Multivariate analysis and geochemical investigations of groundwater in a semi-arid region,case of superficial aquifer in Ghriss Basin,Northwest Algeria
2022-09-28LaouniBenadelaBelkacemBekkoussaLaouniGaidi
Laouni Benadela,Belkacem Bekkoussa,Laouni Gaidi
Laboratory of Water Science and Technology (LSTE), Faculty of Science and Technology, University of Mustapha Stambouli, Mascara,Algeria.
Abstract: This study aims to investigate the hydrochemical characteristics of shallow aquifer in a semi-arid region situated in northwest Algeria,and to understand the major factors governing groundwater quality.The study area is suffering from recurring droughts,groundwater resource over-exploitation and groundwater quality degradation.The approach used is a combination of traditional hydrochemical analysis methods of multivariate statistical techniques,principal component analysis (PCA),and ratios of major ions,based on the data derived from 33 groundwater samples collected in February 2014.Results show that groundwater in the study area are highly mineralized and collectively has a high concentration of chloride(as Cl-).The dominant water types are Na-Cl (27%),Mg-HCO3 (24%) and Mg-Cl (24%).According to the(PCA) approach,salinization is the main process that controls the hydrochemical variability.The PCA analysis reveal the impact of anthropogenic factor especially the agricultural activities on the groundwater quality.The PCA highlighted two types of recharge: Superficial recharge from effective rainfall and excess irrigation water distinguished by the presence of nitrate and lateral recharge or vertical leakage from carbonate formations marked by the omnipresence of HCO3-.Additionally,three categories of samples were identified: (1) samples characterized by good water quality and receiving notable recharge from carbonate formations;(2) samples impacted by the natural salinization process;and (3) samples contaminated by anthropogenic activities.The major natural processes influencing water chemistry are the weathering of carbonate and silicate rocks,dissolution of evaporite as halite,evaporation and cation exchange.The study results can provide the basis for local decision makers to ensure the sustainable management of groundwater and the safety of drinking water.
Keywords: Hydrochemistry;Multivariate statistics;PCA factors mapping;Ratio of major ions;Plioquaternary aquifer;Ghriss Basin
Introduction
Groundwater is an important part of the water cycle and therefore contributes to natural water balances.It is also a great renewable resource(Alley et al.2002;Troudi et al.2020).Unfortunately,the high population growth results in an increased need for water supply for agricultural production and industrial development which in turn has repercussions on the quality of groundwater in most countries of the world (Yang et al.2016;Ojo-Awo et al.2018;Khan et al.2021).In arid and semi-arid regions (such as North Africa),groundwater is the main source of drinking,domestic,agricultural,and industrial water and is necessary for socio-economic development (Hadji et al.2017;Mokadem et al.2018;Ncibi et al.2019a).Ghriss basin,located in the semi-arid region of northwest Algeria,is considered to be the main engine of regional economic development.However,the low annual precipitation and the uneven distribution that characterizes this region has caused a scarcity of water resources.Consequently,this situation has led to an over-exploitation of groundwater resources which has undeniable impacts specially on the superficial aquifer.As in other region of the world (Ayouba and Guel,2015;Yang et al.2016;Haj-Amor et al.2018;Hamad et al.2018;Ncibi et al.2019b),the intensification of groundwater withdrawals has been accompanied by the water quality deterioration especially an increase in salinity and some chemical elements in conjunction with anthropogenic activities like agriculture.Indeed,several contaminations by NO3-,Cl-and increase in salinity were recorded in some wells and boreholes tapping the superficial aquifer of the Ghriss Basin.Understanding the natural and anthropic processes that control the hydrochemistry of groundwater is essential for the sustainable management of this resource.The present and future quality of groundwater depends on how these factors will act or interact in a spatial or temporal context.Natural processes consist of dissolution and precipitation of different minerals(e.g.calcite,dolomite,silicate,gypsum,halite,etc.) and corresponding ion exchange (Zhang et al.2018;Ruiz-Pico et al.2019).These processes are determined by identifying aquifer formations and limits,residence time,flow paths,recharge sources and mechanisms,etc.Anthropogenic activities are dominated by agricultural,industrial,domestic seepages,etc.leading to an abnormally high concentration of nitrogen,arsenic,and fluorine among other pollutants (Chen et al.2020;Swift Bird et al.2020;Jia et al.2020).Hydrochemical studies are often based on classical methods such as Gibbs diagram,ionic ratio,saturation index and correlation analysis to identify major ion origins in groundwater (Liu et al.2020).In addition to these methods,researchers increasingly use multivariate statistics specially to characterize and interpret a complex dataset collected from regions where several factors interact as in the case of Ghriss Plain(Fatoba et al.2017).The main aim of the current research is to investigate the hydrogeochemical characteristic of shallow groundwater,identify the principal geogenic or/and anthropogenic factors impacting the hydrochemistry of groundwater using multivariate statistics and classical tools and estimate the possible origin of salinity and major chemical ions.From a scientific point of view,the results of the present study facilitate to estimate the efficiency of combining statistical and classical hydrochemistry tools on shallow groundwater suffering from overexploitation and qualitative degradation.From an environmental point of view,the results will offer a key method to the decision makers to preserve a vital resource for an entire population.
