastic filaments in a FDM process, such as PLA, polycarbonate (PC),and butadiene-styrene (ABS) for continuous extrusion from the nozzle, which may affect the electrical conductivity of products.
By combining with conductive carbon materials such as carbon black, graphite, carbon nanotubes and graphene, this technology presents a versatile platform to print rechargeable batteries. The conductive carbon content is supposed to be sufficient, or polymers are partially removed by etching or sintering.Maurel et al.[50]fabricated graphite/PLA composite filaments using a mixture of graphite and poly(lactic acid) (PLA) in the dichloromethane solvent. The obtained composite was printed into an anode for LIBs with a graphite loading mass up to 60%-70%. In addition to fabricating Li-ion battery electrodes, the FDM method also shows potential in realizing fully printable microbatteries. In another example, all the battery components, current collectors, anode and cathode layers containing graphite, LTO (Li4Ti5O12), and LFP (LiFePO4), and solid electrolyte were fabricated concurrently with the FDM method, thus enabling free form-factor batteries for LIB application[49]. FDM process produces negligible precursor waste and is considered as an energy-efficient route to develop commercially available 3D-printed EES devices[57].However, its relatively low printing resolution, limited choices of raw materials remain the bottlenecks to be overcome.
3.3 Selective laser sintering
SLS, also known as selective laser melting(SLM), offers a technique based on powder bed fusion which sinters a successive powder layer with a highly energized laser to construct desirable objects,and has been commercialized in printing composite materials such as ceramic, fibers and glass. The schematic diagram of this printing technique is shown in Fig. 3c. The laser beam is used to sinter the selected region where powder material is melted and fused.Afterwards, the build platform is relocated to repeat the above steps, and finally create desired architectures after the un-lased parts are removed. The processes normally do not need solvents or binders, enabling a high proportion of functional materials in the obtained objects and less post-treatment time to remove solvents or binders[58]. It is noteworthy that the laser irradiation could possibly cause structural defects within the printed materials and manufacture electrode micro-architectures, which presents rational design strategies for high-mass loading electrodes.These merits make the SLS printing a promising technology for the precise manufacture of exquisite structures for batteries.
SLS printing is used for metals and alloys with 3D structural requirements[59]and has been recently employed to fabricate carbon-based materials for energy-storage purpose. Iwabuchi et al.[60]fabricated silicon/carbon composite nanofibers as the anode for LIBs using SLS printing. It was demonstrated that the porosity of the film surface and the crystallinity of silicon were easily tunable by adjusting the scan speed of the laser beam. Another critical variable for control of the laser irradiation process of SLS printing is the laser duty cycle. Sha et al.[61]developed a SLS-based template method to construct 3D-printed graphene foams (GFs) (Fig. 4c). It was found that higher duty cycles reduced the amount of unreacted sucrose and contributed to higher graphene quality. The obtained freestanding 3D GFs possessed a high porosity of about ~99.3%, a low density of ~0.015 g·cm-3and an electrical conductivity of ~8.7 S·cm-1. Although the SLS technique presents capability to construct complex 3D structures, some disadvantages like high cost and inherent surface roughness still remain to be tackled.
3.4 Stereolithography
SLA is a photopolymerization printing process that works with ultraviolet (UV) light scanning to solidify photocurable resin (Fig. 3d). During the process,the resin composed of photoinitiators, liquid monomers, and oligomers solidifies upon UV light irradiation to form a single layer in a pre-programmed shape. The operating platform moves downward over a layer to allow the blade to re-coat the top surface of resin tank. This procedure is repeated until the 3D object is completed. The obtained object then undergoes pyrolysis at high temperature in an inert environment,or washing with suitable solvents to remove the polymer resin before post-processing. Wang et al.[62]yielded hierarchical porous 3D carbon electrode arrays as electrodes for Li-ion microbatteries (Fig. 4d), with the SLA technique coupled carbon micro-electromechanical system (C-MEMS) which was able to carbonize the 3D objects printed by SLA. The as-obtained electrodes possessed carbon fiber posts with high aspect ratio (>10∶1) by pyrolyzing SU-8 negative photoresist with a one-step procedure. Although SLA exhibits a relatively low multi-material flexibility on account of its operating principles, compared with extrusionbased technology such as FD), SLA is able to obtain high resolution smooth surfaces, and has better controllability over cell structures and morphologies, because the printing process is not restricted by extrusion. Moreover, the technology is able to print solidstate or gel electrolytes directly on electrodes of rechargeable batteries[63], which provides chances to simplify the fabrication process and enhance the safety for energy storage devices.
