Carbon-based flexible electrodes for electrochemical potassium storage
2022-10-10WUYuhanWUXiaonanGUANYinyanXUYangSHIFanianLIANGJiyan
WU Yu-han, WU Xiao-nan, GUAN Yin-yan, XU Yang, SHI Fa-nian, LIANG Ji-yan
(1. School of Environmental and Chemical Engineering, Shenyang University of Technology, Shenyang 110870, China;2. Department of Chemical Engineering, Hebei Petroleum University of Technology, Chengde 067000, China;3. Department of Chemistry, University College London, London WC1H 0AJ, UK)
Abstract: With the rapid growth of the flexible and wearable electronics market, there have been big advances in flexible electrochemical energy storage technologies. Developing flexible electrodes with a low cost, superior safety, and high performance remains a great challenge. In recent years, potassium-based electrochemical energy storage devices have received much attention by virtue of their cost competitiveness and the availability of potassium resources. Carbon materials have been widely used as electrode materials or substrates for flexible energy storage devices due to their excellent properties, such as low weight, non-toxicity and abundance.Here, we summarize the recent advances in carbon materials (e.g. carbon nanofibers, carbon nanotubes, and graphene) for use in flexible electrochemical potassium storage devices, including potassium-ion batteries, potassium-ion hybrid capacitors, and K-S/Se batteries. Strategies for the synthesis of carbon-based flexible electrodes and their reported electrochemical performance are outlined. Finally, the challenges of future developments in this field are discussed.
Key words: Carbon materials;Flexible;Potassium-ion batteries;Potassium-ion hybrid capacitors;K-S/Se batteries
1 Introduction
Since the Sony Company commercialized lithium-ion batteries (LIBs), this kind of electrochemical energy storage technology has rapidly dominated the battery market due to its high efficiency and long-term cycling stability. With continuous development in the following few decades, LIBs have achieved noticeable progress and thus have been widely applied in modern society. In light of the success and advantages of LIBs, researchers gradually pay attention to other lithium-based electrochemical energy storage devices (EESDs) (e.g. Li-ion hybrid capacitors[1], Li solid-state batteries[2], Li-S/Se batteries[3-4], etc.).However, the tricky problem of the shortage (20×10-6in Earth's crust) and uneven distribution (mainly in Latin America) of Li resources largely limits their further development[5]. It is estimated that Li reserves on land will be exhausted around 2080 based on the consumption rate projected for 2050[6]. Therefore, on account of geopolitics and ever-growing price, novel electrochemical energy storage technologies have been explored as alternatives or supplements to lithium-based EESDs.
Recently, considering the similarity of electrochemical storage mechanisms and the advantage in cost, K-based EESDs, such as potassium-ion batteries(PIBs), potassium-ion hybrid capacitors (PIHCs), and K-S/Se batteries, have been developed successively[7-9]. The similar redox potential of K+/K (-2.93 Vvs. standard hydrogen electrode [SHE]) to that of Li+/Li (-3.04 Vvs. SHE) potentially ensures the comparable energy density of K-based EESDs. K is widely distributed in Earth's crust, and K reserve(17 000×10-6) is about 900 times higher than that of Li[10]. The price of potassium carbonate is much lower than that of lithium carbonate. Also, in contrast to Li,there are no alloying reactions between K and aluminum in the range of low voltages, meaning that cheap and light aluminum foil can be used as both cathode and anode current collectors. Therefore, the production cost will be decreased while the energy density will be increased. Moreover, owing to the low melting point of K (63.5 °C), K metal dendrites would be melted by Joule heat when metallic K growth leads to internal short circuits. The short-circuit would be cut before severe thermal runaway of EESDs, which largely relieves the safety problem caused by metal dendrites[11]. In addition, K-based EESDs exhibit advantages in certain situations. For instance, in propylene carbonate (PC) solution, K+has a smaller Stokes radius (0.36 nm), lower desolvation energy (119.2 kJ mol-1), and higher ionic conductivity (15.2 S cm2mol-1) compared with Li+(Table 1), which is in favor of fast diffusion kinetics[12]. Until now, K-based EESDs have achieved encouraging results and performance. In terms of energy/power densities (calculation based on full cells), PIBs can yield an energy density of >230 Wh kg-1at a power density of 4 000 W kg-1[13], while K-chalcogen (S/Se) batteries achieved an energy density of 700 Wh kg-1[14]. As for PIHCs that combine energy densities and power densities, their maximum energy density reaches 152 Wh kg-1at a low power density (350 W kg-1), and an energy density of 112 Wh kg-1could be maintained at a high energy density of 17 500 W kg-1[15].According to the above-mentioned points, K-based EESDs have aroused wide attention and interests.
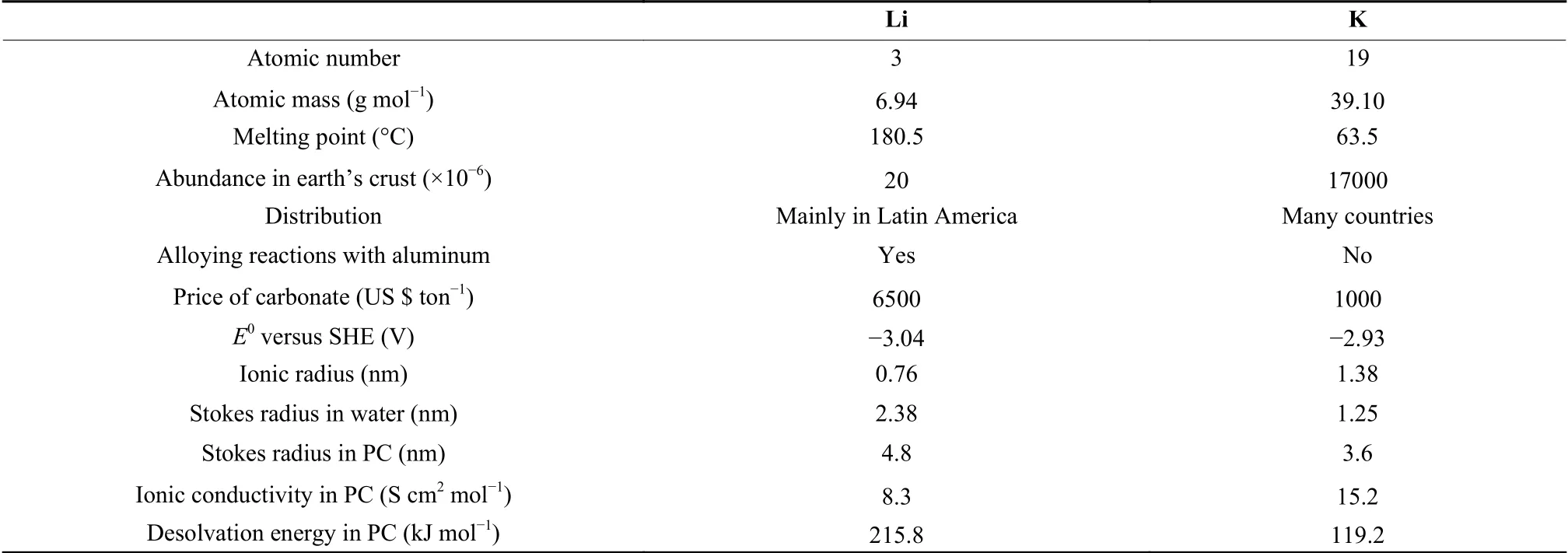
Table 1 Comparison of physical and economic properties between Li and K[11, 16-17].