1 Study area
Ghriss Basin,(Fig.1),located in the northwest Algerian,between 35°07' and 35°31' and 0°0' and 0°26'.The study area has a total surface area of about 1 185 km2.The topography is relatively flat with elevation ranging between 470 m and 1 100 m(Dahmani,2010).The hydrography is very weakly marked with only a few small Wadis crossing the basin.With the scarcity of surface water,superficial and deep aquifers remain the only means of mobilizing water to satisfy water demands (Ouis,2012).
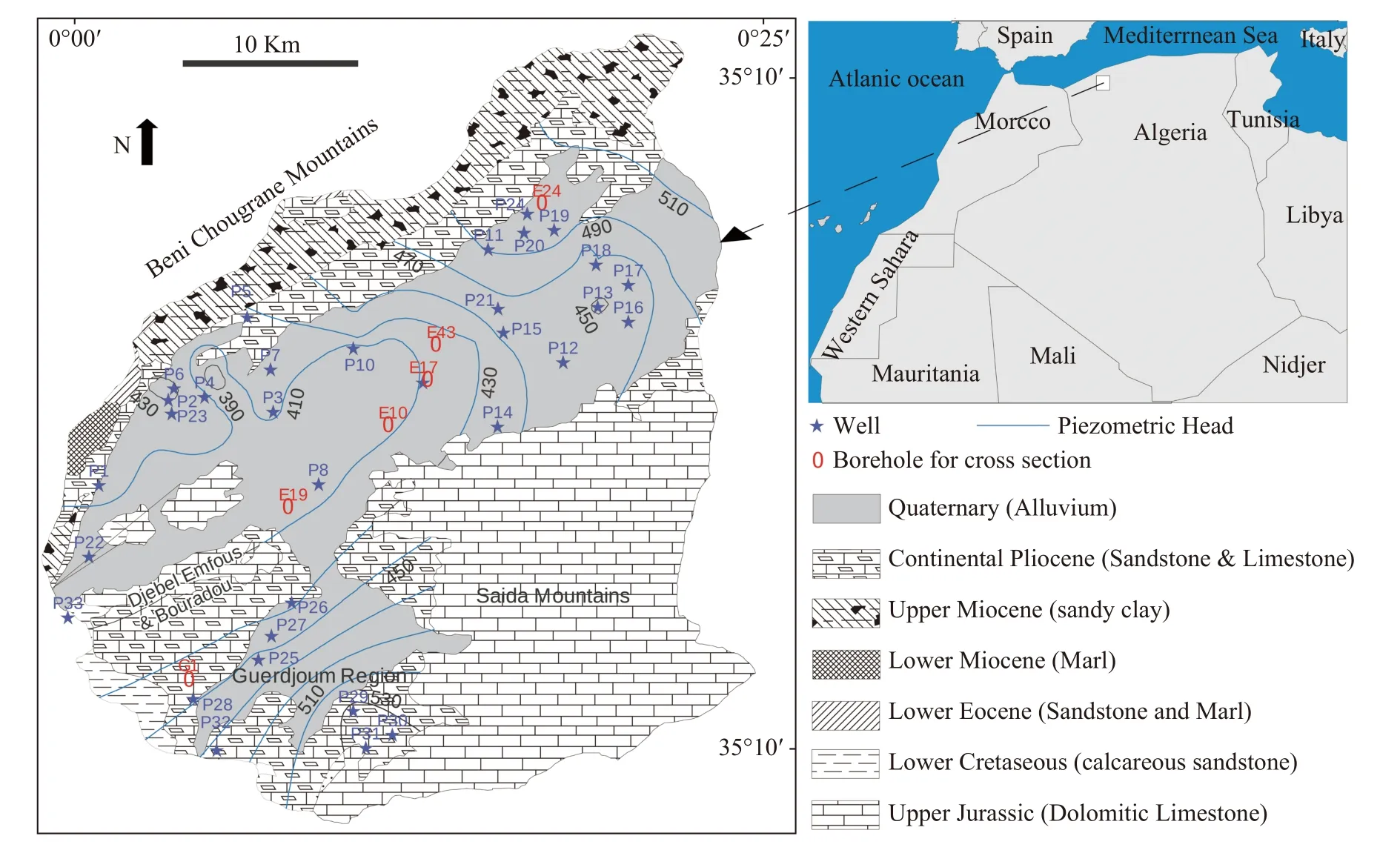
Fig.1 Geological and piezometric map of the study area and groundwater samples location (Adapted from Sourisseau,1972)
The study area is subject to both Mediterranean and Saharan climatic influences (Matari et al.1995;Hamed et al.2014b;Besser and Hamed,2019).The climate is semi-arid,characterized by irregular rainfall from one year to another (Chibane et al.2015),decrease in precipitation from the south to the north,and a prolonged drought that has led to over-exploitation and vulnerability of groundwater resources (Meddi,2009).The average annual temperature is 17°C and the recorded values of precipitation during the period 1979-2009 are between 200 mm and 300 mm with an average of 275 mm (Baba-hamed et al.2015).
1.1 Geology
The Ghriss plain is a flat-top collapse basin with sandy-clay alluvial sedimentation.It is lined with reliefs dominated by the Neogene layers,a component of the mountains of Beni-Chougrane.The silt-sandy soils from the Oued Maoussa continue to allow this subsidence to