3.5 Inkjet printing (IJP)
Besides the above-mentioned 3D printing methods, inkjet printing (IJP) has also been explored for printing conductive carbon materials such as carbon nanotubes[64,65]and graphene[9]. Based on the material jetting method (Fig. 3e), IJP can propel droplets of ink through a nozzle onto various substrates to fast and cost-efficiently print functional materials. IJP presents a high flexibility of materials and can directly print active materials for battery electrodes, and thus a relatively high potential for practical applications. Similar to DIW, IJP has specific requirements for inks in terms of density, surface tension, and dynamic viscosity, but requires a lower viscosity (40-100 cP) to ensure both flowability and jettability. As a result, most of the printed electrodes tend to be in 2D patterns,leading to a low self-standing ability. IJP may be used to print thin-film electrodes or deposit electrolyte into solid electrolyte frameworks with a porous structure.
4 3D printed carbon materials for microbatteries
Carbon materials are an important class of conductive materials which have been extensively studied and widely applied in the electrochemistry field[66-69]. With the growing interest of 3D printing technology, many efforts have been devoted to the development of 3D printed carbon-based microbatteries using graphite, graphene, graphene oxide (GO), reduced graphene oxide (rGO), carbon fibers (CFs), carbon nanotubes (CNTs), and carbon black. Due to their superior electrical conductivity, carbon materials are normally utilized as conductive fillers or composited with polymer matrix[7]. Moreover, the high Li+intercalation ability of many carbon forms can also be conducive to LIB fabrication[70-72].
Traditional electrodes in batteries are facing challenges related to achieving high areametric loading and satisfactory electrochemical properties such as high power and energy density[33]. The functional requirements of batteries have motivated the design of carbon materials with desirable structure and porosity through selecting proper carbon precursors, applying viable 3D printing techniques, as well as implementing novel treatment procedures for activation and functionalization. It is thus necessary to understand the relationships among the characteristics of varied carbonaceous materials, architecture building strategies, and 3D printing techniques, in order to better realize the great potentials of carbon materials in enabling high-performance batteries.
4.1 Graphite-based 3D printed microbatteries
Graphite has been recognized as a promising anode materials for rechargeable batteries due to its low cost, high conductivity and good cycling reversibility[25,73], and is the first anode material used for the commercialization of LIBs. There has been a particular focus upon the utilization of 3D printing techniques to fabricate graphite-based electrodes for microbattery applications. FDM has been employed for development of graphite composite filaments[33]. Nevertheless, due to the requirements of the printing procedure, active material mass loading is often relatively low, thus inhibiting the electrochemical performance[74]. To gain enhanced electrochemical behavior, a 3D printed graphite/PLA filament was reported as the negative electrode in a LIB with a conventional FDM 3D printer (Fig. 5a)[50]. The content of active material (graphite) within the filament was increased up to 49.2% (corresponding to 773 mg cm-3),while the mechanical strength was preserved due to the poly(ethylene glycol) dimethyl ether added as a plasticizer. The active material and the total composite displayed 200 and 99 mAh·g-1reversible capacity,respectively, at a current density of 18.6 mA·g-1.
4.2 Graphene-based 3D printed microbatteries
Graphene, an individual layer of graphite, is a two-dimensional and atomically thin carbon material that offers a series of special properties, such as high electron mobility, large specific surface area, high intrinsic specific capacitance, good mechanical strength,and good chemical stability, which make graphene an attractive candidate for fabricating 3D-printed microbatteries[75]. DIW and FDM are the most adopted AM techniques to construct graphene-based composites, followed by SLA and SLS. A pioneering work by Foster et al.[47]demonstrated that a commercial graphene-based polylactic acid (PLA) filament could be printed into 1 mm-thick 3D disc electrodes by FDM technique without further modification/ex-situcuring step (Fig. 5b). The discs were used as freestanding anodes that did not require a current collector,and could be easily slotted into various coin cell configuration (Fig. 5c). However, owing to the low fraction of active material (only 8% graphene) within the filament ink, a relatively low discharge specific capacity of 15.8 mAh·g-1for active material and 1.26 mAh·g-1for the entire composite were observed at a current density of 10 mA·g-1. The results of coulombic efficiency and rate capability of the 3D printed anode are shown in Fig. 5d and 5e, respectively.Following this investigation, Foster et al.[48]reported that the electrochemical performance of the 3D printed graphene/PLA electrode could be enhanced through increasing the graphene content up to 20% as well as using chemical pre-treatment to improve the porosity of graphene. The specific capacity of the freestanding 3D printed anodes for the LIB was ca.500 mAh·g-1at 40 mA·g-1.
Thickness and geometry control of the printed material is critical in tuning the loading mass of active material and optimize the capacity of the electrode. Therefore, modulating the structural configuration of thick electrodes at macro or micro scales has become important strategy to improve power density.Vernardou et al.[76]described a micro/mili-meter scale FDM-type printing approach to obtain size-controllable graphene pyramids with a 5 mm height as electrodes for LIBs. Compared with the flat printed graphene, the 3D printed graphene delivered an enhanced specific discharge capacity of 265 mAh·g-1and a good retention for 1 000 cycles.
