Recent progress on freestanding carbon electrodes for flexible supercapacitors
2022-10-10ZHAOYirongLIUCongcongLUQiongqiongOMARAhmadPANXiaojunMIKHAILOVADaria
ZHAO Yi-rong, LIU Cong-cong, LU Qiong-qiong, OMAR Ahmad, PAN Xiao-jun,MIKHAILOVA Daria
(1. School of Physical Science and Technology, Lanzhou University, Lanzhou 730000, China;2. Leibniz Institute for Solid State and Materials Research (IFW) Dresden e.V., Helmholtzstr. 20, 01069 Dresden, Germany)
Abstract: The construction of flexible supercapacitors with high electrochemical performance and excellent mechanical properties to power flexible electronics and sensors is very important. Freestanding electrodes play a crucial role in flexible supercapacitors,and carbon has been widely used in this role because of its high electronic conductivity, tunable porosity, adjustable surface area, excellent mechanical properties, low density and easy functionalization. It is also abundant and cheap. Recent progress on the fabrication of freestanding carbon electrodes based on various carbon materials for use in flexible supercapacitors is summarized, and remaining challenges and future opportunities are discussed.
Key words: Carbon materials;Pseudocapacitive materials;Freestanding electrode;Flexible supercapacitors
1 Introduction
The burgeoning implementation of flexible electronics and sensors in everyday life has led to a strong thrust in the development of next-generation flexible energy-storage devices (supercapacitors (SCs) and batteries)[1-5]. Among those, flexible SCs are a promising choice due to their high-power-density and long cycling life[6-10]. However, the practical application of flexible SCs is hindered due to challenges of exploring electrode materials with high electrochemical performance as well as maintaining their high electrochemical performance under large strain. Since the electrochemical performance of SCs rely heavily on the electrode materials, the electrode materials with high electrochemical performance and excellent mechanical property are highly desired for flexible SCs. However, traditional electrode materials suffer from inferior electrochemical performance. In addition, most of the electrode materials are in powder form and additional non-active materials such as binder, conductive additives, and metal current collectors,are required for the fabrication of electrodes. The usage of these non-active materials significantly reduces the energy densities for devices as well as involves significant additional processing costs(Fig. 1a)[11].
SCs store charge via forming an electrical double-layer due to the electrolyte ions adsorption on the electrode surface or pseudocapacitive properties of electrode surface induced by redox reaction(Fig. 1b)[12-13]. Electrical double-layer capacitance of the electrode is determined by its accessible surface area, the electrolyte, and thickness of the double layer(the Debye length), which enables a fast charging/discharge ability and a high cycling stability of the electrode[14]. On the other hand, the pseudocapacitance of the electrode is related to a redox reaction between the electrode materials and electrolyte on the electrode surface. As compared to electrochemical double-layer capacitance, pseudocapacitance possesses a higher energy density, but comes with lower power density and an inferior cycling stability.
Various carbon materials, including active carbon, carbon nanotubes (CNTs), and graphene, have been widely used in energy storage devices especially for electrical double-layer capacitors, due to their high electronic conductivity and large surface area, along with lightweight and low cost[15-23]. The lightweight nature of carbon materials enables a high gravimetric energy density, the large surface area provides more active sites and the porous conductive structure facilitates the electron and ion transport. Furthermore,freestanding carbon electrodes, such as carbon cloth,CNTs film, and graphene film, combined with solidstate electrolytes have demonstrated their success in flexible SCs benefiting from their interconnected conductive structure and excellent mechanical properties[24]. Moreover, the various macroscopic forms of freestanding carbon (yarn, film, foam) make it possible to fabricate diverse configurations of flexible SCs (fiber-shaped SCs, paper-shaped SCs,sponge-like SCs)[25-26]. Unfortunately, despite the advantages of freestanding carbon materials, they normally exhibit a low electrochemical activity owing to small surface area and low capacitance. Micropores(<2 nm) accessible to the electrolyte are essential for charge storage. Mesopores (2-50 nm) working as diffusion channels facilitate the ion diffusion, thus improving the power performance, while macropores(>50 nm) work as reservoirs of electrolyte ions[27]. The wettability toward electrolyte favors electrolyte ions transport, and pseudocapacitance active sites or materials contribute to capacitance. Accordingly, strategies of optimizing pore structure, adjusting surface properties, and integrating with pseudocapacitance materials have been adopted to improve capacitance.
Although many reviews on carbon materials for supercapacitor have been published, only a few reviews focus on freestanding carbon electrodes for flexible supercapacitor[27-35]. In this review, diverse freestanding carbon electrodes based on various carbon materials such as commercial carbon cloth/felt,biomass-derived carbon, polymer-derived carbon,CNTs, graphene, etc. for flexible SCs are summarized(Fig. 2). Additionally, various strategies including activation approaches (wet chemical oxidation, electrochemical oxidation, thermal activation, plasma treatment) and integration routes (heteroatom doping and hybridization with pseudocapacitance materials) for improving the electrochemical performance as well as the structure-performance correlation are discussed in detail. Furthermore, the remaining challenges and future development of these freestanding carbon electrodes for flexible SCs are presented to fasten the development of practical flexible SCs.
2 Freestanding carbon electrodes for flexible SCs
Freestanding carbon materials are promising electrodes for flexible SCs due to their high electronic conductivity, lightweight, tunable porosity, adjustable surface area, low cost, and excellent mechanical properties. Thus, diverse freestanding carbon electrodes, including commercial carbon cloth/felt,biomass-derived carbon, polymer-derived carbon,CNTs, and graphene, have been used and demonstrated success in flexible SCs. To further improve the electrochemical performance, activation approaches(wet chemical oxidation, electrochemical oxidation,thermal activation, plasma treatment, etc.) and integration routes (heteroatom doping and hybridization with pseudo-capacitance materials) have been developed[47].
2.1 Commercial carbon cloth/felt/yarn-based electrode
Commercial carbon fiber cloth has a high potential towards freestanding electrodes for flexible SCs owing to its high electronic conductivity and excellent mechanical properties. Carbon fiber cloth consists of bundle carbon fibers, which are prepared by carbonizing polyacrylonitrile and mesophase pitch[48].However, the pristine carbon fiber cloth suffers from a low surface area, low porosity, and poor electrochemical activity, resulting in a poor electrochemical performance. Various approaches have been proposed to improve electrochemical performance, such as wet chemical oxidation[49], electrochemical oxidation[40],thermal activation[50], plasma modification[51], heteroatom doping[52], and hybridization with pseudo-capacitance materials[53].