evolve.The collapsed substratum consists of dolomitic limestones from the Upper Jurassic.The overlying basal conglomerates were deposited locally,followed by a marine filling of greyish and greenish clays and marls of the lower and middle Miocene.In the centre of the plain,subsidence allowed the accumulation of a large thickness of lacustrine limestones from the Pliocene and alluviums during the Quaternary.The soils of the Ghriss plain are predominantly silty and clayey sand with a thickness not exceeding 6 m,their nature varying with transport factors and the nature of the underlying bedrock.These formations support the agriculture of the region (Benfetta et al.2008).The presence of evaporites as halite and gypsum was reported by some authors especially in the northwest and the southwest of the plain (Assens et al.1977;Bekkoussa et al.2013;Bekkoussa et al.2018).The geological sequence is given in Table 1.
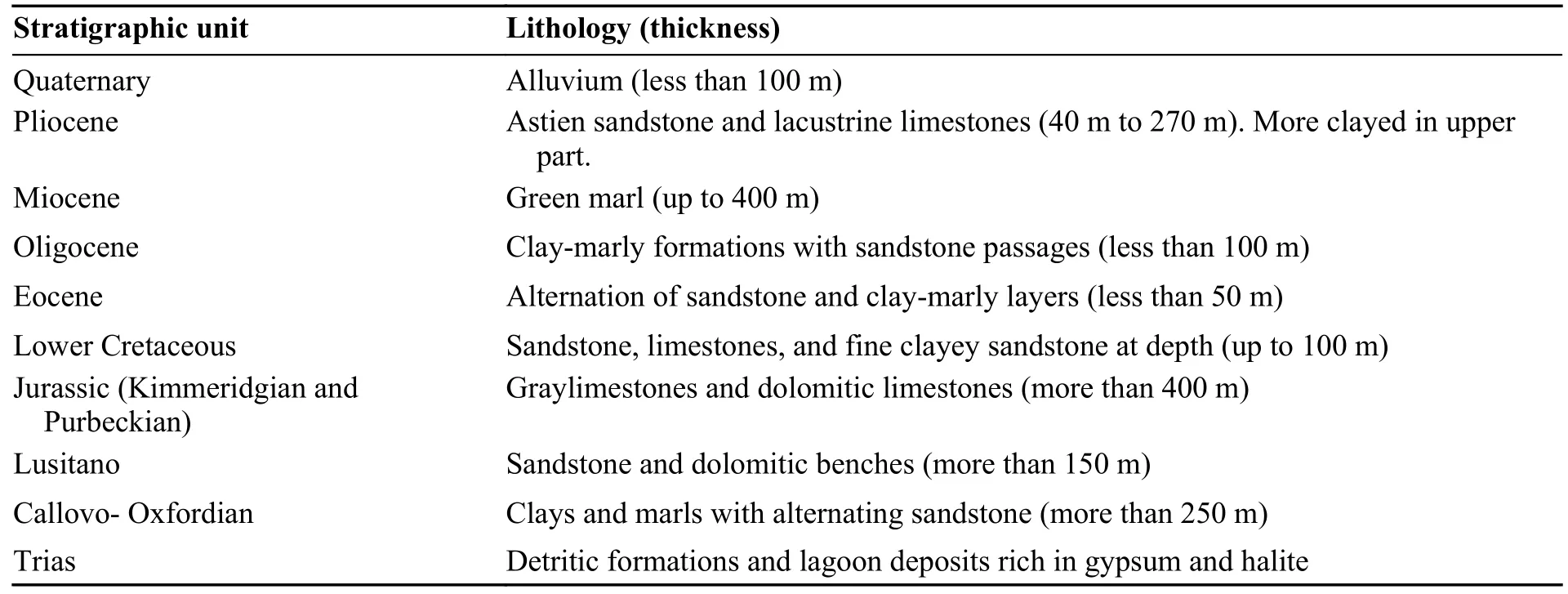
Table 1 Lithostratigraphic units in the study area
1.2 Hydrogeology
The Ghriss plain is an independent hydrogeological unit,formed by a multilayer aquifer system(Sourisseau,1972).Studies carried out in the region have identified three aquifer layers,namely the Plio-quaternary aquifer,the Pliocene limestone and sandstone aquifers and the Jurassic calcareous dolomitic aquifer (Bekkoussa et al.2018).