Obtaining a suspension well dispersed with graphene remains a challenge due to the strong interlayer Van der Waals interaction[77]. As a consequence,polymeric binders or surfactants are inevitably incorporated into graphene, which may prevent graphene sheets from restacking and expose the active sites on the surface of the electrodes and affect electrical conductivity. Rocha et al. attempted to address the challenge by formulating the colloidal inks of chemically modified graphene (CMG) as the active material,coupled with metallic particles (Cu) as a current collector, which had matchable flow behavior and viscoelasticity, to print a self-standing and binder-free electrode for potential LIB application based on DIW technique (Fig. 6a-d). This work provided a design strategy for inks containing graphene and metal particles with the optimized rheological behavior to fabricate 3D printed multicomponent EES devices.
4.3 Graphene oxide-based 3D printed microbatteries
Graphene oxide (GO) is a form of graphene functionalized with oxygen-containing groups (e.g., hydroxyl, epoxide, carboxyl and carbonyl groups), and could be transformed to a reduced form denoted as rGO with enhanced conductivity up to 104S·cm-1[78].Compared with graphene, GO has oxygen moieties on its surface and can easily form stable colloids or dispersions in polar solvents, which endow 3D printing inks with suitable rheological properties for extrusiontype techniques in the absence of polymer binders. In addition to the controllable rheological behavior, GObased inks also offer advantages over functional performance, where rGO has a high electrical double layer capacitance, meanwhile its residual oxygen moieties contribute to pseudo-capacitance, thus leading to an overall high areal specific capacitance of the printed electrodes[79]. Moreover, GO is able to support sufficient loading of active materials because of its sizeable thin-flake dimension[80]. Therefore, there has been a progression towards the design and fabrication of GO-based microbatteries by the utilization of 3D printing.
Electrical conductivity is significant to the electrochemical performance of batteries[81,82]. GO is a good candidate to offer high electrical conductivity for 3D printable electrode inks. The first work on 3D printed GO for LIB application was reported by Fu et al.[19], who fabricated a Li-ion microbattery with electrodes in an interdigitated configuration using extrusion-based 3D printing, where GO-based electrode inks were obtained by coupling highly concentrated GO (80 mg·mL-1) with anode (Li4Ti5O12, LTO) and cathode (LiFePO4, LFP) active materials (Fig. 6e and 6f). The 3D-printed cathode and anode delivered specific capacities of ~160 mAh·g-1and ~170 mA·g-1at a current density of 10 mA g-1, respectively (Fig. 6g and 6h). The added GO played multiple roles in enhancing the electrochemical performance of the printed electrodes. Besides providing a good electrical conductivity, GO improved the printable ability of the inks by tuning the viscoelastic and rheological properties. Moreover, GO could bind the active materials and the electrolyte while maintaining inherent porosity to ensure sufficient surface area without extra binders.
To make the best of the macroscopic functions of GO materials in energy storage devices, an approach is to realize large-scale 3D assembly of GO-based building blocks with AM techniques. Due to its inherent amphiphilic feature, the self-assembly of GO could create various nanostructures with complex morphologies. 3D printed GO has been utilized to obtain various structures such as aerogel microlattices[83]and interconnected networks[61]. In the work by Lacey et al.[54], a printable hGO ink with shear-thinning behavior was extruded into a complex 3D architecture of stacked mesh. The hGO mesh cathode exhibited multiple-level porosity ranging from nanoscale to macroscale, which provided sufficient diffusion tunnels for oxygen and electrolyte to further improve Li-O2battery performance. GO framework can also serve as continuous highly conductive backbone in printed hybrid aerogels to support other active materials. Brown et al.[9]fabricated a 3D MoS2/rGO hybrid aerogel electrode composed of MoS2nanoparticles anchored on conductive rGO network in a macroporous framework structure (Fig. 7a-7g). It was demonstrated that the rGO backbone could increase the electrical conductivity as well as mechanical strength of this 3D hybrid aerogel, while its macropores (3-5 μm) were able to promote fast electron/ion transfer. The electrochemical energy storage ability of the hybrid aerogel was shown as an efficient anode for Na-ion batteries,with an initial specific capacity over 429 mAh·g-1(C/3.3) in 2.5-0.10 V.
4.4 Carbon nanotube-based 3D printed microbatteries
Carbon nanotubes (CNTs) are promising 3D printable electrodes as well as current collectors, because of their large specific surface area, high charge carrier mobility, and good mechanical strength that can be functionalized for enhanced electrochemical energy storage. However, the relatively high cost of CNTs limits the wide application of CNT-based carbon materials in the manufacturing of 3D printed microbatteries. To realize good dispersion of CNTs in solvents commonly used in 3D printing, polymers or surfactants are often required. Alternatively, chemical modification of CNTs can be performed, such as carboxylation, which increases hydrophilicity and enables an aqueous dispersion of CNTs[84]. This offers a cost-effective, easily handling, as well as environmentally friendly method for CNT processing for inkjet printing.