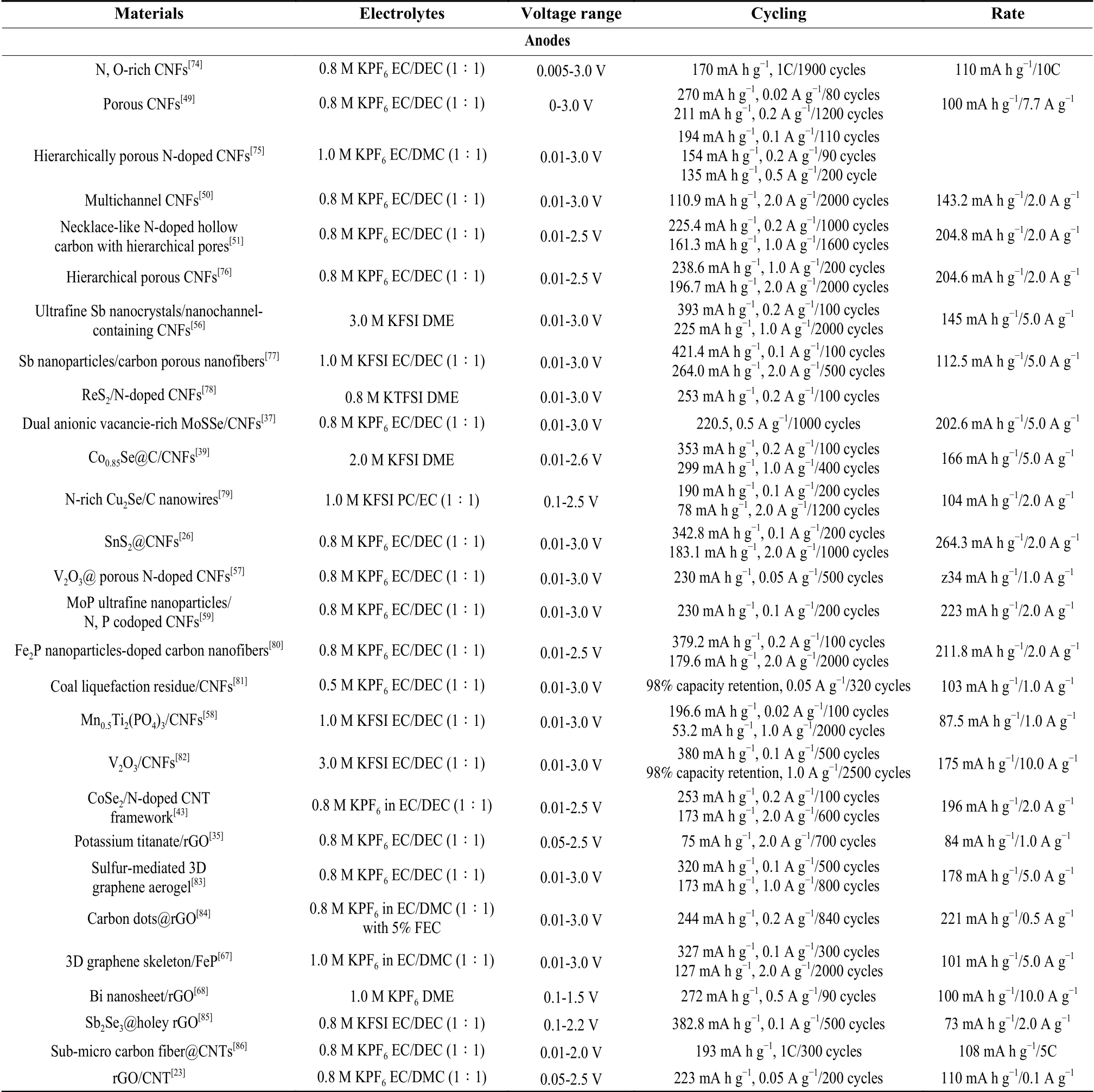
Table 2 Recent progress on electrochemical properties of carbon-based electrodes for flexible PIBs.
Over the past few years, the soaring demand for flexible and wearable electronics, such as bendable smartphones, roll-up displays, and health monitoring skin sensors, has driven great progress in flexible electrochemical energy storage technologies[18-19]. In comparison to conventional EESDs, flexible EESDs have the advantages of foldability, lightweight, and stretchability. With regard to electrode preparation of conventional EESDs (e.g. LIBs), normally, a mixed slurry consisting of active materials, conductive additives, and binders is cast onto foil (copper or aluminum). Herein, insulating and electrochemically inactive binders only play the effect of adhering active materials and conductive additives to current collectors.They not only prevent ions from contacting active materials but also increase the polarization of electrodes.Conductive additives (e.g. carbon black) are used to maintain good electrical contact between active materials and current collectors. However, their contribution to capacity is very limited. In this case, the presence of these two components has negative effects on volumetric/gravimetric energy densities, and meanwhile, side reactions between electrolytes and them reduce the cycling stability of electrodes. In addition,in conventional EESDs, a metal current collector is used to provide an electron transfer path, but their heavy weight whittles the gravimetric energy density of full cells down largely[18]. As for flexible EESDs,they have the same principles as conventional EESDs,but, normally, they are binder-, conductive additive-,and current collector-free. The research on flexible EESDs mainly includes developing electrode materials, separators, and electrolytes. To achieve high-performance flexible EESDs with application prospects,it is crucial to develop amenable flexible electrodes.Various materials have been utilized for flexible electrodes, such as carbon materials, metal materials, and polymers[19]. Among them, carbon materials, e.g.graphene, carbon cloth, carbon nanotubes (CNTs),and carbon nanofibers (CNFs), are the most studied due to their merits of high electrical conductivity, superior chemical and thermal stability, low weight,non-toxicity, and large surface areas. These properties play a decisive role in the performance of flexible EESDs[20-22].
In spite of several reviews on carbon materials for K-based EESDs, their applications in flexible Kbased EESDs, such a crucial system for electrochemical K storage and flexible electronics, has not been systematically summarized. In this review, the recent progress on carbon materials in flexible K-based EESDs including PIBs, PIHCs, and K-S/Se batteries are summarized by highlighting representative studies(Fig. 1). The fabrication methods of carbon-based flexible electrodes, the electrochemical storage principles of each K-based EESD, and the achieved electrochemical performance are presented. Furthermore,challenges to the future development in this field are discussed and perspectives are given. We hope our work may provide comprehensive information to promote their further development toward practical applications.
2 Fabrication methods
Until now, various technologies have been developed to build carbon-based electrodes for flexible EESDs, such as vacuum filtration[23-24], electrospinning[25-26], hydro(solvo)thermal[27-28], electrodeposition[29-30], drop-casting[31], chemical vapor deposition(CVD)[32]. These methods are often used in a manner of combination. Fig. 2 illustrates four main strategies for making carbon-based flexible electrodes, and the detail will be introduced in the following paragraphs.
Vacuum filtration is a simple, scalable, low-cost physical method to prepare carbon-based flexible electrodes and has been widely adopted in the reported literature[23,33-35]. In general, carbon materials (e.g.CNT, graphite, and graphene) and active materials are dispersed into deionized water to form a suspension.Considering the poor dispersion of CNTs in an aqueous solution, CNTs should be modified with acids or surfactants[23,33]. Thereafter, the suspension is suction filtered by a vacuum filtration setup consisting of a Buchner funnel, a filter membrane, and a vacuum instrument (Fig. 2). Finally, the flexible electrode is obtained by drying and peeling off operation.
Electrospinning is a common strategy to fabricate flexible nanofiber films. Typically, active materials or corresponding precursors are homogeneously mixed with a polymer solution, such as polyacrylonitrile (PAN), poly(methylmethacrlate) (PMMA), and polyvinyl alcohol (PVA), and then the mixture is electrospun to a film by electrostatic force. The properties of nanofiber films, such as pore structures, electrical conductivity, and mechanical properties, can be adjusted by changing electrospinning parameters, polymers, additives,etc[36]. To further obtain electrochemically usable carbon-based flexible electrodes, the prepared film should undergo high-temperature carbonization. Sometimes, a following hydro(solvo)thermal,electrodeposition or gas-phase sulfidation/selenization treatment is needed to grow active materials on/in carbon nanofibers[37-39]. It should be noted that some polymers can realize heteroatom doping upon carbonization simultaneously. It is well known that heteroatom doping can enhance the electrochemical reactivity, electronic conductivity, and pseudocapacitance contribution of carbon materials. For example,annealing PAN in an inert atmosphere can obtain Ndoping carbon materials directly.
Hydro(solvo)thermal is a commonly used liquidphase method to synthesize nanomaterials. In the synthesis process, flexible carbon substrates, such as carbon cloth, graphene papers, and CNF/CNT films are firstly immersed into the precursor solution of active materials. After that, the mixture solution is poured into a sealed steel vessel lined with Teflon, and then, it is heated to a certain temperature for several hours,during which nanomaterials can grow on the surface of carbon substrates. Additionally, to improve the crystallinity of active materials, a subsequent annealing treatment is needed[40]. According to the properties of reaction media (e.g. polarity, viscosity, and softness) and heating parameters (e.g. reaction temperatures and time), the morphologies, sizes, phases,and mass loading of active materials can be regulated[41].