To increase the surface area and to introduce electrochemically active groups into carbon cloth, a wet chemical oxidation using a strong acid is commonly applied. Similar to the exfoliation of multiwalled CNTs by modified Hummer’s method, the exfoliation and oxidation of carbon cloth was realized by using a solution consisted of H2SO4, HNO3and KMnO4with high oxidizing properties[49]. The obtained oxidized carbon fiber showed 50 nm thick porous shells with a large amount of mesoporous pores,and a larger surface area of 61.2 m2g-1as compared to untreated carbon fiber (5.3 m2g-1) as well as numerous oxygen-containing functional groups (Fig. 3a-c).Although oxygen functional groups are favored to boost the performance, their excess would decrease electronic conductivity. Therefore, the reduction was further conducted by hydrazine gas and thermal reduction in an NH3atmosphere, and the porous surface morphology of oxidized carbon fiber was maintained after reduction process. As a result, a capacitance of 88 mF cm-2(8.8 mF g-1) at a scan speed of 10 mV s-1was achieved in 1 mol L-1H2SO4electrolyte, while a capacitance of 31 mF cm-2(1.55 mF g-1) at a scan speed of 10 mV s-1and a high rate capability (i.e.,a capacitance retention of 73% with the scan rate from 10 to 10 000 mV s-1) were realized for a flexible symmetric solid-state SCs based on modified carbon fiber and a poly(vinyl alcohol) (PVA)/H2SO4gel electrolyte (Fig. 3d). Because only the exfoliated carbon shell with a thickness of 50 nm works as “active” part,the capacitance of the activated carbon fiber cloth is relatively low. Besides, different reduction methods were developed based on acid-oxidation process using modified Hummer’s method. For example, Jiang et al.[55]adopted a thermally reduction method to treat oxidized carbon fiber at 1 000 °C in a N2/H2atmosphere for 3 h. Consequently, a capacitance of 485 mF cm-2at a current density of 2 mA cm-2is achieved in 1 mol L-1H2SO4electrolyte, and the flexible asymmetric solid-state SC using PVA/H2SO4as electrolyte displayed a capacitance of 161.28 mF cm-2at 12.5 mA cm-2and an exceptional cycling stability for 30 000 cycles. Additionally, hydrothermal approach was also used to reduce the oxidized carbon fiber,while simultaneously the oxygen species were replaced by nitrogen dopants using hydrazine and ammonia[52]. The roles of different nitrogen species in capacitance were investigated by X-ray photoelectron spectroscopy and electrochemical tests. It has been found out that the formation of pyridinic nitrogen species is beneficial for fast electron transfer. Consequently, the obtained N-doped carbon fiber showed a high capacitance of 136 mF cm-2at 0.5 mA cm-2and a capacitance retention of 81% with the current density from 0.5 to 15 mA cm-2in 1 mol L-1H2SO4electrolyte. Furthermore, Miao et al.[56]tailored the oxygen functional groups by adjusting the acid-oxidation treatment time, and investigated the correlation between functional species and capacitive performance. They found out that the -C-O species gradually increased with the increase of treatment time,while the -C=O and -COOH species first increased with the increase of treatment time and reached a maximum value at 12 h. The -C-O and-C=O groups contribute to capacitance due to a reversible redox reaction, while -COOH increases charge transfer resistance leading to a reduced electrochemical performance. As a result, the active carbon fiber with 12 h treatment time displayed the highest areal capacitance of 5 310 mF cm-2at a current density of 5 mA cm-2and a low capacitance decay of 7%after 5 000 cycles in 1 mol L-1H2SO4electrolyte. In addition, solid-state symmetric SCs using PVA/H2SO4as electrolyte showed a high energy density of 4.27 mWh cm-3at a power density of 1.32 W cm-3and excellent mechanical flexibility.
Another common method to activate carbon fiber is electrochemical oxidation, which is more facile and cost-effective[40]. For instance, Wang et al.[40]proposed an electrochemical oxidization in a mixed acid solution. After oxidation in H2SO4/HNO3electrolyte at 3 V for 10 min, core-shell carbon fiber with abundant oxygen-containing groups (-C-OH, -C=O and -COOH) and high surface area of 88.4 m2g-1was obtained (Fig. 3e). Benefiting from abundant electrochemically active oxygen-containing groups and high accessible surface area, an areal capacitance of 756 mF cm-2at 6 mA cm-2was achieved in 5 mol L-1LiCl electrolyte, while a high energy density of 1.5 mW h cm-3and a stable cycling over 70 000 cycles were realized in MnO2@TiN//active carbon fiber flexible asymmetric SCs using 5 mol L-1LiCl as electrolyte at the voltage window of 2 V. More importantly, LED indicators were powered for 10 min by two asymmetric SCs devices, demonstrating the potential for practical use (Fig. 3f). Furthermore, electrochemical activation in a mild neutral electrolyte solution was explored[57]. After oxidation in 0.1 mol L-1(NH4)2SO4aqueous solution at 10 V for 15 min and then reduction in 1.0 mol L-1NH4Cl aqueous solution at -1.2 V for 30 min, the active carbon fiber cloth with micro-crack, exfoliated carbon fiber shells, and abundant oxygen-containing functional groups was obtained. Consequently, a capacitance of 505.5 mF cm-2at 6 mA cm-2and a high capacitance retention of 88% at a scan rate varying from 6 to 48 mA cm-2was achieved in 1 mol L-1H2SO4electrolyte.In addition, other aqueous inorganic salt electrolytes were also explored, such as, KNO3solution[58], and Na2SO4solution[59]. Moreover, electrochemical oxidation combined with the chemical oxidation was found to further boost the performance[60]. The NiOOH decorated carbon fiber was annealed and residual Ni was removed by acid. Subsequently, the carbon fiber was oxidized by electrochemical oxidation, resulting in the formation of hierarchical pores and abundant pseudocapacitive oxygenic groups. As a result, the obtained active carbon fiber cloth exhibited a large areal capacitance of 1.2 F cm-2at 4 mA cm-2and stable cycling for 25 000 cycles in 5 mol L-1LiCl electrolyte. Remarkably, an exceptional energy density of 4.7 mWh cm-3was realized in flexible solid-state asymmetric SCs using 5 mol L-1LiCl in PVA as gel electrolyte.
Thermal activation is another effective method to increase the surface area of commercial carbon cloth[50]. Chemical activating agents (KOH, ZnCl2,etc.) are mostly used as additives in the thermal activation process[61-62]. For example, Zhang et al.[62]proposed KOH as activation agent along with thermal annealing to improve the specific area and electrical conductivity of carbon fiber cloth. After treatment, the obtained activated carbon fiber not only maintained its excellent mechanical property, it also showed a much higher surface area of 2 780 m2g-1and an improved electrical conductivity of 320 S m-1as compared to pristine carbon fiber cloth (~1 000 m2g-1; ~100 S m-1, respectively). Thanks to the improved surface area and electrical conductivity, the obtained carbon fiber cloth exhibited a high capacitance of ~197 F g-1/~0.5 F cm-2at 0.1 A g-1and a high capacitance retention of 99% after 15 000 cycles at 3.3 A g-1in 6 mol L-1KOH electrolyte. Furthermore, the symmetric solid-state SCs using PVA/H3PO4as gel electrolyte demonstrated excellent flexibility and performance stability. Due to the corrosion of used chemical activating agents and additional wash step, an activation without additive is preferred. Zhao et al.[50]adopted thermal treatment at 400 °C to activate commercial carbon felts and treatment was optimized. After treatment, graphene nanosheets along with abundant micropores and oxygen-containing functional groups were formed on the surface of carbon fiber, which endowed an improved rate capacitance (i.e., 188 F g-1at 0.1 A g-1and 122 F g-1at 5 A g-1) and an improved cycling stability with a capacitance retention of 99%after 1 000 cycles at 2 A g-1in 1 mol L-1H2SO4electrolyte (Fig. 3g). More importantly, the assembled flexible symmetric solid-state SCs using PVA/H2SO4as electrolyte maintains a stable performance even under different bending states (Fig. 3h-i).