Conventional slurry-cast electrodes could hardly meet requirements of both high power and energy density, due to the electron-transfer pathways and iondiffusion distance. An ideal electrode architecture is a 3D interpenetrating network with adjustable porosity and high specific surface area to realize shorter electron and ion diffusion distances. 3D-structured electrodes based on CNT interconnected frameworks have great potential for high-areal-energy and high-powerdensity microbatteries. For instance, Wang et al.[34]reported the design of a 3D patterned electrode composed of interconnected LFP active material and multiwalled CNT conductive network based on an optimized extrusion 3D printing method. The 3D microstructures facilitated the penetration of the electrolyte into the active materials and the ion/electron diffusion.The electrode filament possessed a shorter Li-ion diffusion distance (< 150 μm) compared with that of the conventional electrode obtained with a slurry coating method. As a result, the printed electrode achieved high areal energy density over slurry-cast electrodes in the application of Li-ion microbattery.
Wearable energy storage devices with high breathability and stretching ability can be developed by fiber electrode design. Although prior works have demonstrated the successful fabrication of various 3D microbatteries using 3D printing, it remains challenging to achieve fiber-shape energy storage devices with twisted or coaxial structures, good flexibility and mechanical strength, as well as satisfactory electrochemical performance. Wang et al.[39]fabricated fibershape electrodes with inks composed of LFP/LTO nanoparticles as active materials, CNTs as a conductive additive, and PVDF as a binder using 3D extrusion-based printing technology (Fig. 7h and 7i). It was demonstrated that the abundant porosity of the PVDF coating layer could promote the absorption of liquid electrolyte, thereby contributing to the fast ion diffusion. Moreover, the interconnected conductive CNTs could effectively enhance the transport of electrons.An all-fiber quasi-solid-state Li-ion microbattery was fabricated by twisting the as-prepared LFP/LTO fibers with a gel polymer electrolyte, which exhibited a specific capacity of 110 mAh·g-1at a current density of 50 mA·g-1. The energy storage device with a twisted fiber structure holds large promise of being incorporated into a commercial textile for wearable electronic applications.
4.5 Carbon fiber-based 3D printed microbatteries
Carbon fiber (CFs), when graphitized to graphite fibers, are composed of thin, strong crystalline filaments of graphite carbon. CFs are typically lightweight, high in tensile strength, temperature tolerant,and have extraordinary electrical and thermal conductivity[85]. Owing to these merits, CF has been utilized to directly enhance the electrical and mechanical performance of 3D printing products[86,87]. In the study by Praveen et al.[32], vapour grown carbon fibers(VGCFs) were incorporated in electrode inks as the conductive reinforcing material for a cathode active material (LiNi0.8Co0.15Al0.05O2). Meanwhile, VGCF possessed a coiled and stacked arrangement of graphene nanosheets, which made it a suitable anode material for LIBs (Fig. 7k). The fabricated fullcell assemblies delivered a discharge capacity of 191 mAh·g-1(0.1 C) and achieved good flexibility along with endurance to bending, providing opportunities for developing LIB devices with reliable electrochemical performance for wearable micro-electronics in flexible device applications.
4.6 Carbon black-based 3D printed microbatteries
Carbon black (CB) is a widely used carbon material that is cost-effective, lightweight, easily handling, and high in electric conductivity. It is generally used as a conductive filler in various 3D printing methods (extrusion-based DIW and FDM, inkjet printing, SLS) in the form of polymer conductive composites for fabricating rechargeable battery electrodes[88,89]. Hong et al.[90]developed a conductive CB/polyamide (PA) matrix composite, which was suitable for SLS printing technique. Results revealed that the electrical conductivity of the printed composites grew from 2.86 × 10-10to 2.05 × 10-4S·m-1when the CB content varied from 0 to 2%, indicating the CB had a great advantage in enhancing the electrical properties of polymer composites. However, excessive filling amount may make the processing of the composites more complex and affect their mechanical properties[7]. CB was further utilized to create percolating networks among different components. Recently, an Ag-Ga microbattery was developed[91], with electrodes consisted of a 3D printable gallium-CB-SIS(Ga-CB-SIS) as an anode and a Ag2O-styrene-isoprene block copolymers (SIS) as a cathode. The combination of Ga and SIS did not form conductive composites. Thus, the CB was added to enable a conductive electrode (CB:Ga ratio was 7.5). The Ag-Ga battery displayed a areal capacity of ~19.4 mAh·cm-2,and a superior stretch ability (larger than 130% maximum strain).