Electrodeposition is a simple strategy for producing coherent active material layers coating carbonbased flexible electrodes by passing current in a solution with a three-electrode system. In the reaction system, flexible carbon substrates as working electrodes are deposited by various materials through electrochemical redox reactions. Electrodeposition can realize fine controllability over deposited materials enabled by varying electrodeposition parameters, including current densities/voltages, solution composition,additives, temperatures, reaction time,etc[42].
Apart from the above-mentioned methods, other effective strategies have also been adopted to fabricate carbon-based flexible electrodes.
(1) CVD. The CVD method plays two main roles to make flexible carbon-based electrodes. One is used for synthesizing flexible carbon electrodes, such as CNT frameworks and films[32,43]. The other one is loading active materials onto carbon-based flexible substrates[44].
(2) Pyrolysis. Typically, flexible polymer sponges, papers,etc. are used as precursors, and they are annealed at a high temperature under an inert atmosphere to obtain flexible carbon foam and papers[45].
(3) Doctor blade. It is also known as knife coating, being a well-established technique for producing large-area films over rigid or flexible substrates. In a typical procedure, active material-containing slurry is transferred to a substrate by moving a blade over a flat base[46].
(4) Spray printing. Ink mixed with active materials and carbon materials is printed onto a polymer(e.g. PVA) coating substrate. Later, the polymer is dissolved, and a flexible film is obtained[47].
(5) Melt infiltration. This method is mainly used for K-S/Se battery cathodes. Flexible carbon substrates are mixed with bulk S/Se and vacuum-sealed in a quartz tube. Afterward, the tube is heated for several hours to obtain composite cathodes[48].
Each manufacturing method owns its intrinsic advantages and shortcomings. Vacuum filtration is a feasible method for industrially large-scale production because only mechanical stress is required when carbon-based flexible electrodes are constructed. This method can realize the precise and simple regulation of the composition of flexible electrodes by changing the amount of added materials. However, the interaction between carbon materials and active materials is weak, which is negative on the stability of flexible electrodes during electrochemical cycling. Electrospinning is a high-efficient and widely applicable technology to fabricate flexible 2D fibric films. After carbonization, the electrospun film is transferred into flexible CNF films or active material-CNF composite films. The flexible CNF film can be further served as a flexible substrate to plant active materials by hydro(solvo) thermal, CVD, electrodeposition,etc, while flexible active material-CNF composite films can be applied directly. However, the high temperature used in carbonization greatly increases the production cost.In addition, some kinds of embedded active materials are not stable at high temperatures, leading to their decomposition or phase transfer. Hydro (solvo) thermal is a good route to grow active materials onto flexible carbon substrates and meanwhile, can realize the nanostructuring of active materials. It is well-known that nanostructured active materials have large specific surface areas and short ion and electron diffusion length. However, its reaction conditions and processes are harsh and time-consuming, and it is difficult for large-scale applications. Also, the volume expansion of active materials during potassiation cannot be solved because they are exposed on the surface of carbon substrates. This problem also persecutes the composite electrode prepared by electrodeposition and CVD methods. In addition, as for electrodeposition and CVD, the high cost and strict synthesis conditions severely limit their practical applications.Moreover, many materials cannot be deposited by these two methods. Accordingly, none of them is perfect, and the cost, electrochemical performance, material property, application environment,etc. should be considered and balanced in advance.
3 Application in flexible electrochemical potassium storage devices
3.1 PIBs
3.1.1 Potassium storage mechanisms
Similar to LIBs, PIBs have a typical sandwich configuration, consisting of an anode, a cathode, a porous membrane, and an appropriate electrolyte.During charging, K+is extracted from cathodes and impregnated into K salt-containing electrolytes immediately. Meanwhile, the K+of electrolytes is inserted into anodes. Upon discharging, a reverse process takes place. Owing to the whole discharge-charge reaction process being similar to a rocking chair, this kind of battery system is named “rocking-chair batteries”[11].
3.1.2 CNF-based flexible electrodes
For flexible electrodes, CNFs are a common flexible material as active materials or substrates, and the combination of electrospinning and carbonization is the most widely adopted method to fabricate CNF films. The entire surface of such 2D films can contact electrolytes, and 1D nanofibers with high conductivity are favorable to the transportation of electrons.Normally, the prepared CNF film has good flexibility and thus can be used directly as flexible electrodes.Xuet al. utilized PAN as a precursor to synthesize a porous carbon nanofiber paper by electrospinning followed carbonization, and explored its electrochemical potassium storage capability[49]. The porous CNF paper exhibited good flexibility, and there was no fracture after bended by 180°. The porous structure could mitigate the volume expansion during K+insertion effectively, and the radius expansion of CNFs is negligible (~0.7%) after full potassiation. Served as a PIB anode, it showed a high reversible capacity of 270 mA h g-1at 0.02 A g-1and a very low decay rate of 0.01% per cycle over 1 200 cycles. After an electrochemical cycling test, the porous CNFs remained a well-interconnected entire architecture without fracture or pulverization. Like other carbon materials,morphologies also have a great impact on the electrochemical properties and behaviors of CNFs. Zheng and co-workers constructed multichannel CNFs by regulating the ratio of PMMA/PAN[50]. The fibers without adding PMMA showed the typical smooth surface of PAN-derived CNFs, while the multichannel morphologies gradually formed and became obviously with increasing the amount of PMMA (Fig. 3a).However, the excessive PMMA led to the split of CNFs, thus reducing the mechanical property. As a flexible electrode, this is not conducive to practical applications. Electrochemical tests showed that the material with the optimized ratio as anodes possessed superior cycling stability (110.9 mA h g-1at 2 000 mA g-1after 2 000 cycles) and rate performance. Such high performance was attributed to plentiful space for volume changing, highly conductive interconnected structures for fast electrons and K+transfer, andin situN-, O- doping provided more active sites for K+storage. Template methods are another good option for controlling the morphology of CNFs prepared by electrospinning. Guo’s group took ZnO as a template to design necklace-like N-doped hollow carbon possessing hierarchical pores[51]. Monodisperse ZnO nanospheres were firstly mixed with PAN and dimethylformamide to form an electrospinning precursor, and then it was electrospun into a fiber film. In the following successive high-temperature treatment under NH3and Ar, ZnO was removed,while necklace-like hollow carbon fibers were obtained (Fig. 3b). The ultra-high pyridinic/pyrrolic-N doping, large specific surface area, and porosity could facilitate the (de)intercalation of K+significantly.Combining these merits, it delivered a high capacity of 293.5 mA h g-1at a current density of 100 mA g-1and showed excellent rate capability of 204.8 mA h g-1at 2 000 mA g-1and cycling life (161.3 mA h g-1at 1 000 mA g-1after 1 600 cycles) when it was served as an anode.