Plasma treatment is another facile method to modify the surface of carbon fiber[51]. For example,Ouyang et al.[51]used nitrogen plasma to treat commercial carbon cloth. As a result of the reaction between N2+species and carbon fiber, nitrogen-containing functional groups were formed along with nano-structuring, leading to a high surface area and improved liquid electrolyte wettability. The resultant carbon fiber showed a high capacitance of 391 mF cm-2at 4 mA cm-2in 1 mol L-1KOH electrolyte,which is ascribed to the interaction between the electrolyte and N species in addition to a large surface area.
Combining commercial carbon cloth/felt with other materials with pseudocapacitance is an effective method to obtain satisfactory capacitance, in which carbon cloth/fiber serves as flexible conductive substrate. Transition metal oxides (RuO2, MnO2, Fe3O4,etc.) are a kind of desired pseudocapacitance materials to combine with the carbon fiber cloth due to their high capacitance[63]. A carbon cloth decorated with hierarchical carbon-coated Fe3O4nanorods derived from metal-organic framework (MOF) grown on carbon cloth, which was fabricated as an electrode for SCs by Peng et al.[63]. Benefiting from the conductive scaffold of carbon fiber cloth, large surface area of nanorod-shaped Fe3O4, and carbon coating protection,a high capacitance (i.e., 463 F g-1at 1.5 mA cm-2) and a fast reaction kinetics were realized in 1 mol L-1Na2SO4electrolyte. Furthermore, the flexible asymmetric solid-state SCs combining MnO2coated on carbon cloth as cathode using carboxymethyl cellulose sodium (CMC)/Na2SO4was as a gel electrolyte displayed a high energy density of 74.6 W h kg-1at a high power density of 1 249.1 W kg-1and a superior rate performance (i.e., 258 mF cm-2at 3 mA cm-2), as well as stable capacitance under different bending conditions.
Polymers (polyaniline (PANI), polypyrrole(PPy), polythiophene (PTP), etc.) are another kind of promising pseudocapacitance materials to composite with carbon fiber cloth due to their low cost and easy preparation procedure[64]. Xiong et al.[64]combined nanoscale thin PANI with graphitic petals (GF) grown on carbon cloth (CC) by electrochemical polymerization. The CC provides a conductive and flexible substrate, and conductive GF with large surface area facilitates electron transport to PANI. Consequently,PANI displayed a high capacitance up 2 000 F g-1at 1 A g-1, and excellent rate performance with a capacitance of 1 200 F g-1at 100 A g-1as well as exceptional cycling stability with a capacitance retention of 93% after 2 000 cycles in 1 mol L-1H2SO4electrolyte.In addition, solid-state SCs using PVA/H2SO4as electrolyte also showed an excellent flexibility and superior electrochemical performance.
Except for the commercial carbon fiber macroscopic cloth for planar flexible SCs, commercial carbon fiber thread/yarn is also widely used for flexible fiber/yarn-shaped SCs[54,65-66]. Jin et al.[54]fabricated PANI coated carbon fiber thread (CFT@PANI) via electrochemical deposition as positive electrode and functionalized carbon fiber thread (FCFT) with oxygen-containing groups (-C-O, -C=O, -COOH)by electrochemical oxidation after annealing for solidstate fiber-shaped SCs (Fig. 3j). Benefiting from the high electrical conductivity of CFT, the porous networks of PANI, and abundant oxygen functional groups of FCFT, the assembled flexible asymmetric SCs using PVA/H3PO4as electrolyte delivered a high energy density of 2 mWh cm-3and a power density of 11 W cm-3. More importantly, the fiber-shaped solidstate SCs maintains their capacitance under bending and stretching states, while still showing an excellent capacitance stability and stretchability when knitted into textile (Fig. 3k,l).
2.2 Biomass-derived carbon electrode
To achieve sustainable development, it is necessary to maximize the utilization of biomass materials due to their merits of environmental friendliness,widespread availability, renewable nature, and low cost. Biomass has been widely used in various applications, and especially in SCs as source for carbon materials. For example, shrimp skin, shaddock peel, corn cob, and wood have been used in SCs[32,67]. Some biomass materials (cotton, silk, bacterial cellulose,chitosan, etc.) can also be used for fabricating freestanding carbon electrodes for flexible SCs[68].
Cotton is the most common biomass material,and a good template for freestanding carbon electrode[69-71]. Zhang et al.[72]fabricated a freestanding carbon electrode by pyrolyzing cotton cloth followed by an activation using KOH (Fig. 4a). The obtained activated carbon cloth shows a hollow tubular structure, a high conductivity of 1 506 S m-1, and a high surface area of 1 075 m2g-1. The electrode, applied as the electrode for SCs, demonstrated a higher capacitance of 1 026 mF cm-2at 5 mA cm-2than that of carbon cloth without activation (741 mF cm-2) and a long-term stable cycling with a capacitance retention of 97% after 10 000 cycles at 10 mA cm-2in 6 mol L-1KOH electrolyte. More importantly, the assembled flexible symmetric solid-state SCs using PVA/KOH as electrolyte exhibited an energy density of 0.42 mWh cm-3at a power density of 10.9 mW cm-3and only 8% capacitance loss after 200 cycles at a maximal bending angle of 180° (Fig. 4b,c).Besides, Zhao's group developed nitrogen-doped graphene complexed carbonized cotton (NGCs) for flexible SCs using freeze-drying technique and ammonia-assistant thermal activation processes[70]. Freezedrying technique made the graphene oxide (GO)nanosheets well mixed with carbonized cotton skeleton fibers, and a cross-linked structure between GO and carbonized cotton was formed. The NH3not only has an etching effect on carbon fibers to form a porous structure during the activation process, but also enables nitrogen doping on the surface of graphene oxide and cotton-derived carbon fiber. Thanks to the synergy effect of graphene nanosheets and cotton fibers, the obtained NGCs have a high capacitance of 291 F g-1at 1.0 A g-1in 1 mol L-1H2SO4along with high flexibility. Moreover, the assembled lightweight and flexible symmetric SCs based on the NGCs electrodes showed excellent capacitance stability under bending in 1 mol L-1H2SO4electrolyte. In addition,pseudocapacitance materials grown on carbon fiber derived from cotton were also developed for flexible SCs and showed promising performance[71]. Jiang et al.[71]fabricated Ni0.54Co0.16O nanosheets grown on carbon fiber derived from cotton cloth (NCONSs/CFC) for flexible SCs. Thanks to the ultrathin and porous Ni0.54Co0.16O nanosheets with high utilization, interconnected conductive carbon fiber cloth as current collector, and hierarchical structure for fast ion and electron transport, the NCO-NSs/CFC shows a high capacitance of 438 μAh cm-2at 1 mA cm-2and a capacitance retention of 70% after 10 000 cycles at 10 mA cm-2in 2 mol L-1KOH electrolyte. Moreover,the flexible symmetric solid-state SCs using PVA/KOH as electrolyte shows an energy density of 92.4 Wh Kg-1at a power density of 207.2 W kg-1and an excellent flexibility.