The plio-quaternary aquifer (an unconfined aquifer) constitutes the main water resource of the region.Geological formations of this aquifer are mainly alluvial deposits resulting from the erosion of sandstones,lacustrine limestones,dolomitic limestones,and marls from the borders of the plain watershed.In direct geological contact with the Pliocene layer,this aquifer is hydraulically related with the upper part of the Pliocene limestone and sandstone aquifer.A clayed impermeable layer of the Miocene separates the Plio-Quaternary aquifer from the Pliocene aquifer in the south.The water table depth ranges from -6 m in the northeast to-80 m in the center of the plain and the thickness of the reservoir ranges from 20 m to 150 m.The rainfall infiltration rate is estimated at 20% (Benadela and Bekkoussa,2017).This aquifer is rechar-ged naturally by precipitation infiltration,lateral recharge,and upward leakage from the Jurassic aquifer in the southern part and with a lesser degree the return of irrigation water.Pumping of the groundwater is major resource withdraw method for the supply of irrigation and drinking water.The direction of groundwater flows converges from northeast and south to the center where a depression cone (piezometric head equals to 410 m) is observed and caused by intensive withdrawals.Transmissivity values range from 5×10-5m2/s to 10-2m2/s (Sourisseau,1972).The region of Guerdjoum,is the least permeable part of the plain with an average transmissivity of 10-5m2/s.The significant hydraulic gradients in this part (3.5% on average) testify to this decrease in permeability.The storage coefficient varies between 0.1 and 0.25 on average,which reflects a high porosity of the Plio-quaternary aquifer.