Electrospun CNFs are also used as a matrix for conversion- and alloying-type materials to alleviate their problem of volume expansion during potassiation. Meanwhile, flexible composite electrodes with high capacities can be obtained. Metal chalcogenides(MCs) are also promising research objects by virtue of their high theoretical specific capacities and unique morphologies[52-53]. In general, there are two composite types. One is embedding MCs into CNFs, where CNFs can improve the electronic conductivity of MCs and inhibit the large volume expansion of MCs during conversion/alloying reactions. Firstly, MCs or MC precursors are dispersed into an electrospinning precursor uniformly. Thereafter, the mixing solution is electrospun into a film. Finally, the MC-embedded CNF film is obtained through carbonization or sulfidation/selenization. For example, Ciet al. reported freestanding and flexible SnS2@C nanofibers as a foldable PIB anode by a combination preparation process of electrospinning, pyrolysis, and sulfidation[26]. The SnS2@C nanofiber film displayed superior flexibility in deformation tests (Fig. 3c). The K+storage capability was firstly evaluated in half cells. Its capacity remained 342.8 mA h g-1(at 100 mA g-1) after 200 cycles, corresponding to a 96.62% capacity retention ratio. Also, the rate capacity could reach 219.4 mA h g-1at 5 000 mA g-1, and a capacity of 183.1 mA g-1was sustained after 1 000 cycles in a long-term cycling test at 2 000 mA g-1. Considering its good flexibility, a pouch-packed full cell was assembled. As shown in Fig. 3d, the pouch cell could light 27 red LEDs on easily, and kept working in folded and subsequently released states. The other one is coating MCs onto the surface of CNFs. During preparation processes, electrospun polymer fiber films are firstly carbonized, and then the obtained carbon fiber film is subjected to CVD, electrodeposition, or hydro(solvo)thermal treatment to plant MC nanosheets. This method is generally adopted by layered MCs because the agglomeration problem of MC nanosheets can be suppressed, thus exposing more active sites. Huanget al. developed a dual anionic vacancy-rich MoSSe array anchored carbon nanofiber membrane (v-MoSSe@CM) as a free-standing anode for PIBs (Fig. 3e)[37]. PAN nanofiber membrane was obtained by electrospinning, and then it was pre-oxidized and carbonized under an inert atmosphere. Afterward, v-MoSSe arrays were anchored through hydrothermal and annealing processes. The composite maintained K storage capacities of 234.2 and 220.5 mA h g-1at 0.5 A g-1after 200 and 1 000 cycles, respectively. A rate performance test featured a high capacity of 202.6 mA h g-1at 5 A g-1.
Alloying-based metal materials, such as Sb, Bi,and Sn, have been investigated as electrode materials because they can offer high theoretical capacities.However, they usually undergo a huge volume change during cycling processes, and thus electrodes may suffer from mechanical stress, delamination, and pulverization. These problems are normally the origin of high voltage hysteresis and poor cycling performance[54-55]. As mentioned before, electrospinning can encapsulate active materials within carbon fibers, which can largely relieve the volume expansion of alloying-based metals. For instance, Zhou’s group impregnated ultrafine Sb nanocrystals within nanochannel-containing CNFs[56]. The ultrasmall Sb nanocrystals and the hollow nanochannels enabled fast reaction kinetics and easy strain relaxation. Exactly due to these advantages, the electrode as the anode of PIBs achieved a high capacity and good cycling stability (225 mA h g-1at 1 A g-1after 2 000 cycles) (Fig. 3f). Apart from MCs and alloying-based materials, metal oxides, metal phosphides,etc. were also used in flexible PIB electrodes and exhibited great prospects[57-59].
3.1.3 CNT-based flexible electrodes
CNTs have two types, i.e. single-walled CNTs(SWCNTs, single graphene sheet) and multi-walled CNTs (MWCNTs, additional graphene sheets rolled up around SWCNTs withvan der Waalsforce between neighboring layers). 1D electron transport path ensures their high electrical conductivity. Additionally, they have high tensile strength and chemical resistance. CNTs have been widely used as electrode materials or act as carbon modification materials[60].The most common and facile method to construct CNT-based flexible electrodes is vacuum filtration.For instance, Xu and co-workers designed a K0.5V2O5and CNTs hybrid film through vacuum filtration and investigated its electrochemical properties as a PIB cathode[61]. Evaluated in PIB half cells, the K0.5V2O5/CNT electrode exhibited good reversible specific capacities, retention, and rate performance. It was coupled with a hard carbon anode to assemble a flexible cable-shaped PIB full cell (Fig. 4a). The full cell could deliver a capacity of 51 mA h g-1at 20 mA g-1after 30 cycles and could power a red LED at various bending angles. Recently, a two-step strategy combing CVD and solvothermal treatment was adopted to fabricate metallic octahedral CoSe2threaded by a N-doped MWCNT framework(NCNF@CS) (Fig. 4b)[43]. where NCNF was first synthesized by CVD, and then CoOxand CoSe2were formed on NCNF successively through solvothermal treatment. In flexible testing, NCNF@CS displayed high flexibility (Fig. 4c). When it was employed as a PIB anode, a capacity of 253 mA h g-1at 0.2 A g-1after 100 cycles with ~85.3% capacitance retention was achieved, and a long-term cycling test featured a stable capacity (173 mA h g-1at 2.0 A g-1after 600 cycles).
3.1.4 Graphene-based flexible electrodes
Graphene, a 2D sheet, is comprised of sp2-bonded single-layer carbon atoms with a honeycomb lattice structure. It has a high electron mobility (>15 000cm2V-1at room temperatures), low resistivity (even lower than that of silver), and a high surface area(~2 630 m2g-1)[62-64]. All of these excellent properties have aroused extensive attention in electrochemical energy storage. In graphene, every atom is available, leading to high electrochemical activity and low diffusion resistance for ions to access the surface[65]. It is calculated that its theoretical specific capacity is 744 mA h g-1[66]. Moreover, the merits of exceptional mechanical robustness, good flexibility, and ultralow weight make graphene become a promising candidate for flexible electrodes. The flexible graphene film/paper can be used as an electrode and a substrate to form a hybrid electrode.
Zhanget al. fabricated a free-standing film of potassium titanate (KTO)/rGO with a sandwiched structure through a strategy of the confined transformation of MXene/rGO hybrid films[35]. The film could display outstanding electrochemical performance in both cycling and rate tests when it was applied as a PIB anode. This was attributed to the synergetic effect of the unique sandwiched structure, short diffusion distance originating from ultrathin KTO layers, enhanced electronic conductivity provided by graphene, and good compatibility between layered nanosheets. Likewise,to achieve high-performance electrode materials,graphene has been hybridized with other active materials to form composite electrodes. Considering the issues of large volume variation, poor intrinsic electronic, and ionic conductivity of transition-metal phosphides, hollow FeP nanospheres were encapsulated within a 3D graphene framework (3DG/FeP) to form a flexible and hierarchically porous PIB anode(Fig. 4d)[67]. The porosity and high mechanical strength endowed the 3DG/FeP aerogel with good flexibility, as shown in Fig. 4e. In comparison to those of pure FeP, the rate and cycling performance of 3DG/FeP had significant improvement. Also, the 3DG/FeP displayed ultrahigh cycling stability with a capacity retention of 97.6% at 2 A g-1after 2 000 cycles. Qiuet al. reported a bismuth nanosheet/graphene (BiNS/rGO) electrode prepared by vacuum filtration (Fig. 4f)[68]. Bi, as an alloy-based electrode material, has a high theoretical specific capacity, but it endures a large volume expansion of ~411% during discharge processes, resulting in severe capacity fading. BiNS/rGO network with controlled pore structures which effectively tackle the expansion and alleviate the structural failure of electrodes during electrochemical cycling, and facilitate the transfer of K+and electrons. When cycled at 0.5 A g-1, the BiNS anode only kept a reversible capacity of 104 mA h g-1after 90 cycles, while BiNS/rGO still delivered 272 mA h g-1after the same cycling number. As for rate performance, the capacity of BiNS was only 30 mA h g-1at 2 A g-1, and there was nearly no capacity when current densities were higher than 5 A g-1. By contrast, the capacity of BiNS/rGO maintained 100 mA h g-1even at 10 A g-1.