Bacterial cellulose (BC) is another commonly used biomass material, and is widely used as carbon electrode material precursor because of its advantages of high production, environmental friendliness, nontoxic nature, low cost, and a good mechanical strength. The carbon materials derived from BC possesses a conductive network connected by nanofibers and a large specific surface area, which facilitates charge transfer and storage properities[41,73]. Hao et al[76]. prepared a carbon nanofiber network from BC(CN-BC) via silica-assisted strategy. Silica shell is served as a nanoreactor creating a confined environment during the pyrolysis. The generated CO2and H2O functioned as activation agents inside the nanoreactor, leading to an improved surface area and porosity. The obtained carbon nanofiber shows three-dimensional (3D) interconnected structure, a surface area of 624 m2g-1, and mesopore-dominated hierarchical porosity. The CN-BC delivered a capacitance of 302 F g-1in 6 mol L-1KOH electrolyte, and the symmetric SCs displayed a capacitance of 184 F g-1at 0.25 A g-1as well as a capacitance retention of 78% at 10 A g-1in 6 mol L-1KOH electrolyte. Besides, Luo et al.[73]prepared freestanding carbon nanofiber/graphene nanosheet (CNF/GN) composite films by a membrane-liquid interface culture method followed by carbonization. GNs was put into the culture medium of BC, and BC was in situ grown on the surface of GNs. After carbonization, CNF/GN composite films show excellent flexibility, mechanical robustness,good structure stability, and high specific surface area(Fig. 4d,e). Consequently, the symmetric SCs using 1 mol L-1Na2SO4electrolyte based on CNF/GN electrodes offered a high energy density of 20 Wh kg-1and a high power density of 900 W kg-1, and kept a stable capacitance at different bending states (Fig. 4f).
A freestanding carbon electrode derived from silk, composed of silk fibroin and sericin, is also a promising electrode for flexible SCs due to abundant nitrogen doping and high flexibility[77]. Li et al.[78]fabricated heteroatom (N, O and S) co-doped carbonized silk as a flexible carbon electrode by dyeing silk with heteroatom-enriched dye, followed by pyrolysis. The heteroatoms (N, O and S) co-doped carbonized silk shows a higher surface area of 256.6 m2g-1than that of carbonized silk (16.3 m2g-1), a more defective structure, and hierarchical porosity. In addition, the heteroatoms (N, O and S) doping contributes extra pseudocapacitance and improves the wettability toward electrolyte. As a result, the heteroatoms (N, O and S) co-doped carbonized silk delivered a higher capacitance of 255.95 F g-1at 2 mV s-1as compared to carbonized silk (45.69 F g-1), and a good cycling stability with 8% capacitance decay over 5 000 cycles at 100 mV s-1in 1 mol L-1Na2SO4electrolyte. Additionally, Xia et al.[74]fabricated MnO2coated carbonized silk fabric (CS-MnO2) for flexible and shape-editable SCs (Fig. 4g). The freestanding carbonized silk fabric serves as substrate, and the introduction of MnO2improves the hydrophilicity as well as capacitance performance. As a result, the CS-MnO2delivered a capacitance of 105 F g-1at 0.25 A g-1in 6 mol L-1KOH electrolyte. More interestingly, the assembled SCs using 6 mol L-1KOH as electrolyte showed 101.34% of initial capacitance under a 180° bending angle and can be modifited to desired shape (Fig. 4h,i).
Carbon gels derived from biomass are also promising as electrodes for flexible SCs due to high surface area, high porosity, and low density[68]. Wu et al.[75]developed a sponge-like carbonaceous aerogels using watermelon. The obtained carbonaceous gels have a micro-structure with carbonaceous nanofibers and nanospheres, and show an excellent mechanical flexibility (Fig. 4j-m). Meanwhile, Fe3O4was incorporated into the porous carbonaceous gels (CGs) to get magnetite carbon aerogels (MCAs), in which CGs with porous 3D conductive structure as scaffold and Fe3O4provides an additional pseudocapacitance. The MCAs keeps the porous structure of the original CGs,which facilitates both ion and electron transportation to the electrode surface. As a result, a high capacitance of 333.1 F·g-1at 1 A·g-1and excellent cycling stability with a capacitance retention of 96% after 1 000 cycles were realized in 6 mol L-1KOH electrolyte (Fig. 4n).
2.3 Polymer-derived carbon electrode
Melamine sponge consisting of a formaldehydemelamine-sodium bisulfite copolymer is a good template for freestanding carbon electrodes in flexible SCs owing to its light weight, high nitrogen content,and high porosity[79]. For example, Xiao et al.[45]fabricated a N-doped carbon foam as electrode for compressible SCs by carbonization of melamine sponges.The obtained N-doped carbon foams show features of a lightweight, interconnected network, robustness, and extraordinary electrolyte wettability (Fig. 5a,b). As a result, N-doped carbon foam displayed a high capacitance of 52 F g-1at 1 mA cm-2in 5 mol L-1LiCl electrolyte. Moreover, it can bear a high compressive strain of 80% and a durability over 100 cycles with a negligible volume change under a constant strain of 55%. Furthermore, the assembled symmetric solidstate SCs based on carbon foam using PVA/LiCl as electrolyte showed an excellent compressible and stable electrochemical performance under different strains (Fig. 5c). Normally, the freestanding carbon electrodes from direct carbonized melamine sponge shows an uneven pore size distribution and a low specific surface area. Activation process is necessary to further improve the surface area and porosity. Zhang et al.[80]adopted ZnCl2additive for activation treatment during pyrolysis process of melamine sponges.Because the ZnCl2could dehydrate carbon atoms, the porous structure was formed on the surface of the carbon sponges. The mass ratio of ZnCl2and melamine was optimized and resultant carbon sponge showed a highest capacitance of 242 F g-1at 0.5 A g-1and a high capacitance retention of 97% after 10 000 cycles at 5 A g-1with the ratio of 1∶10 for 6 mol L-1KOH electrolyte. More importantly, a solid-state SCs using PVA/KOH as electrolyte showed an energy density of 4.33 W h kg-1at a power density of 250 W kg-1. Other additives (KOH, K2CO3, etc.) were also applied for activation, and an improved electrochemical performance was also realized[81-82]. Introducing the heteroatom in the carbon materials is other most used approach to improving the capacitance via the pseudocapacitive effect. Yang et al.[83]developed a 3D B-doped N-containing carbon foams as electrode for SCs by annealing boric-acid-infused melamine sponge. They found out that the porosity of mesoporous structure and concentration of B doping increased with the increase of temperature from 500 to 700 °C, while the collapse of pore structure and the formation of insulating boron nitride were caused with the temperature increasing from 700 to 900 °C. As a result, the sample annealed at 700 °C showed the highest capacitance of 462 mF cm-2at 0.2 mA cm-2in 6 mol L-1KOH electrolyte. Furthermore, the fabricated solid-state symmetric SCs using PVA/KOH electrolyte showed a capacitance retention of 77% after 2 000 cycles at 6 mA cm-2. Moreover, the two solid-state symmetric SCs can power a light consisting of 43 LEDs for 5 min. Integration with other pseudocapacitive materials (metal oxides, metal sulfides, polymers) to boost the capacitance was explored here as well. Shen et al.[85]fabricated NiCo2S4nanosheet decorated melamine sponge derived N-doped carbon foam(NiCo2S4/NCF) as a flexible electrode for SCs. The interconnected framework and good adhesion enabled a fast electron and ion transport, a high capacitance of 877 F g-1at 20 A g-1and an extraordinary cycling stability in 6 mol L-1KOH electrolyte. More importantly, the assembled flexible asymmetric solid-state SCs using 6 mol L-1KOH as electrolyte based on NiCo2S4/NCF and order mesoporous carbon decorated N-doped carbon foam (OMC/NCF) displayed a high energy density of 45.5 Wh kg-1at 512 W kg-1.