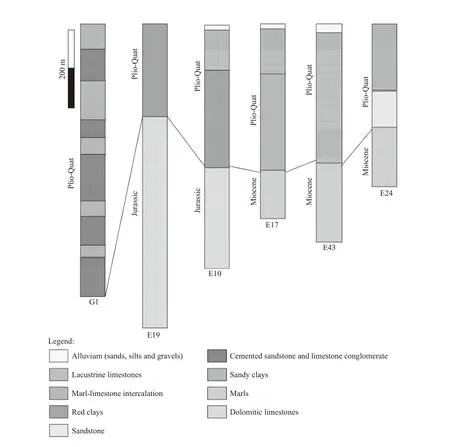
Fig.2 Lithologic correlation of borehole logs from the study area (Bekkoussa,2009)
2 Materials and methods
2.1 Groundwater sampling and analytical techniques
The sampling points were chosen so as to have an overall picture of the Plio-Quaternary water table.We took a total of thirty-three water samples of 1 000 mL during February 2014.Samples were collected from well and borehole water pumped from the Plio-Quaternary aquifer (sampling depth between 55 m and 120 m).The water was pumped for 6 min before sampling (Ruiz-Pico et al.2019).Samples were preserved in a cooler at a temperature of 4°C (Rodier,2009) and transported to the laboratory of the National Hydric Resources Agency (NHRA) in Algeria.The physicochemical analyses carried out included the concentrations of the major ions (Ca2+,Mg2+,Na+,K+,Cl-,SO42-,HCO3-and NO3-),electrical conductivity (EC),TDS and pH (Table 2).The location of the wells and boreholes is shown in Fig.1.The pH was measured in the field at the time of sampling using the Hanna pH meter (HI 8 314).The EC was measured with a Mettler Toledo conductivity meter(S30),with an accuracy of ± 1μS/cm,whereas the pH data accuracy is ± 0.05 (Benadela et al.2018;Benadela et al.2019).The anions (Cl-,SO42‾and NO3-) were analyzed by ion chromatography in the liquid phase and the bicarbonate (HCO3-) by titration of alkalinity.The cations (Ca2+,Mg2+,Na+and K+) were determined using a Perkin Elmer atomic absorption spectrophotometer (AAnalyst 200).
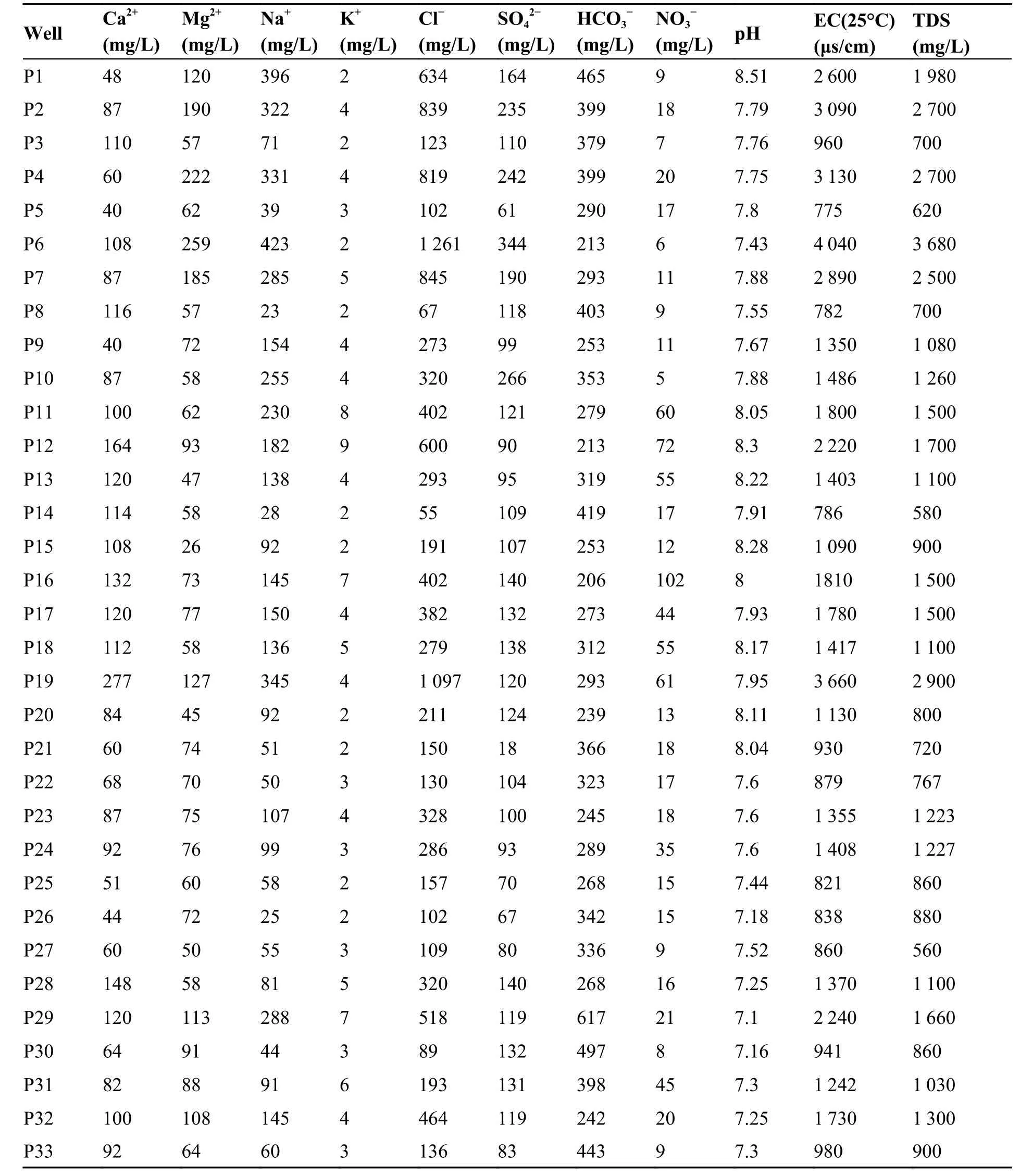
Table 2 Physicochemical parameters of groundwater samples
The reliability of water sample analysis has been verified by the charge balance error (CBE) which is based on the principle of equivalence between the sum of anions and cations when the concentrations are expressed in (meq/L).CBE was determined according to the following expressions:

Where:CBE-The charge balance error (%).