3.1.5 Carbon sponge/carbon foam/carbon clothbased flexible electrodes
Carbon sponges, carbon foam, and carbon cloth,such carbon materials with high flexibility, are commonly used to synthesize flexible electrodes owing to their great electrical conductivity, superior mechanical strength, and large surface areas to accommodate active materials. In addition, they are scalable and commercially available. Qiuet al. developed a nitrogen and phosphorus dual-doped vertical graphene (N,P-VG) uniformly grown on carbon cloth (N, PVG@CC) as the binder-free anode of flexible PIBs(Fig. 5a)[13]. The self-supported VG nanosheet arrays were firstly planted on carbon cloth using a microwave plasma-enhanced CVD method. Afterward, it was annealed in NH3mixed with PH3generated by the decomposition of NaH2PO2·H2O to realize N and P co-doping simultaneously. Electrochemical tests in half cells showed that N, P-VG@CC had superior cycling and rate performance. Moreover, a flexible full cell was assembled using Prussian blue (KPB) as the cathode. As a result, the full cell delivered a reversible capacity of 116.25 mA h g-1at 50 mA g-1and only had a 14.1% capacity decay after 150 cycles. The maximum energy density and power density reached 232.5 W h kg-1and 4 000 W kg-1, respectively. More importantly, the full cell was capable to light a neon sign consisting of 57 LEDs and power the LED green light of a wearable watch (Fig. 5a). Yu and co-workers reported a flexible nitrogen and oxygen dualdoped carbon-coated graphene foam film (Fig. 5b)[69].In preparation, graphene foam was synthesized by the pyrolysis of CH4using commercial nickel foam as a template, and then, graphene@Ni foam was coated by PPy by electrochemical polymerization. Finally,NOC@GF was obtained after etching and heat treatment. Serving as a PIB anode, it displayed a high reversible capacity (319 mA h g-1at 0.1 mA g-1with a capacity retention of 94.9% after 550 cycles) and excellent rate capability (123 mA h g-1at 5.0 mA g-1).Impressively, its capacity still reached 281 mA h g-1at a current density of 1 A g-1after 5 500 cycles with Coulombic efficiencies (CE) nearly approaching 100% and a capacity retention of 98.1% (Fig. 5c). The excellent electrochemical properties were attributed to the high electric conductivity of graphitic carbon,abundant active sites of amorphous heteroatom-doped carbon layers, and the favorable K-intercalation of 3D interconnected structure of NOC@GF. As for carbon sponges, several works took it as a substrate to load active materials to fabricate flexible PIB electrodes. In general, carbon sponges are obtained by pyrolysis of a polymer sponge in an inert atmosphere, during which heteroatom N from polymers themselves can be doped into carbon sponges. To anchor active materials, hydro(solvo)thermal, electrodeposition,etc. should be further performed. For example, high-temperature calcination combing a solvothermal method was adopted by Yuet al. to synthesize ultrathin CoSe2nanosheets coated 3D N-doped carbon foam (CSNS/NCF)(Fig. 5d )[45]. The as-prepared sample had good flexibility, and could fully recover to its original shape after being bent to different angles, as illustrated in Fig. 5e.The sample with the optimized solvothermal temperature had great long-term cycling life (212 mA h g-1at 1 000 mA g-1after 1 000 cycles) and great rate performance (225 mA h g-1at 2 000 mA g-1).
3.1.6 Hybrid carbon-based flexible electrodes
Sometimes, the above carbon materials are used in combination rather than individually with a view to achieving synergetic improvement. For example,graphene has great electrochemical ion storage ability theoretically, but, in fact, the electrochemical performance of graphene films is unsatisfactory due to the barrier of electron and ion diffusion in the crossplane direction. In this case, Han and co-workers fabricated an rGO/CNT hybrid paper by introducing CNTs (Fig. 6a)[23]. Highly conductive CNTs could not only prevent restacking and increase the interlayer spacing of graphene, thus providing more active sites for ions, but also bridge graphene layers to facilitate electron and ion transport in the cross-plane direction.All rGO/CNT hybrid papers with different weight ratios (10%, 20% and 30%) exhibited better K storage capability than pure rGO papers (Fig. 6b-c).
Electrospinning with subsequent carbonization is an easy method to produce CNF films. However, their graphitization degree is usually low, which reduces the electrical conductivity and mechanical properties of CNF films[70-71]. To offset this issue, Chenet al. introduced CNTs with outstanding mechanical properties and conductivity into electrospun CNFs, as shown in Fig. 6d[72]. Meanwhile, ZnO was utilized as a sacrificial agent to construct multi-channel hollows for improving access to active sites, and sulfur powder was employed as a sulfur-doping precursor to increase the layer spacing, conductivity, and chemical reactivity.When served as a PIB anode, the free-standing S/N doped CNT/CNF electrode showed a high discharge capacity (212.5 mA h g-1at 1 A g-1after 1 000 cycles), long cycling life (100.1 mA h g-1at 5 A g-1after 5 000 cycles), and high-rate performance(108.7 mA h g-1at 5 A g-1). Zheng and co-workers reported graphene/porous nitrogen-doped carbon nanofibers (G-PCNFs) and investigated their electrochemical performance (Fig. 6e)[73]. Reversible capacities of 358 and 276 mA h g-1were achieved after 200 and 2 000 cycles at 0.1 and 2 A g-1, respectively,which were higher than those of all the control samples (PCNFs, G-CNFs, and CNFs). Its rate capability was the best among all the prepared samples. The authors pointed out that the enhanced performance was attributed to the improvement of the electronic conductivity provided by graphene and a large number of mesopores exposing abundant N-doped active sites for the adsorption of K+.
3.2 PIHCs
3.2.1 Potassium storage mechanisms
PIHCs have the same assembling components as PIBs. According to whether the electrolyte is consumed during electrochemical processes, the electrochemical K storage mechanism of PIHCs can be classified into electrolyte-consuming, ion exchange, and hybrid mechanisms. (1) Electrolyte-consuming mechanisms. Battery-type materials are used as anodes,while capacitor-type materials act as cathodes. In charging processes, K+is inserted into anodes, while anions (PF6-, FSI-,etc) are absorbed on the surface of cathodes. In discharging processes, K+and anions go back to electrolytes to reach a charge balance state.(2) Ion exchange mechanisms. K-containing battery materials and capacitor-type materials are the cathode and the anode of PIHCs. In this system, electrolytes are only used to transport K+and its concentration keeps constant during electrochemical reactions. K+is extracted from the cathode during charging, and meanwhile, the released K+is absorbed on the anode.The behavior of K+is the opposite during charging.(3) Hybrid mechanisms. The key feature of this kind of PIHCs is that one or two of electrodes use the material with both battery- and capacitor-behaviors[96].
3.2.2 Carbon-based flexible electrodes
CNF-based flexible films have been also widely utilized as PIHC electrodes. Wenet al. took a dimethylformamide solution containing ZIF-8, triphenylphosphine, urea, and PAN as an electrospinning precursor constructing phosphorus/nitrogen co-doped hierarchical porous CNFs (PN-HPCNFs), as illustrated in Fig. 7a[97]. PN-HPCNFs exhibited a 3D interconnected network structure with successive macropore cavities, which were favorable for the accessibility of ion and electron transfer. In comparison to HPCNFs without N or P/N doping, PN-HPCNFs had wider interlayer spacing, which is beneficial to the reversible insertion/extraction of large-sized K+. P and N co-doping resulting in abundant defects and extra active sites could reduce K+diffusion barrier and improve capacitance contribution. Consequently, PNHPCNFs exhibited excellent K storage capability when evaluated in PIBs as an anode. Furthermore, a PIHC full cell was assembled, where activated PNNPCNFs (APN-NPCNFs) acted as the cathode. A maximum energy density of 191 W h kg-1was achieved at a power density of 100 W kg-1. When the power density increased to 7 560 W kg-1, the energy density still retained 86 W h kg-1. More impressively,capacity retention reached 82.3% after 8 000 cycles,corresponding to only 0.002 2% fading per cycle. The full cell could light a pattern consisting of 43 LEDs and drived a miniature windmill (Fig. 7b). Recently,MC-CNF composite films have been investigated as PIHC electrodes. Zhang and co-workers designed tiny CNTs capped with nickel sulfide nanocrystallites(NSCN hollow fiber)[98]. The authors investigated the physicochemical properties of three nickel sulfide nanocrystallites with different phases (α-NiS, β-NiS,and NiS2) inside the NSCN fiber. Both theoretical and experimental results indicated that α-NiS had the best kinetics and durability. Considering the superior comprehensive properties and good mechanical characteristics, a full cell that the α-NiS-based NSCN hollow fibers and central hollow carbon fibers were used as the anode and the cathode, respectively, was assembled. The full cell showed an energy density of 187 W h kg-1at a power density of 1.7 kW kg-1, and the energy density was as high as 127 W h kg-1even at 8.4 kW kg-1. Moreover, a sandwich-type flexible PIHC was assembled using the same materials as the electrodes and a perfluorinated sulfonic resin polymer as the electrolyte. The mechanical and electrochemical properties of the flexible PIHC are shown in Fig. 7c-d. More importantly, it exhibited good electrochemical performance even at low temperatures(Fig. 7e-f).