Electrospun polymer nanofibers or micro-fiber is also a good template for freestanding carbon electrode, which is fabricated by electrospinning. Electrospinning is a simple, low-cost, and high efficiency technology, which allows to fabricate polymer nanofibers under electric field using polymer solutions,such as polyacrylonitrile (PAN), polyimide (PI),polyvinylpyrrolidone (PVP), polyvinyl alcohol(PVA), poly (vinyl fluoride) (PVDF) etc.[86-92]. The obtained electrospun polymer nanofibers or microfiber can be transformed into carbon nanofibers and mats after carbonization, and these electrospun-derived carbon materials are lightweight, free-standing,and flexible, making them ideal electrode candidates for flexible SCs[93-94]. A large variety of electrospunderived carbons have been developed as electrode materials for flexible SCs. However, most of electrospun-derived carbon materials exhibit small accessible surface area leading to an inferior electrochemical performance[95]. To improve the surface area, activation of CNF with additional various types of oxidizing gases (steam, CO2, etc.) or various chemical activators (KOH, H3PO4, NaOH, etc.) is developed[96-100].Ma et al.[99]reported a porous electrospun phenolicbased carbon nanofibers paper with a large surface area by coating KOH on electrospun fiber for activation. The surface area and the concentration of micropores of the obtained CNFs gradually increased with the increase of KOH. When 20% of KOH was used, a high specific surface area for CNFs of 1 317 m2g-1with an average pore width of 2.12 nm was obtained.When electrode materials for SCs is employed, the obtained CNFs achieved high energy densities of 7.1 W h kg-1and 27.9 W h kg-1in aqueous electrolyte(6 mol L-1KOH) and organic electrolyte (1 mol L-1tetraethylammonium tetrafluoroborate/propylene carbonate ((C2H5)4NBF4/PC)), respectively.
Expect for conventional surface activation process to improve the porosity and surface area, the morphology, composition, and structure (e.g. hollow,core-shell, multi-channel) of carbon nanofibers can be tuned and designed by easily adjusting polymer blends or using additives[101-102]. The thermolabile polymers can be serveved as sacrificial polymer and decomposed during carbonization to create pores or hollow structure, such as PVP and polymethylmethacrylate (PMMA), which allows to design nanofibers with a large surface area and porous structure[103-106].The porosity provides channel for quick transfer of electrolyte ions thus improving the charge storage capacities and rate capability. Kim et al.[107]reported a unique N-doped hierarchical porous core-shell CNFs(PHCNFs) using a PVP/PAN as the shell material and polystyrene-acrylonitrile (SAN) as the sacrificial core polymer by co-axial electrospinning. The PVPs were etched by immersing the electrospun fibers in N, Ndimethylformamide (DMF)/water solution generating pores and texture surface, and the residual PVP decomposes to nitrogen species under carbonization at N2atmosphere, while the SAN decomposes during carbonization forming hollow structure. The hollow core and porous structure facilitates rapid adsorption and desorption of electrolyte ions, and abundant nitrogen species contribute to pseudocapacitance and improve surface wettability as well as electronic conductivity. As a result, a high energy density of 4.12 Wh kg-1at powder density of 15 kW kg-1and a high capacitance retention of 92.33% after 10 000cycles were achieved for optimized structure in 2 mol L-1KOH electrolyte. Similarly, the use of additional hard sacrificial templates like metal oxides(SiO2, ZnO, etc.), slat (ZnCl2, NaCl, etc.) and MOFs into the electrospun polymeric solutions also can control over the porosity and surface area of CNFs[108-112].
Pseudocapacitive materials, such as metal oxides and conductive polymers, can be grown on prepared electrospun fibers or carbon nanofibers by chemical synthesis methods (electrodeposition, hydrothermal processes, co-precipitation processes, etc.), giving rise to an improved electrochemical performance as compared to carbon nanofibers[113-116]. For instance, MnOcarbon nanofibers (MnO/CNF) composite was prepared by adding manganese acetylacetonate to PAN precursor for electrospinning and calcination process[117]. The synergistic effect of pseudocapacitive MnO and porous carbon nanofibers endows the assembled fiber SCs (FSC) with improved specific capacitance of 200 F g-1as compared to pure PANbased FSC (90 F g-1) and a stable electrochemical performance over a wide bending range in 0.25 mol L-1aqueous bis(trifluoromethane)sulfonimide lithium(LiTFSI) electrolyte. Besides the conventional pseudocapacitive materials, pseudocapacitive two-dimensional (2D) layered nanomaterials (MXenes,black phosphorus, etc.), have been intensely explored for flexible SCs because of their high specific area,and excellent electrical and electrochemical properties[118-120]. MXenes regarding as a large family of two-dimensional transition metal carbides and nitrides, it can be easily processed into various structures by forming stable colloidal solutions due to their hydrophilic property[121]. Ti3C2Tx/ANF hybrid fibers were prepared by mixing ultra-high mechanical strength aramid nanofibers (ANFs) with Ti3C2Txsuspensions as electrospinning precursors by solidification baths injected with FeCl2solution (Fig. 5d)[84].ANFs not only improved the flexibility and mechanical properties of Ti3C2Tx-based fibers, but also prevented the re-stacking of Ti3C2Txnanosheets during the wet spinning process. The prepared Ti3C2Tx/ANF hybrid fibers have relatively regular cylindrical shapes,and the hybrid fibers inherit the MXene microstructure and show an expanded layer spacing. As the Ti3C2Tx/ANFs were employed as electrode of flexible solid-state symmetric SCs, they delivered a high specific capacitance of 295 F cm-3at 0.25 A cm-3, and a high energy density of 26.2 mWh cm-3, benefiting from rapid carrier mobility in 3 mol L-1H2SO4electrolyte (Fig. 5e).
2.4 Carbon nanotubes-based electrode
CNTs, as a one-dimensional (1D) nanocarbon material, is a research hotspot because of its high theoretical specific surface area, extraordinary electronic conductivity, excellent thermo conductivity, high Young’s modulus, and admirable tensile strength[24].Owing to the intrinsically flexible merits, CNTs can be used as building blocks to assemble freestanding macroscopic structures with different dimensions,such as 1D fibers, 2D films, and 3D foams[33,124-125].These macroscopic CNTs forms with light weight and superior mechanical properties play a vital role in high-performance flexible electrodes for flexible SCs[126-127]. Wang et al.[122]reported the single-walled carbon nanotubes (SWCNTs) as electrode materials and cellulose nanofiber as separator materials to fabricate SCs via a consecutive spray printing strategy(Fig. 6a). This technique could build all SCs components consecutively and controllably, and the SCs can be printed as different shapes on various rough or smooth surface. Moreover, this kind of flexible SC device demonstrated a stable capacitance performance at different bending states (Fig. 6b-e). In addition, CNTs films fabricated by vacuum filtration using CNT suspension is often used for SCs[128-129].However, the CNTs randomly cross and bundle with each other, resulting in a lower specific surface area.To overcome this, Li et al.[130]designed a CNTs-aerogel electrode prepared by electrochemical activation and freeze-drying method. Comparing with conventional vacuum filtration method, the CNT-aerogel possesses a large number of small pores maintained by freeze-drying method and CNT fibers inside connected each other with Y-shaped junctions forming a 3D conductive network, endowing the CNT-aerogel with high specific surface area and strong mechanical strength. The micro-morphological interlinked CNTaerogel offers fast and continuous electron transport paths, thus greatly enhancing the electrochemical performance of the assembled SCs. When fibrous CNTaerogel electrode was assembled as solid-state fibrous SC using P(VDF-HFP)/EMIMBF4as electrolyte, it showed a high specific capacitance of 11.3 F g-1at 2 mA and a high energy density of 29.6 W h kg-1.Moreover, the mechanical performance test was performed at 0.5 mA, and the 92.9% capacitance could still be maintained even after being bent for 2 000 times.