4.7 Low-dimensional carbon (carbon nanopaticle)-based 3D printed microbatteries
The choice of 3D printed carbon filament is mainly focused upon 1D or 2D conductive fillers,e.g.,CNTs, CFs, and graphene, largely because they possess relatively large specific surface area, superior conductivity, and can be easily fabricated into a conductive framework structure with a relatively low percolation threshold. In contrast, low-dimensional carbon-based filaments remain less explored considering that they intrinsically have lower conductivity and higher mass loading is needed to build sufficient electron pathways in the 3D printed electrodes[92]. To overcome these challenges, conductive polymer can be coated on the surface to serve as a support to keep the structural integrity, and as a conductive coating layer to boost the kinetics of electron transport. Gao et al.[29]fabricated 3D nanocarbon frameworks modified with a conductive polymer by a FDM technique. It was found that a slight deposition of conductive poly(ortho-phenylenediamine) (PoPD) noticeably improved the electrochemical activity of an electrode in the example of Li-ion cathode through bridging carbon particles to form a conductive network. This work offers a pathway to develop active 3D-printed electrodes especially with low-dimensional and cost-efficient carbon materials in the applications of aqueous rechargeable Li-ion microbatteries.
5 Applications of 3D printed carbonbased microbatteries
To date, several types of the most commonly used rechargeable batteries have been successfully printed with AM techniques based on carbon materials, which mainly include LIBs, and new beyond LIBs such as Na-ion, and solid-state-metal Li-S, Li-O2, Li-CO2, Na-O2, Zn-air systems and so on. Previous section has provided thorough discussion on the current progress of 3D printed carbon-based electrode materials with a majority of examples in Li-ion microbatteries. This section, according to the existing studies,elaborates on the application scopes of 3D printing for rechargeable microbatteries, which are classified into three major subsections at the component level, including metal ion, metal-sulfur and metal-O2/CO2microbatteries.
Furthermore, examples are given with regard to the integration of the 3D printed batteries in micro electronic devices. Table 2 summarizes and compares the electrochemical performance over a variety of representative 3D-printed carbon-based microbatteries.
5.1 Metal ion microbatteries
As seen from Table 2, the most commonly printed carbon-based microbatteries are alkali-ion batteries, represented by LIBs. Carbon offers high electronic conductivity as well as mechanical property to facilitate electron transport, and is expected to protect the Li-ion from the dendritic formation during the recharging process. A variety of carbon-based materials including graphite, GO, rGO and CNTs have been explored as active materials or conductive additives in printable anodes for 3D printed metal-ion battery systems, such as Si/graphite[93], highly loaded graphitepolylactic acid (PLA) composite[50], GO/lithium titanium oxide (Li4Ti5O12, LTO)[19], fiber-shaped LTO/CNTs[39]for LIBs, and hybrid MoS2/rGO aerogel[9],rGO/Na3V2(PO4)3hierarchical porous frameworks[12]for NIBs. The working principle of a metal-ion battery cathode is similar to that of an anode, but different in the metal ion flux direction during charge/discharge cycling. 3D printed carbon-based nanostructured composites as cathode materials for LIBs have also been reported in the literature,e.g., LFP/carbon black[33], rGO-Ag nanowire-LTO[94]gel-based thick electrode, LFP/GO[19], PoPD/nanocarbon/LMO[29],LFP/CNTs[34]and LiNi0.8Co0.15Al0.05O2/carbon fiber[32].