Biomass carbon materials have been explored as electrode materials for flexible PIHCs because of their wide availability and low cost. For example, Ye’s group constructed a flexible freestanding electrode consisting of porous carbon tubes using Metaplexis japonica fluffs (FSF-PCTs) as a precursor by successive microwave treatment, carbonization, and CO2perforation[103]. FSF-PCT as both the anode and the cathode presented superior electrochemical performance in specific capacities, cycling life, and rate performance. In this case, a symmetrical PIHC using FSFPCTs as both the anode and the cathode is assembled.The all-carbon-based symmetrical PIHC delivered an energy density of 117.8 W h kg-1at a power density of 450 W kg-1and possessed favorable cycling stability(37 mA h g-1at 1 A g-1after 1 500 cycles). Paper is also a promising material for the preparation of flexible carbon electrodes due to their industrial availability, low cost, environmentally friendliness, and recyclability. Wang’s group designed a flexible hard carbon microbelt (HCMB) film using sanitary tissue paper as a precursor through simple carbonization[15].The HCMB film had mechanical flexibility and could be bent into any shape without breaking (Fig. 7g). The HCMB film inherited the primary morphology of tissue paper and exhibited high electronic conductivity and a low surface area. The HCMB anode delivered a larger charge specific capacity of 204 mAh g-1at 100 mA g-1below 1.00 V compared with graphite(191 mAh g-1) and soft carbon (156 mAh g-1). The initial CE was as high as 88% (graphite: 46%, soft carbon: 35%). Moreover, both rate and cycling performance were satisfactory. Over 75% of the discharge plateau located below 0.25 V endowed the HCMB//activated carbon full cell with a high operating working voltage (4.5 V) and a large energy density of 152 W h kg-1).
3.3 K-S/Se batteries
3.3.1 Potassium storage mechanisms
The cell configuration of K-S/Se batteries is composed of a S/Se cathode, a metallic K anode, a separator, and an organic electrolyte. The charging/discharging process involves reversible plating/stripping of metallic K on the negative side and oxidation/reduction reactions of S/Se on the positive side. In K-S batteries, solid S8is reduced to soluble high-order potassium polysulfides K2Sn(5<n<6) and insoluble low-order potassium polysulfides K2Sn(1<n<4) during discharging. During charging, the discharged products are partially oxidized to K2S5and K2S6[8,107]. As for K-Se batteries, the final discharged product K2Se has been demonstrated by many works,but whether there are intermediate processes producing potassium polyselenides is still being debated[108].In the following charging process, the produced K2Se at the fully discharged state is converted to Se[108-110].Until now, the specific reaction processes have been unclear in both K-S and K-Se batteries, and more exploratory studies are needed.
3.3.2 Carbon-based flexible electrodes
To date, there are limited reported works on carbon-based flexible electrodes for K-S/Se batteries,most of which are based on CNF films prepared by electrospinning. Yu’s group developed N/O dualdoped CNFs with interconnected micro/mesopores(MMCFs) by carbonizing electrospun ZIF-8/PVP nanofibers and used them as a host material to manipulate the Se molecular configuration of flexible K-Se batteries (Fig. 8a)[111]. The micropores could confine small Se2-3molecules, inhibiting the formation of polyselenides and serve as a physical barrier to maintaining cycling stability. As for the mesopores, they could confine long chain Se4-7, confirming a high Se loading and contributing to a high discharge voltage of Se@MMCFs. The N/O co-doping and the 3D interconnected structure were able to improve the electrical conductivity and keep the structural integrity during reaction processes. Based on these merits, the optimal electrode (Se2-3/Se4-7-MMCFs) achieved satisfactory stability (395 mA h g-1at 1 A g-1after 2 000 cycles) and a high specific energy density of 400 W h kg-1.
Except for CNFs, flexible electrodes based on other carbon materials have been developed. Yu and co-workers encapsulated Se into CNTs interwoven N,O dual-doped porous carbon nanosheets (Se@NOPCCNT) to construct a flexible free-standing Se/carbon composite film (Fig. 8b)[109]. N, O dual-doped porous carbon nanosheets were derived from chestnut inner shells, and NOPC-CNT was obtained by vacuum filtration. It is believed that the electrode is low-cost and readily scalable. Zhuet al. adopted three types of activated carbon fiber (ACF) cloth with different specific surface areas and microstructures as porous carbon matrices of K-S battery cathodes (Fig. 8c)[48]. By comparing their electrochemical performance, the authors found that the controllable physical confinement and chemical adsorption toward soluble polysulfides could be achieved by adjusting the specific surface area,porous architectures, and surface functional sites of the ACF@S composite cathode. In addition, reducing the cloth thickness and the fiber diameter was conducive to increasing the operation stability and the sulfur utilization of composite cathodes.
4 Conclusions and perspectives
In this review, we systematically introduce the recent research advances in carbon materials for flexible electrochemical potassium storage including PIBs, PIHCs, and K-S/Se batteries. The common preparation methods of carbon-based flexible electrodes have been presented. The obtained electrochemical performance, voltage windows, and electrolytes are collected, as presented in Tables 2-4. Carbon materials have many excellent properties, such as low weight, non-toxicity, and abundance, which make them competitive in flexible electrodes. They can be used as active materials directly and the substrate of composites. When used as active materials, their material properties and electrochemical performance are largely influenced by heteroatom doing, graphitization degree, morphologies,etc. As substrate materials,they are normally incorporated with active materials that possess a high theoretical specific capacity, such as alloying materials, metal chalcogenides/phosphides, and organic materials. Herein, it should be mentioned that different carbon materials possess their advantages and shortcomings in terms of electrical conductivity, cost, flexibility, mechanical strength,etc. Therefore, in such an application-oriented research field, it is necessary to comprehensively consider and balance various factors during research progress. So far, although the related study has achieved encouraging results as described above, there are still many challenges to be further addressed and substantial room for improvement. Based on the recent advances, five possible research directions in this realm are proposed in the following parts (Fig. 9).
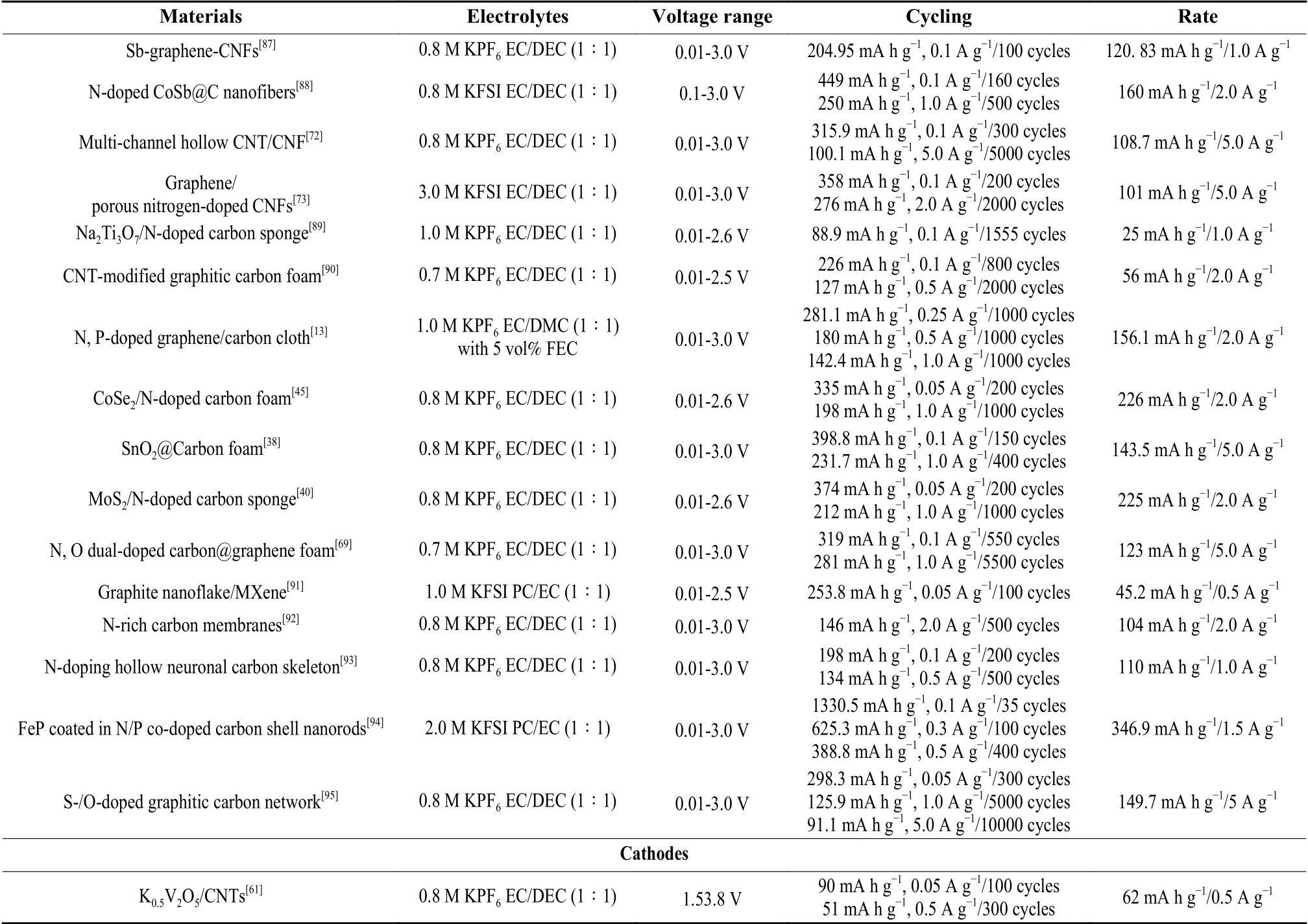
Table 2 Recent progress on electrochemical properties of carbon-based electrodes for flexible PIBs. (Continued).