Introducing multidimensional carbon with rational design can make CNTs showing a higher electrical conductivity and mechanical strength[131-134]. Xue et al.[135]reported a one-step chemical vapor deposition(CVD) method to obtain 3D graphene-CNTs hollow fibers, which shows cylindrical structure with seamless graphene sheath and radially aligned CNTs. The seamlessly joined 3D structure endows graphene-CNTs hollow fibers with efficient isotropic electrical transport capability. When the fibers are woven to electrode for flexible SCs using PVA/H2SO4as electrolyte, it shows a high specific capacitance of 89.4 mF cm-2, and excellent cycling stability under repeated bending up to 90°. In addition, a partially unzipped carbon nanotube/reduced graphene oxide(PUCNT/RGO) hybrid fiber with low level of "dead volume" and well-ordered porous structure was fabricated by wet spinning and chemical reduction from partially unzipped oxidized carbon nanotube/graphene oxide (PUOCNT/GO) solution[136]. PUOCNT with high hydrophilic property and large surface area served as spacer to inhibit the restacking of GO and reduced the spacer itself, leading to reduced “dead volume” and large pores. As a consequence, the assembled solid-state fiber-shaped SCs constructed from hybrid fibers using PVA/H3PO4as electrolyte showed excellent volumetric capacitance of 62.1 F cm-3and energy density of 8.63 mWh cm-3as well as a high capacitance of 97.8% after 2 000 cycles, along with good mechanical stability with a capacitance retention of 98.2% over 1 000 bending cycles. Besides, the introduction of heteroatoms (e.g., N, B) can further improve the polarizability of the carbon network and electrical conductivity, leading to an improved capacitance[137-139]. Zhang et al.[140]showed that N-doped core-shell CNTs array can be synthesized for highly stretchable SCs. CNT arrays were synthesized from ethylene by CVD at 740 °C, and then the N-doped layers were coaxially regrown from acetonitrile by chemical vapor deposition at 1 060 °C. The introduction of N-containing species can significantly enhance the specific capacitance by providing pseudocapacitance, enhancing specific capacitance by over 80 times compared with the original CNT array. As a result, the assembled solid-state symmetric SCs using PVA/H3PO4as electrolyte displayed a specific capacitance of 30.8 mF cm-2under a strain of 400%, and a 96% capacitance retention after stretching for 1 000 cycles.
To further improve the electrochemical performance, CNTs can be combined with pseudocapacitive materials (metal oxides, conducting polymers) to form a hybrid electrode. In hybrid electrodes, the CNT network provides a robust substrate, which effectively buffers the volume change of the supported high-capacitance pseudocapacitive materials, thus extending the cycling stability of the electrode. Various metal oxides, such as MnO2, Co3O4, NiO, have been studied as CNTs hybrid electrode for flexible SCs[123,141-145].For instance, MnO2nanosheets were in situ grown on CNTs films via a hydrothermal reaction, forming a thin and tough CNTs-MnO2composite film[123]. The active sites of redox reactions, the electron transfer efficiency, and MnO2utilization efficiency increased and thus the capacitance, energy density, and rate performance improved. Moreover, the flexible CNTs-MnO2electrodes can not only be assembled into flexible planar SCs, but also can be rolled and twisted to build yarns that can be reconfigured into stretchable wire SCs (Fig. 6f). The assembled yarn asymmetric SCs based on CNTs-MnO2and carbon fiber (CF) decorated with FeSe2nanonuts (CF@FeSe2) using PVA/LiCl as electrolyte showed a very high energy density of 29.84 Wh kg-1at a power density of 571.3 W kg-1and good capacitance retention after 8 000 cycles, as well as excellent flexibility (Fig. 6g). In addition to hybridization with inorganic pseudocapacitive materials, conducting polymers were electrochemically deposited on CNTs. The conformal coating enhances charge-storage capability, while nanotubes accelerates the ionic diffusion, leading to a high power density[146-148]. However, wet-based electrochemical deposition processes may introduce solvents that interfere with the nanostructure and reduce the compatibility of the electrode with the device[149]. Wardel et al.[150]reported poly(3-methylthiophene) deposited on horizontally aligned carbon nanotubes (P3MT/HACNT) flexible electrode via a solvent-free oxidative CVD method. HACNT endows an excellent mechanical support and enables a high ion and electron transport. The P3MT/HACNT flexible electrode displayed a high area capacitance of 3.1 F cm-2at 5 mA cm-2and retained 1.8 F cm-2even at 200 mA cm-2in 1 mol L-1Et4NBF4/PC electrolyte, while the flexible asymmetric SCs from P3MT/HACNT and HACNT electrode using Et4NBF4/PC as electrolyte exhibited maximum energy densities of 1.08 mWh cm-2and 1.75 W cm-2. In addition, electrochemical performance remains unchanged under bending, showing a high electrochemical and mechanical stability.
2.5 Graphene-based electrode
Graphene, as a kind of 2D nanosheet materials,has becomes one of the most promising carbon materials used in SCs due to its large theoretical specific surface area, excellent electrical, thermal conductivity,and high chemical stability. Owing to facile dispersion of graphene oxide (GO) and reduced graphene oxide (rGO) without surfactants, they are widely employed for fabricating freestanding graphene electrodes in SCs. Moreover, GO or rGO have excellent ability to be self-assembled into different dimensional macroscopic forms (1D fibers, 2D films, 3D aerogels)with extremely predominant mechanical properties by spinning[151], filtration[152], printing[153], hydrothermal[154]approach, which provides the chance to build different types of flexible SCs[155].