A chemical analysis of the water is regarded as representative and acceptable when the ion balance is less than or equal to ±5% (Kouassi et al.2013;Hamed et al.2013,2014a).The 33 water samples presented a relative error (%CBE) of < ±5%,so they were all used in this study.
2.2 Multivariate statistical analysis
Principal component analysis (PCA) is widely applied on hydrochemical data set to identify the influence of natural or anthropogenic activities on groundwater quality (Loni et al.2014;Singh et al.2017),as the case of this study.The data table is transformed into a new orthogonal and uncorrelated set of factors called principal components(PC).
The principal component (PC) can be expressed as (Dugga at al.2020):
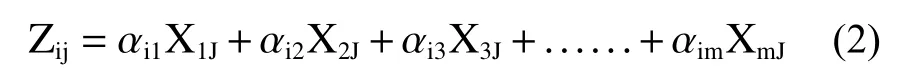
Where:Zis the component score,αis the component loading,Xis the measured value of variable,iis the component number,jis thesample number,andmis the total number of variables.
Due to different dimensions and units of chemical variables,each parameter needs to be standardized through z-score transformation (Güler et al.2002;Nguyen et al.2020) as follow.

Where:Ziis the standardized variable,Xiis the original data,μandSare the mean and standard deviation of the datasets,respectively.
The number of PC extracted is equal to the number of parameters included in the analysis,though,only PCs having eigenvalues greater than 1 are usually considered in the interpretation,based on the Kaiser criterion (Kaiser,1960).The first principal component has the highest eigenvalue and represents the most sources of variation of the original data,followed by the second component and so on.The adequacy of the factor solution was verified by the Kaiser-Meyer-Olkin (KMO) index and the Bartlett sphericity test.If the KMO is greater than 0.5,the factor analysis is appropriate to provide a significant reduction in data size (M’nassri et al.2019).The factor loadings represent the correlation coefficient between the variable and principal components.Component loading coefficients vary between -1 and 1,when maximum values express a strong correlation and approaching 0 values express weak or absence of correlation.The range of the loadings can be maximized using the Varimax normalized rotation method(Gholizadeh et al.2016) and also used in this study.The PCA was performed using xlstat software (version 2015.6) and applied to the whole hydrochemical dataset of 33 samples and 10 parameters (Ca2+,Mg2+,Na+,K+,Cl-,SO42-,HCO3-,NO3-,pH and EC).