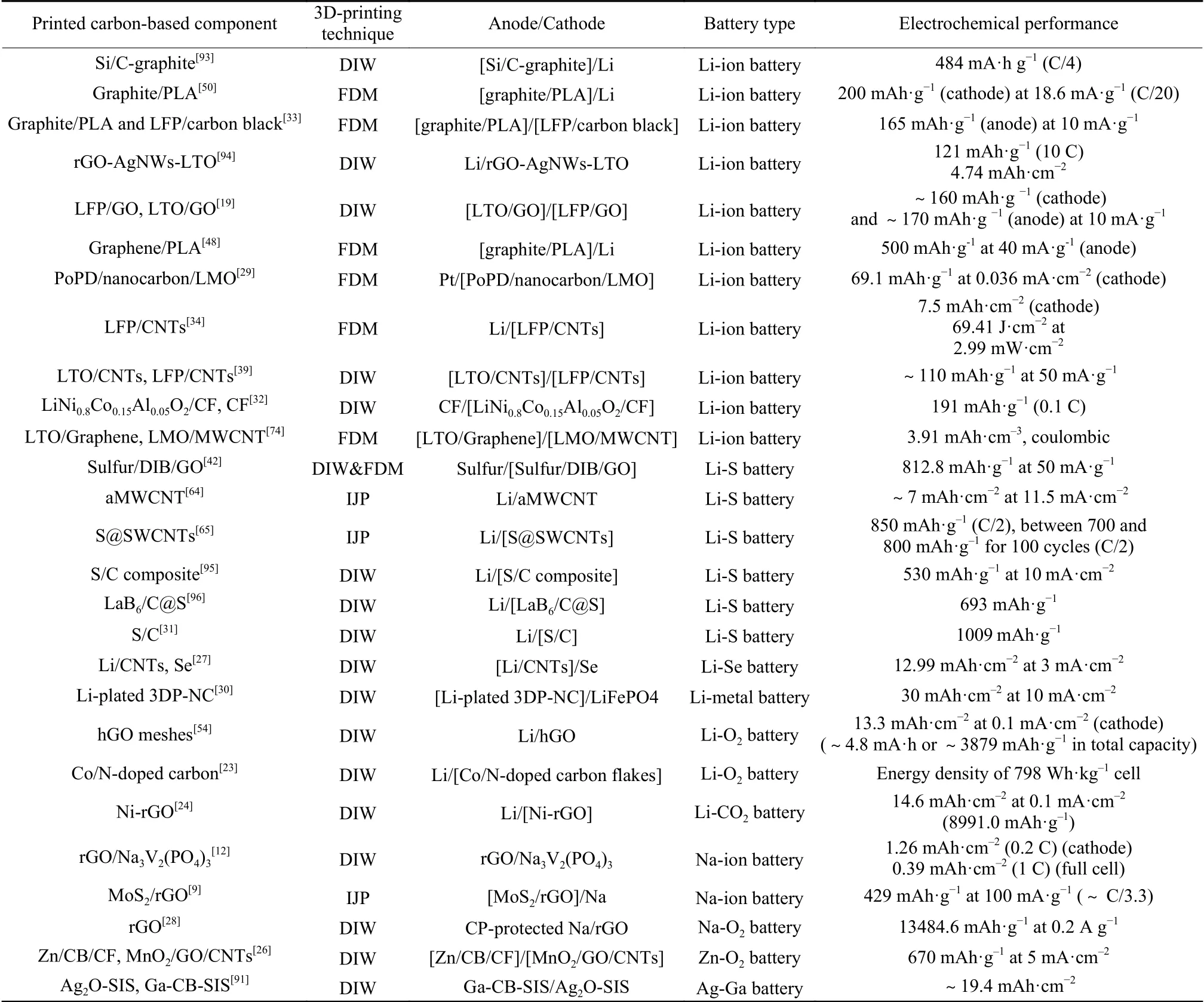
Table 2 Electrochemical performance over a series of 3D printed carbon-based microbatteries.
5.2 Metal-sulfur/selenium microbatteries
Compared to alkali ion batteries, lithium-sulfur(Li-S) battery shows a higher theoretical capacity(1 675 mA·h·g-1) and holds promises of powering the next generation electronic devices[97]. However, the conventional sulfur cathode suffers from dissolving during charge and discharge, which causes a debilitating shuttle effect that inhibits Coulombic efficiency in Li-S systems.
Cathodes based on 3D printed carbon materials have shown improved Li-S performance by providing a conductive matrix to sequester sulfur. Early in 2016,Milroy et al.[64]printed an aligned MWCNT cathode by IJP for high areal capacity Li-S microbatteries.Following this investigation, the same group reported an integrated collector/active-material Li-S cathode with sulfur infiltrated within single-walled CNTs(S@SWCNT) to achieve a stable capacity[65]. More recently, a 3D sulfur copolymer-graphene architecture with a periodic microlattice was developed for Li-S batteries with extrusion-based 3D printing[42]. Nevertheless, due to a low conductivity of sulfur copolymer,the as-designed Li-S battery showed a limited initial capacity and insufficient rate performance. Afterwards, Gao et al.[31]printed a high sulfur loading cathode incorporated with CB and CNTs, resulting in a large initial specific discharge capacity of 1 009 mAh·g-1and a cycling stability of 87% after 200 cycles. Compared with S, selenium (Se) shows an electronic conductivity of 1×10-5S·m-1, which is 1022higher than that of S, endowing Li-Se batteries with more favorable electrochemical kinetics. Gao et al.[27]utilized a 3D-printed CNT interlayer to protect the Li anode to achieve a high energy/power-density Li-Se microbattery, which delivered an areal capacity of 12.99 mAh·cm-2at 3 mA·cm-2.