Niu and co-authors combined photolithography with electrophoretic buildup to fabricate a new kind of lateral ultrathin rGO interdigitated microelectrodes(Fig. 7a)[156]. The H3PO4/PVA gel electrolyte was used, and the flexible, compact, ultrathin, and solidstate micro-SCs was successfully fabricated (Fig. 7bd). The design of lateral interdigitated microelectrodes shortens the diffusion distance of the electrolyte, and shows effectiveness in using the electrochemical surface area of graphene layers. As a result,the micro-SCs showed a high power density and high stability under bending (Fig. 7e,f). Besides, Zhao et al.[154]fabricated a rGO electrode for flexible micro-SCs (MSCs) using hydrothermal method with GO dispersion. The obtained rGO sponge possesses 3D porous structure with conductive interconnected networks, which endows an outstanding mechanical properties and superior electrochemical performance.As a result, electrode with a high graphene loading of 21.1 mg cm-2reached a capacitance of 569.5 mF cm-2at 0.5 mA cm-2in 1 mol L-1Na2SO4electrolyte. Furthermore, the capacitance showed no attenuation after a bending test from the angle from 0oto 180o, and it also kept 98.4% of capacitance after 2 000 repetitious of fatigue test at the bending angle of 90ousing PVA/LiCl as electrolyte. Besides, He et al.[151]fabricated rGO fiber for flexible SCs via a wetting-spinning processing of GO dispersions with different coagulants. The morphologies and properties were investigated when different cations (Ca2+, Fe3+, Al3+) are used.They found that solid-state flexible SCs based on Al3+-containing rGO using PVA/H2SO4as electrolyte showed the highest capacitance of 148.5 mF cm-2at 40 mV s-1and fiber-shaped flexible SCs demonstrated high capacitance retention after 1 000 bending cycles. This excellent electrochemical performance is ascribed to the highest electrical conductivity and higher toughness due to highest binding energy between Al3+and GO, which facilitates the electron transport, electrolyte ions diffusion, and adsorption.Heteroatom doping, such as N, can be introduced to improve the conductivity and contribute to pseudocapacitance. Jin et al.[152]fabricated a free-standing Ndoped graphene aerogel (NG) film via hydrogel method. Firstly, the GO suspension was mixed with pyrrole(Py) monomer, the formed hydrogel was further mixed with GO suspension, followed by vacuum filtration, freeze drying, and pyrolysis. The obtained NG film possesses a macro-porous structure, which ensures fast ion adsorption. In addition, nitrogen species converted from pyrrole endows better conductivity,while pyridinic and pyrrolic N contribute to pseudocapacitance. Benefiting from these outstanding properties, the NG film reached a capacitance of 455.4 F g-1at 1 A g-1and no obvious capacitance loss after 5 000 cycles in 0.5 mol L-1H2SO4electrolyte, while the asymmetric SCs based on NG and rGO/PPy electrode exhibited energy density of 34.51 Wh kg-1at a powder density of 849.77 W kg-1in 0.5 mol L-1H2SO4electrolyte.
However, graphene nanosheets are prone to selfaggregation stack through a strong π-π bond during the preparation of the electrode, which would reduce the surface area and thus restrict the electrochemical performance of capacitance. One of the strategies is introducing other guests. Zhang et al.[157]introduced layered double hydroxide (LDH) nanoplatelets into rGO layers by filtrating the LDH and GO solution,followed by reduction. The LDHs not only create mesopore for high accessible surface area, but also contribute to extra pseudocapacitance. The rGO with a conductive and robust network facilitates fast transport of electron. As a result, the obtained electrode showed a capacitance of 122 F g-1at 0.5 A g-1and a high volumetric capacitance of 43.5 F cm-3at 1 A g-1in 1 mol L-1KOH electrolyte, while the assembled flexible asymmetric solid-state SCs based on rGO/LDH and rGO using PVA/KOH as electrolyte exhibited maximum energy densities 22.6 Wh kg-1and 1.5 kW kg-1as well as a stable cycling over 5 000cycles with a capacitance retention of 94%. Polymer coupled with GO sheet was also used to inhibit the aggregation of rGO during reduction process. For example, Cao et al.[39]prepared flexible rGO@PVA film by simultaneously reducing GO/PVA solution and assembling rGO@PVA with molecular level coupling using Zn foil as reductant. Due to the introduction of PVA, rGO and neighboring rGO were separated and linked by PVA via hydrogen bonding, which endow a high strength and Young’s modulus. In addition, after immersing rGO@PVA into H2SO4, the PVA/H2SO4electrolyte between rGO sheets provide transport channels for fast ion transport (Fig. 7g). As a result,the composite film displayed a high energy density of 7.18 mWh cm-3and power density of 2.92 W cm-3.Moreover, the symmetric SC exhibits a stable capacitance under different bending states and with loading,and two assembled SCs could power LED before and after bending (Fig. 7h-k).
A porous and dense rGO film with a highly accessible surface area and continuous ion transport network is desired for high volumetric energy densities electrodes[158]. Yang et al.[159]fabricated a compact graphene electrode by controlled removal of a volatile solvent trapped in the gel. The rGO hydrogel films obtained by filtration were exchanged with a mixture of volatile (deionized water) and nonvolatile liquids(liquid electrolyte, sulfuric acid, 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF4)). After removing the volatile liquid by vacuum evaporation, the thickness of rGO film was reduced, and nonvolatile liquid remained trapped in the rGO sheets forming ion transport channels. Benefiting from the highly accessible surface area, network for fast ion transport, high electrolyte wettability, and a high packing density, the rGO electrode trapped EMIMBF4between the layers shows a maximum power density of ~ 75 kW L-1. In addition, Li et al.[160]prepared a monolithic ultra-thick and dense rGO electrode by combining capillary drying and ZnCl2as pore former. The rGO shows a maximum surface area of 1 000 m2g-1and maintains a high density of 0.6 g cm-3. As a result, the symmetric SCs based on rGO with a thickness of 400 μm using BMIMBF4as electrolyte exhibited a capacitance of 150 F cm-3and ~65 W h L-1. Although a good balance between the porosity and density was achieved,the flexibility is sacrificed to some extent.
To further improve the mechanical and electrochemical performance, rGO was integrated with other materials. Zou et al.[161]fabricated cellulose fibers/rGO/Ag nanoparticles film via vacuum filtration and reduction by a hot hydrazine vapor. Thanks to the high electronic conductivity (sheet resistance:0.17 Ω sq-1) and excellent flexible, the composite film with a thickness of 400 μm delivered a maximum capacitance of 1 242.7 mF cm-2at 2 mA cm-2and high cycling stability with a capacitance retention of 99.6%after 10 000 cycles at 60 mA cm-2in 2 mol L-1KCl electrolyte, while the assembled flexible symmetric solid-state SCs using PVA/KCl as electrolyte showed a high areal energy density of 95 μWh cm-2and stable performance under bending. In addition, Du et al.[162]prepared ultraflexible MnO2@rGO film for in-plane quasi-solid-state micro-SCs. The rGO film was firstly fabricated by electrophoretic deposition of GO, followed by HI reduction, and the MnO2was in situ growth on rGO via a hydrothermal reaction. The rGO film treated with HI shows an excellent electronic conductivity and outstanding mechanical properties due to highly stacked layers, which acted as a subtract and current collector for MnO2. The assembled symmetric MSCs using PVA/LiCl as electrolyte exhibited a high capacitance of 31.5 mF cm-2at 0.2 mA cm-2, a high capacitance retention of 77.0%after 6 000 cycles.
3 Summary, present challenges and outlook
So far, recent advances of diverse freestanding carbon electrodes for flexible SCs are summarized systematically. However, further work still remains to do for development of flexible carbon electrodes in flexible SCs. We outline some key challenges below which need to be focused on for pushing the flexible SCs towards practical realization.
Key advantages and disadvantages of these freestanding carbon electrodes based on various carbon materials are outlined in Table 1. Due to the advantages of high electronic conductivity, excellent mechanical property and moderate cost, the commercial carbon cloth/felt seems promising for practical application in flexible SCs, but it faces the disadvantage of high weight and low surface area. Biomass-derived materials have the merits of renewable raw materials and low cost, while their weak mechanical properties and inferior conductivity limit their practical application in flexible SCs. Electrospun fiber derived materials possesses the advantage of simple fabrication and structure tunable, it also suffers from the shortcoming of weak mechanical strength and low conductivity. Large-scale application of high-quality carbon nanotube and graphene-based materials by the CVD method is still challenging considering the high fabrication cost. Industrial CNTs with low cost normally shows unsatisfactory properties owing to moderate purity. Other graphene materials fabricated by large-scalable fabrication method have individual limitations. For example, the graphene prepared by reduction of graphene oxide has shortcoming of low conductivity and many layers stacking, the graphene prepared by mechanical exfoliation face the disadvantage of high dispersing difficulty and many layers stacking. Additional efforts are needed to develop high electronic conductivity, low cost, and excellent mechanical freestanding carbon electrodes.