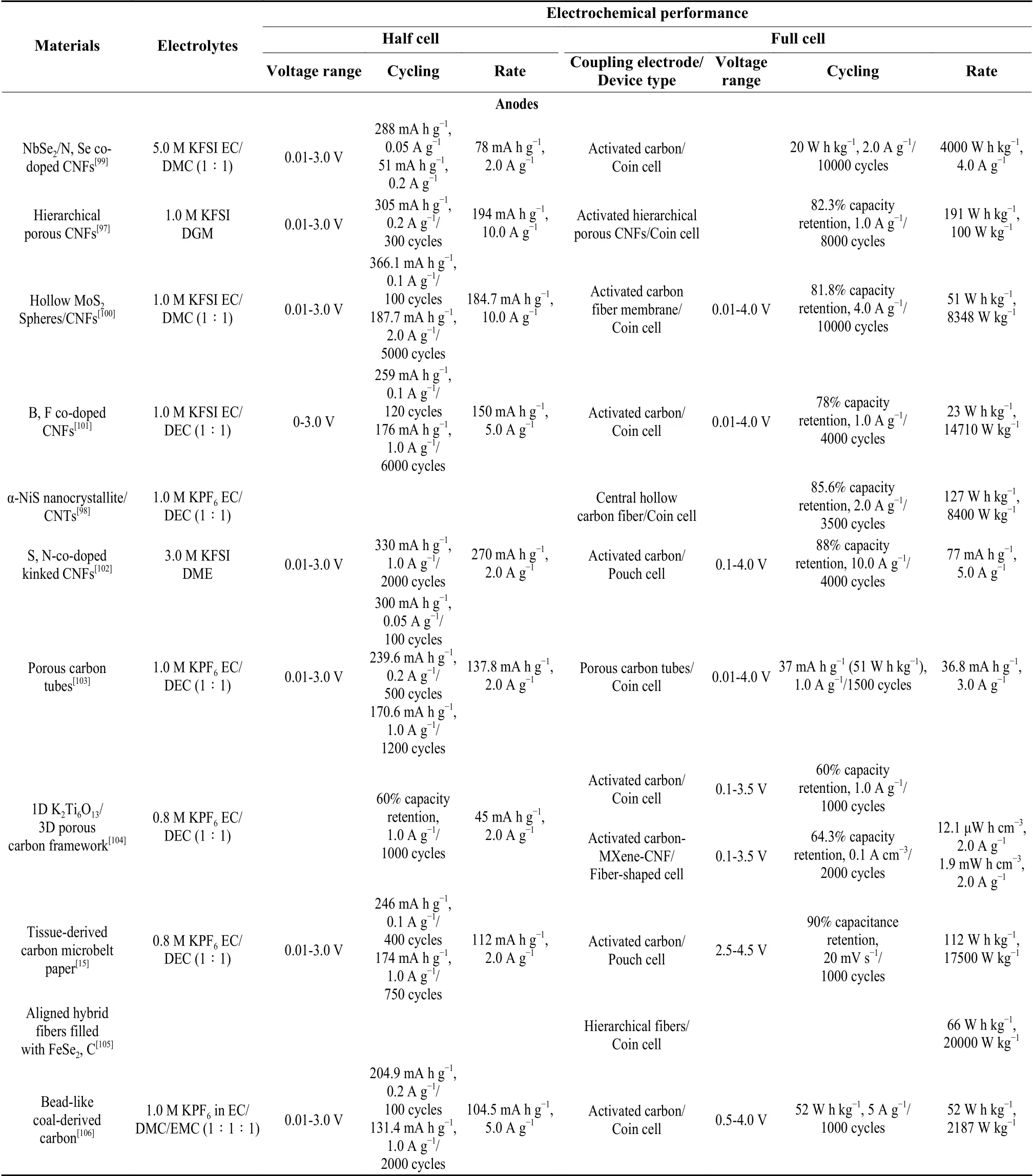
Table 3 Recent progress on electrochemical performance of carbon-based electrodes for flexible PIHCs.
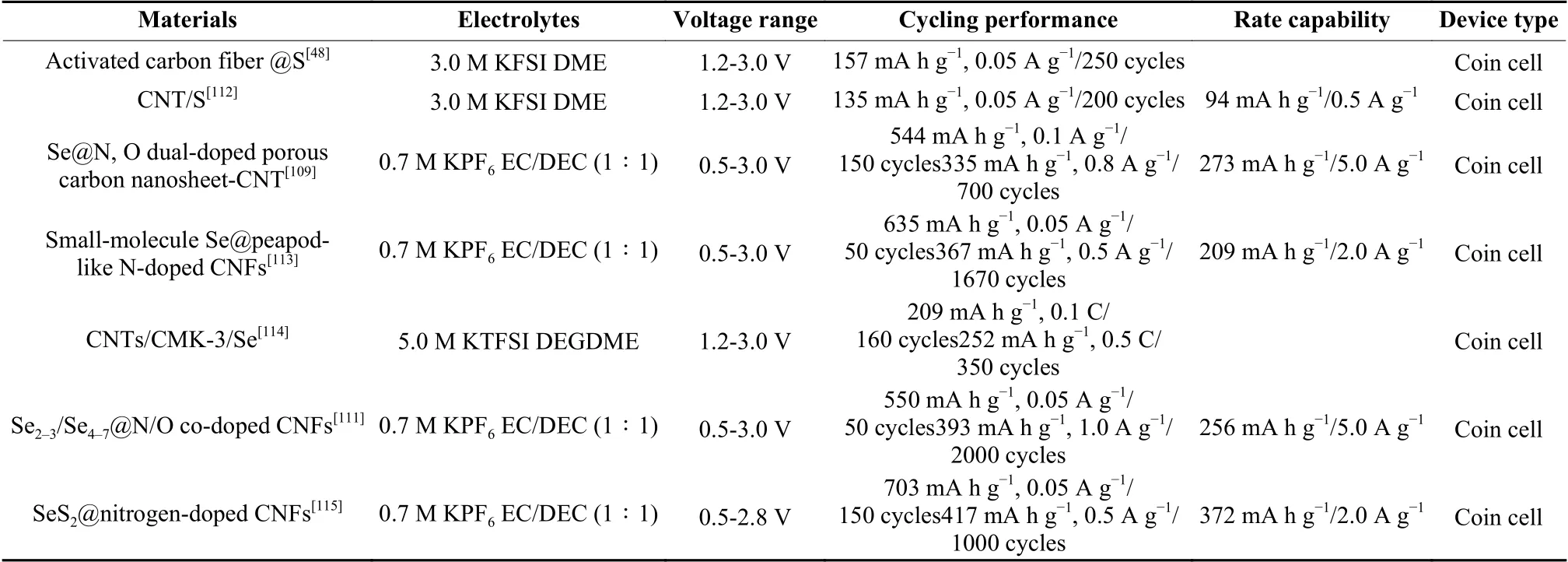
Table 4 Recent progress on electrochemical properties of carbon-based electrodes for flexible K-S/Se batteries.