5.3 Metal-O2/CO2 microbatteries
Li-air (O2) batteries consisting of a Li anode and a porous cathode with catalytic activity can deliver an energy density 5-10 times higher than that of conventional LIBs. An effective approach that reduces the practical specific energy (energy per unit of a fuel) is to use a proper catalyst cathode with a porous framework to support the discharge product (Li2O2). Lacey et al.[54]fabricated a 3D printed holey graphene oxide(denoted as hGO) as a cathode for Li-O2batteries. The architecture is conducive to the access of electrolyte and O2gas and thus enhancing the electrochemical performance of the assembled Li-O2batteries. The assynthesized hGO cathode delivered an areal capacity of 13.3 mAh·cm-2at a current density of 0.1 mA·cm-2.Lyu et al.[23]developed a cobalt-based metal-organic framework (Co-MOF)-derived carbon catalyst with a hierarchically porous network by two-step 3D-printing and thermal annealing procedures, which resulted in an energy density of 798 Wh·kg-1cell.
Compared with Li-O2batteries, where a cathode has less effect on the growth of toroidal Li2O2, the morphologies and compositions of the Na-O2battery discharge products depend largely on the intrinsic properties of the cathode (air electrodes). Therefore,designing a cathode with surface chemistries favorable to the reversible reactions of cubic NaO2formation/decomposition is significant in achieving highperformance Na-O2batteries. A 3D printed rGO mesh architecture with macropores and interconnected conductive network has demonstrated to be a promising cathode for superoxide-based Na-O2batteries[28]. The unique structure of rGO cathode provides sufficient electronic conductivity within the 3D architecture, and facilitates electrolyte permeation and NaO2accommodation. As a result, a remarkable specific capacity of 13 484.6 mAh·g-1(corresponding to 9.1 mAh·cm-2)has been achieved at 0.2 A·g-1. In addition to Li-O2and Na-O2systems, a 3D printed electrode consisted of integrated rGO/CNT/MnO2composites has also exhibited application advantages as cathodes for Zn-O2batteries[26](Fig. 8a), where the highly conductive rGO and CNTs could enhance electron transport and provide diffusion pathways with low resistance for oxygen and active species (Fig. 8b).
Li-CO2batteries are an emerging class of energy storage devices, which are able to contribute to greenhouse gas CO2capture through the corresponding redox reactions between Li and CO2. The recognized reaction process involves CO2reduction with Li2CO3and carbon as the main product (3CO2+ 4Li++ 4e-→2Li2CO3+ C,E= 2.8 V vs. Li+/Li)[98]. However, the high charge overpotential, low recyclability and rate capability, and relatively low energy density on the device level have been the obstacles limiting the application of this technology. To overcome these obstacles, 3D printing technique can be utilized to print thick electrodes with its ability to produce highly ordered and 3D frameworks at a relatively low cost.Moreover, depositing metal/metal compounds (e.g.,metal oxide, metal carbide) on carbon materials has been proved to be an important approach to improve the efficiency of electrodes toward the reversible reaction between Li and CO2. With 3D printing coupled with thermal shock treatment, an ultrathick cathode(~0.4 mm) for Li-CO2battery was fabricated by dopositing Ni nanoparticles (~5 nm) on a 3D printed rGO network[24](Fig. 8c and 8d), which displayed a high areal capacity of 14.6 mAh·cm-2for the initial discharge as well as a stable cycling performance over 100 cycles (Fig. 8e), and good rate capability (up to 1 000 mA g-1) (Fig. 8f), owing to the thick cathode design and a uniform distribution of catalyst nanoparticles.
5.4 Integration of 3D printed batteries in electronic devices
3D printing technology holds promises for simplifying fabrication procedures of microbatteries,achieving high-performance electrochemical activities and obtaining customized architectures compatible with subsequent electronics. The micro energy storage devices such as Li-ion microbatteries have been demonstrated to perform as power units or energy storage units in integrated systems. Fig. 8 shows an example of 3D printed carbon-based Li-ion microbatteries integrated into 3D printed wearable liquid crystal display (LCD) sunglasses[74]. The batteries were assembled with the individually printed anode, cathode and separator (Fig. 9a and 9b), and were connected in series to provide power for the LCD panel, which darkened with voltage applied by a switch(Fig. 9c and 9d). In another case presented by the same group, the printed microbattery demonstrated the ability to power a green LED over 60 s (Fig. 9e and 9f). More recently, Costa et al.[91]reported a fully 3D printed stretchable Ag-Ga battery consisted of gallium/carbon/styrene-isoprene (SIS) (denoted as Ga/C/SIS) as an anode and a Ag2O/SIS as a cathode.The as-designed Ag-Ga batteries were used to power a wearable biomonitoring belt (Fig. 8g), which integrated electrocardiogram (ECG) monitoring, respiration monitoring, as well as a digital temperature sensor. The batteries could provide energy for the belt over 22 h for continuous datum acquisition, and realize a clinical-grade electrocardiogram datum transition by blue tooth at 100 Hz (Fig. 8h). As seen from the above-mentioned examples, there is a tremendous potential to extend the applications of carbon-based 3D printed microbatteries to a broader scope including electronics, sensors, optics, catalysts, desalination,biomedical engineering and environmental pollution remediation.