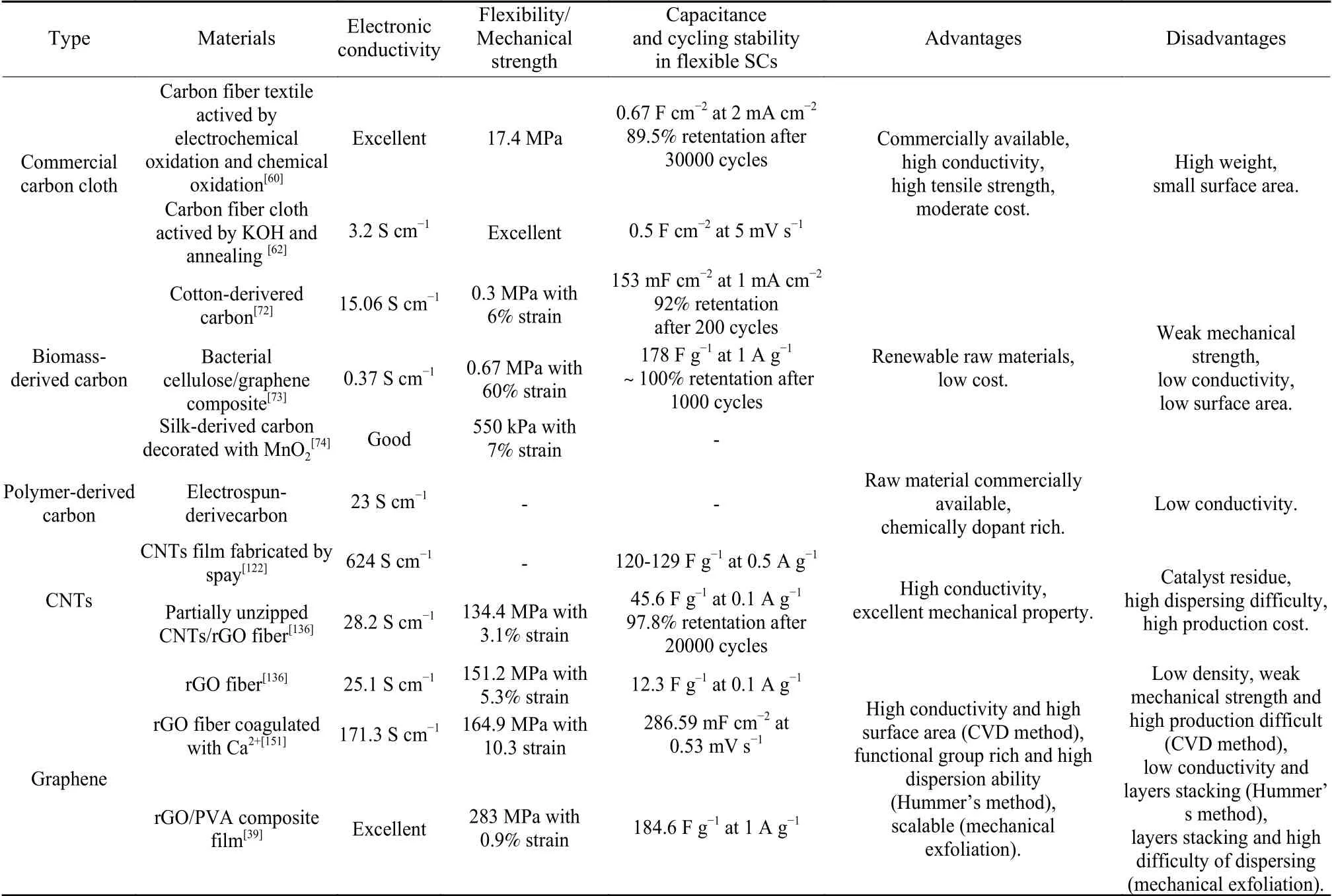
Table 1 Comparison of comprehensive performance of freestanding carbon electrodes based on various carbon materials for flexible SCs.
Activation is required to improve the surface area and porosity, and chemical activation using KOH and NaOH as activation agents was mostly used for activation. However, this method suffers from corrosive and pollution issues, and post-treatment is required.Green activation method is essential to develop. In addition, the activation processes may reduce mechanical stability or destroy the fibrous structure. Thus, the balance of porous and mechanical properties is needed to realize for flexible electrodes.
To hybridize pseudocapacitance materials with freestanding carbon materials, chemical methods such as hydrothermal synthesis and CVD, were widely used. However, the hydrothermal possesses the shortcoming of long reaction time and harsh reaction conditions. CVD has drawbacks of special equipment required and high cost. The method for large-scale practical application is needed to explored. In addition, additional efforts are also needed to achieve the good adhesion between the carbon electrode and additional pseudocapacitive materials as well as homogeneous distribution of pseudocapacitive materials.
Further advances can be achieved by using appropriate geometrical design and rational structure design. Appropriate geometrical design strategies have demonstrated high potential for robust flexibility and stretchability, such as the wavy-shaped design,winding/spring-shaped design, as well as “origami”and “kirigami” inspired structures. These geometrically designed structures can release external stress through geometrical transformation. The all-in-one structured flexible supercapacitor possesses advantages of high resistance to deformation and low interface resistance.
Further advances can also be achieved by using new emerging technologies. For example, 3D printing techniques including direct ink writing printing,inkjet printing, stereolithography, and fused deposition modeling printing, are promising for the fabrication of various architectures of the freestanding electrodes. Moreover, diverse materials (polymer, metals,metal oxides, carbon, ceramics, etc.) can be used for the fabrication of freestanding electrodes by 3D printing technology. More importantly, 3D printing also possesses the advantages of a highly automatic manufacturing process, facile route, and low cost for practical application.
To fasten the development of flexible SCs, other components (electrolytes, separators, substrate, encapsulating materials, etc.) need to be further explored.The novel configurations of flexible SCs are also need to designed to match well with other electric devices.
For a better understanding, investigation of mechanical behavior of a flexible electrode and the whole device under bending deformation by the finite element method is needed.
Acknowledgements
The authors acknowledge the financial support from the China Scholarship Council (CSC,202006180045, 202108080263), the financial support from German Research Foundation (DFG) under the joint German-Russian DFG project “KIBSS”(448719339), and Federal Ministry of Education and Research (BMBF) under the projects “HeNa”(03XP0390C) and “KaSiLi” (03XP0254D).
杂志排行
新型炭材料的其它文章
- Preface
- Guide for Authors
- 《新型炭材料》征稿简则
- 基于界面膜清洗的废旧锂离子电池石墨负极的再生修复
- A high-rate and ultrastable anode for lithium ion capacitors produced by modifying hard carbon with both surface oxidation and intercalation
- The interfacial embedding of halogen-terminated carbon dots produces highly efficient and stable flexible perovskite solar cells