(1) Mechanical robustness of flexible electrodes.Previous works mainly focus on simply exhibiting the bendability and elasticity of flexible electrodes or the flexibility of full cells. However, according to different practical application environments, flexible EESDs will face various working modes and will be twisted, folded, distorted, and bent. In this case, flexible electrodes are at risk of crack or even fracture,which directly affects the lifespan, stability, and safety of flexible cells. In addition, the flexibility of flexible electrodes after electrochemical cycling tests has not received enough attention. Therefore, it should be examined the intrinsic mechanical robustness after flexibility testing and the integrity after electrochemical testing. Moreover, relevant evaluation criteria have not been established, and thus, it is highly necessary to set standards to quantify the mechanical robustness of flexible electrodes.
(2) Cathodes used for flexible PIBs. Until now,the report on cathodes for flexible PIBs is quite rare.For one thing, developed cathode materials are limited. For another thing, these cathode materials are typically ternary or even multi-elemental, which are difficult to directly grow on most flexible carbon substrates. To realize practical applications, it is necessary to develop high-performance flexible cathodes to match with flexible anodes for assembling flexible full cells. Prussian blue analogues (PBAs) are a kind of most studied cathode materials due to their superior structures and electrochemical properties. They can be easily loaded on the surface of various carbon materials by hydrothermal, coprecipitation, electrodeposition,etc. Layered transition metal oxides and polyanionic compounds are other two popular cathode materials with high operating potentials. Among them,those possessing high-temperature stability may be potential candidates. Firstly, they are embedded into nanofibers by electrospinning to form a composite film. Afterward, the film is transferred into a layered transition metal oxide or polyanionic compound/CNF composite flexible electrode.
(3) Flexible electrolytes. Liquid electrolytes are the most used electrolytes in EESDs, but their drawbacks of flammability, toxicity, volatility, fluidity, and potential risk of leakage restrict their safe application.To avoid these issues, solid-state electrolytes have attracted extensive attention. Meanwhile, they have good thermal and electrochemical stability at wide voltage ranges and appropriate mechanical strength to resist metal dendrite growth. Gel polymer electrolytes,as a solid-state electrolyte, composing of a conventional liquid electrolyte and a polymer matrix, not only possess the advantage of solid-state electrolytes,but also inherit the flexibility of gel polymer matrix.They are conducive to developing flexible K-based EESDs. Although the research on K-based gel polymer electrolytes is in its infancy, the advanced experience achieved in the field of LIBs may provide a guiding reference.
(4) Flexible full cells. The electrochemical performance evaluation of most flexible electrodes was carried out in coin cells in terms of half cells, as introduced in Section 3 and shown in Tables 2-4. This is far from enough because the electrochemical performance of electrode materials in full cells is more valuable for practical applications. Normally, the key electrochemical parameters in half and full cells show significant differences. In full cells, researchers cannot only focus on the electrochemical performance of electrode materials. The choice of coupling electrodes,the ratio of cathodes and anodes, the compatibility of electrolytes, the voltage window of full cells,etc.should also be comprehensively considered. As for flexible K-based full cells, designing and fabricating processes are more complex. For example, flexible substrates are essential, whereas their contribution to the electrochemical performance is limited or even negative. In addition, attention should also concentrate on the selection and optimization of cell components, such as packaging materials, which directly affect the energy density of flexible full cells.
(5) Application environments. The flexibility of flexible K-based EESDs largely expands their application fields, making their working environments changeable. In this case, special consideration should be given to their electrochemical performance, safety,and durability. For instance, flexible K-based EESDs as the power of flexible electronics face environmental temperatures and environmental chemistry changing frequently. This places greater requirements on flexible K-based EESDs. Therefore, the electrochemical properties of electrode materials and electrolytes at wide temperature ranges and the chemical resistance of packaging materials should be investigated when flexible K-based EESDs are designed.
All in all, although there are still many problems and challenges, the excellent material, physical, and chemical properties of carbon materials and the achieved encouraging results in flexible K-based EESDs endow them with a bright future in such an emerging field.
Acknowledgements
The authors acknowledge the support by the Key R&D project of Liaoning Province of China(2020JH2/10300079), the “Liaoning BaiQianWan Talents Program” (2018921006), the Liaoning Revitalization Talents Program (XLYC1908034), the Engineering and Physical Sciences Research Council(EP/V000152/1, EP/X000087/1), the Leverhulme Trust (RPG-2021-138), and the Royal Society(RGS/R2/212324, SIF/R2/212002), and Shenyang University of Technology. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising. The authors thank Dr.Chenglin Zhang (Jiangsu University) for his helpful discussions.
Abbreviations
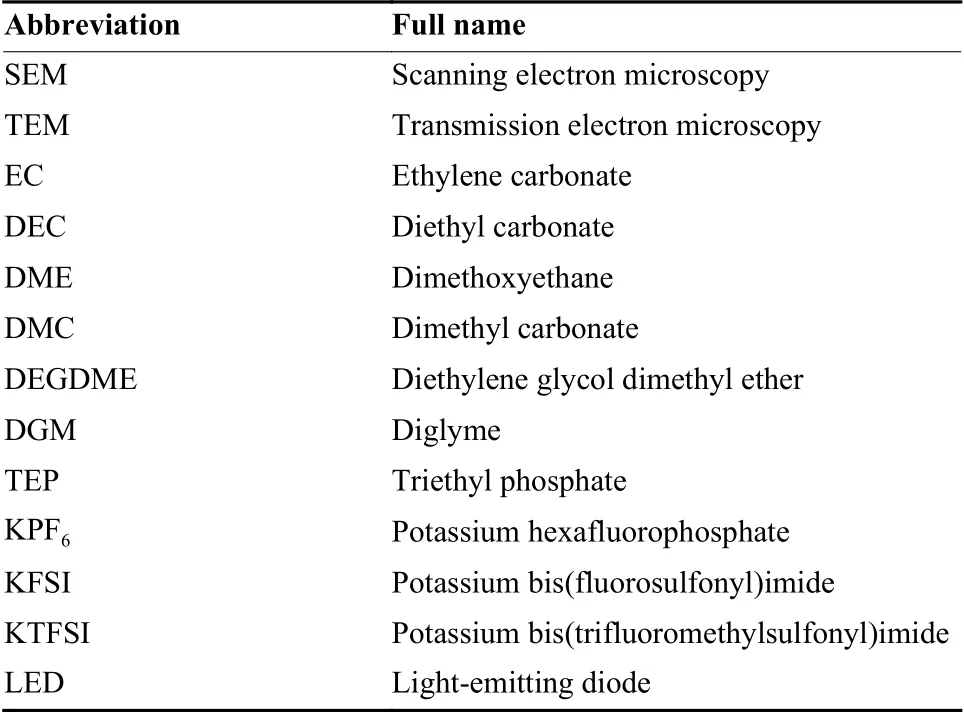
Abbreviation Full name SEM Scanning electron microscopy TEM Transmission electron microscopy EC Ethylene carbonate DEC Diethyl carbonate DME Dimethoxyethane DMC Dimethyl carbonate DEGDME Diethylene glycol dimethyl ether DGM Diglyme TEP Triethyl phosphate KPF6 Potassium hexafluorophosphate KFSI Potassium bis(fluorosulfonyl)imide KTFSI Potassium bis(trifluoromethylsulfonyl)imide LED Light-emitting diode
杂志排行
新型炭材料的其它文章
- Preface
- Guide for Authors
- 《新型炭材料》征稿简则
- 基于界面膜清洗的废旧锂离子电池石墨负极的再生修复
- A high-rate and ultrastable anode for lithium ion capacitors produced by modifying hard carbon with both surface oxidation and intercalation
- The interfacial embedding of halogen-terminated carbon dots produces highly efficient and stable flexible perovskite solar cells