6 Summary and outlook
Compared with conventional manufacturing methods, 3D printing is playing a significant role in fabricating portable and flexible microscale electrochemical energy storage devices in a facile and repeatable manner. This paper provides a perspective on the recent progress in the area of 3D printing technology for rechargeable microbattery fabrication based on carbon materials from 3 aspects.
(1) The concepts and respective advantages of 4 major 3D printing techniques are briefly discussed.DIW is the most commonly used approach to print carbon-based electrodes but has strict requirements for the rheological behaviors of printing inks. FDW is able to print at a relatively low cost but limited to the extrusion of thermoplastics at elevated temperature,resulting in a narrow range of printable materials. SLS allows rich choices of printing materials, but the low resolution remains a challenge. SLA prints a substrate with high resolution smooth surfaces. The printing speed and manufacturing cost will need to be taken into consideration when one adopts this technique. IJP has an affordable cost and a multi-material printing capability, but also has high requirements for density,surface tension and dynamic viscosity, and lacks suitability for thick electrodes. DIW and IJP hold promise to meet the prerequisites of commercialization, but the compatibility of the 3D printing techniques needs to be improved. As strengths of one technique may cover the weaknesses of another, it is envisioned that a combination of multiple printing methods may be a promising approach to surmount these obstacles.
(2) Conductive carbon materials with attractive properties that enable reliable 3D printed rechargeable microbatteries are introduced. Graphene,GO (or rGO), CNTs, CFs are promising printable carbon materials that can be easily fabricated into highcapacity electrodes as functional fillers or conductive additives. Synthesis methods are modified and optimized to meet both requirements of 3D printing techniques as well as satisfactory electrochemical performance. Strategies within this framework have focused on: (i) regulating the rheological behaviors of 3D printable inks to improve printability, (ii) controlling the printed electrode thickness to optimize energy efficiency, (iii) improving conductivity for rapid charge transfer, (iv) constructing a hierarchical porous framework structure to facilitate electrolyte infiltration and ion/active-species transportation, and (v)maintaining mechanical properties to withstand the charging/discharging fluctuation stress.
(3) Taking advantages of the certain properties of carbon materials, a wide-ranging scope of rechargeable microbatteries by 3D printing is reviewed.The most common types of printed batteries are LIBs.3D printed Li-S, Li-O2, Li-CO2batteries are also fabricated mainly based on graphene and CNTs. Beyond Li-systems, 3D printed Na- and Zn- batteries with GO composites have also displayed functionality and feasibility. Moreover, the integration of the batteries into wearable electronics, such LCD sunglasses and biomonitoring belt, is being carried out to explore broad multifunctional applications.
Despite the remarkable progress made in recent years, the direct applications of 3D printing technology in obtaining high-performance carbon-based microbatteries remains challenging. The main issues to be addressed at present are in the following. First,most of the existing 3D printing techniques fail to control the porosity of the printed material, precisely tailor its morphology, and obtain directly printed pure carbon electrodes with binder-free inks. Additional post-treatment procedures like freeze-drying, hightemperature carbonization and photo-/thermo-curing are normally required. It is noteworthy that some postprocessing may increase manufacturing complexity and cause shrinkage or even distortion of the 3D architectures, thus restricting the printing repeatability,reproducibility and scalability. Therefore, post-processing should be avoided or optimized to be compatible with printed materials in practical manufacturing.Secondly, studies on a fully 3D printed microbattery at a package level are still in an early stage. The main barriers exist in the compatibility of the printing processes for different components, including electrodes,current collectors, separators/electrolytes and packaging materials. Manufacturing processes that enable to print on complex substrates are key for realizing fully 3D microbatteries. In addition, highly integrated batteries with electronic devices such as circuit board can be further explored, which will open new applications areas and functionalities. Overall, the combination of conductive carbon materials and 3D printing technologies provide great opportunities for emerging energy-related applications based on microscale rechargeable batteries. More efforts in future work can be given to design better 3D-printable conducting carbon inks featuring fast drying, suitable viscoelasticity,green solvent and storing stability, and develop highresolution 3D printers to enable more functional energy storage devices.
Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (52003251) and Henan Center for Outstanding Overseas Scientists(GZS2022014).
杂志排行
新型炭材料的其它文章
- Preface
- Guide for Authors
- 《新型炭材料》征稿简则
- 基于界面膜清洗的废旧锂离子电池石墨负极的再生修复
- A high-rate and ultrastable anode for lithium ion capacitors produced by modifying hard carbon with both surface oxidation and intercalation
- The interfacial embedding of halogen-terminated carbon dots produces highly efficient and stable flexible perovskite solar cells