3 Results and discussions
3.1 Statistical analysis of groundwater chemical properties
In terms of dominance,the order of ions in groundwater were Mg2+> Na+> Ca2+> K+for cations and Cl-> HCO3-> SO42-> NO3-for anions.The statistics of chemical constituents in groundwater are given in Table 3.The pH values of the groundwater samples in the study area range from 7.1 to 8.51,which indicate neutral to weakly alkaline characteristics.The EC and TDS are two important indicators reflecting the quality of groundwater.Table 3 shows that the waters of the studied aquifer are highly mineralized,in which the EC ranges between 775 μS/cm and 4 040 μS/cm with an average of 1 630 μS/cm.36% and 15% of the samples have EC above the maximum value recommended by World Health Organization (WHO)and Algerian standards for drinking water,respectively.In addition,the TDS concentrations range between 560 mg/L and 3 680 mg/L,and 60% and 15% of the samples exceed the WHO standard limits and Algerian standards of TDS,respectively.The mineralization of the waters mainly results from migrated minerals and evaporites dissolution,so the most mineralized waters indicate a deep recharge and a long residence time.Chloride (Cl-)is a major component of dissolved solids and has an important role in groundwater salinity.The concentration of Cl-shows a wide range from 55 mg/L to 1 261 mg/L with an average value of 369 mg/L.57% and 24% of the samples exceed international (WHO) and national (Algerian) potability standards for Cl ions,respectively.Na+concentrations range between 23 mg/L and 423 mg/L with an average value of 151 mg/L and the percentages of the samples exceeding the WHO and Algerian standards are both 27%.The enrichment of Na+and Cl-in groundwater is enhanced by deep groundwater circulation,which captures ions from lower strata,has longer aquifer residence time and promotes extended water-rock interactions (Avci et al.2018).
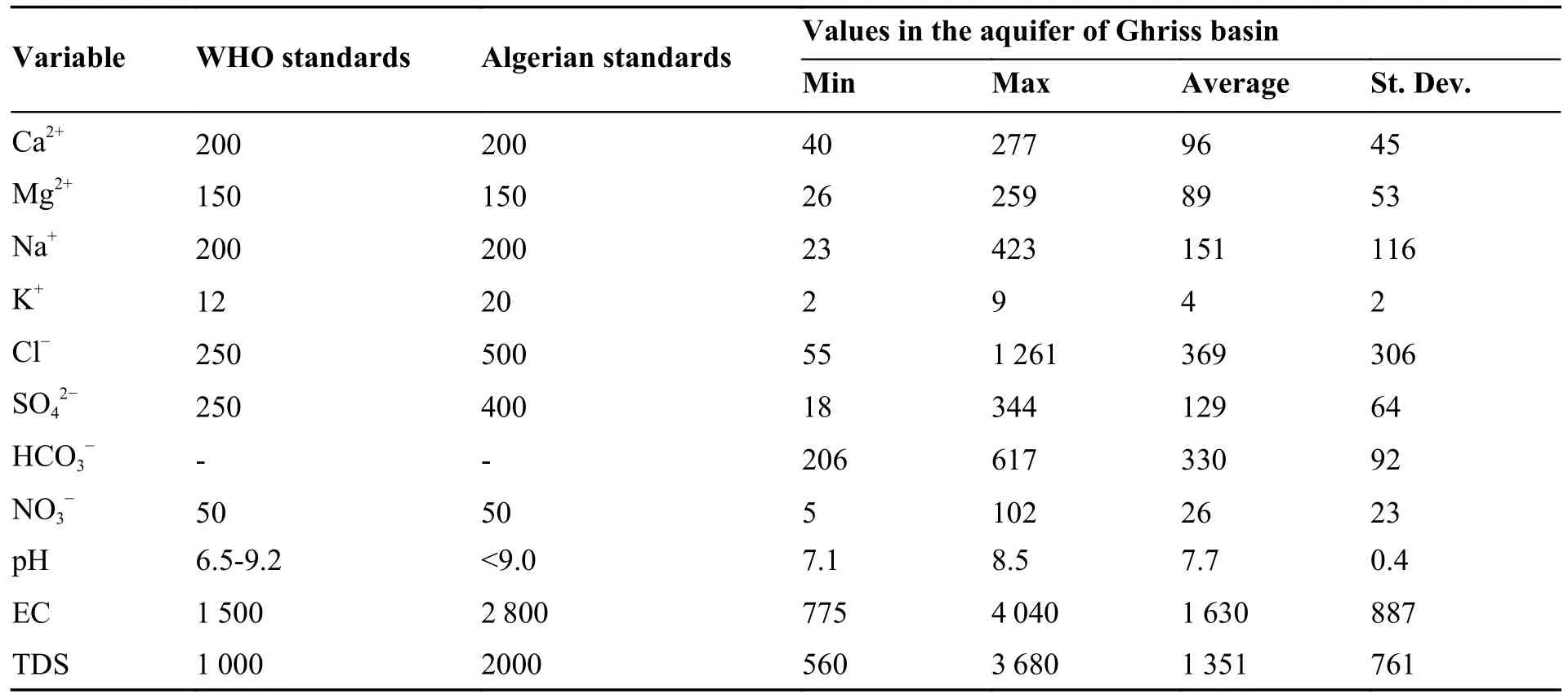
Table 3 Statistical summary of chemical parameters of groundwater samples (Units in mg/L,except EC (μs/cm))
Sulphate is ubiquitous in groundwater,with both natural and anthropogenic sources.The sulphate concentration in the area ranges from 18 mg/L to 344 mg/L.Only two samples with sulphate exceed the WHO standards.The concentration of Ca ionsvaries between 40 mg/L and 227 mg/L,with an average of 96 mg/L.The concentration of Mg ions ranges between 26 mg/L and 259 mg/L,with an average of 89 mg/L.The HCO3-concentration extends between 206 mg/L and 617 mg/L,with anaverage value of 330 mg/L.The lateral recharge and vertical leakage water from Jurassic calcareous dolomitic aquifer is rich in HCO3-and lead to the high concentration of this element in the plioquaternary aquifer.Consequently,the dissolution of carbonate formations (calcite and dolomite),outcropped in the south and forming the confined aquifer at depth,is the most important source of Ca,Mg and HCO3ions in the groundwater.The primary mechanism for the dissolution of a carbonate mineral is as follows:
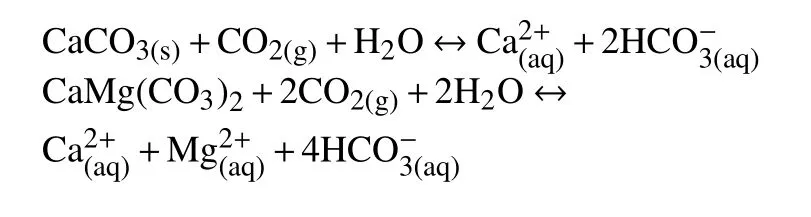
The concentration of NO3-in a water body can reflect the impact of human activities on the water environment,including agricultural production and domestic wastewater discharge (Liu et al.2021a).The average nitrate concentration in the groundwater did not exceed the maximum permissible concentration (50 mg/L) for the majority of campaigns except for 6 samples.However,the maximum levels were excessively high,and recorded in wells located in the northeast of the study area,where leaching of nitrates into groundwater are principally from precipitation infiltration and the return of irrigation water.
3.2 Hydrochemical facies
Characterization of hydro-geochemical data can be done by evaluating the hydrochemical facies of water,commonly known as water type,using various plots like Piper diagram,Schoeller Diagram,Durov diagram,Stabler Diagram (Tamma Rao et al.2015).The Piper diagrams show the evolution of a water,transitioning from one facies to another,using analysis from temporally spaced samples or spatially spaced samples (Wang et al.2014;Niu et al.2017).The diamond-shaped field between the two triangles is used to represent the composition of water with respect to both cations and anions(Raju et al.2009;Yang et al.2016).It can be seen from Fig.3 that the main anions are dominated by Cl-(66%),followed by HCO3-(33%),while the cations are dominated by Mg2+(48%),followed by Na2+(27%) and Ca2+(25%).The hydrochemistry types are Na-Cl (27%),Mg-HCO3(24%) and Mg-Cl (24%).A significant influence of the chloride high is observed in the aquifer.The predominance of Na-Cl water type is generally attributed to the dissolution of evaporites especially halite,and cation exchange as well.Though,The Mg-HCO3facies characterize lateral recharge and vertical leakage from the underlying Jurassic carbonate formations,while Mg-Cl type is a mixture of waters from a dolomitic environment (hence the predominance of magnesium) as well as seepage from effective rainfall and/or return of irrigation water (Bekkoussa et al.2018)
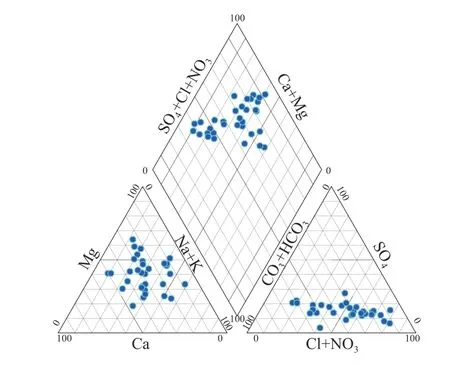
Fig.3 Piper diagram of water and chemical facies
3.3 Correlation coefficient
In order to establish the relationships between the physicochemical parameters,and to identify the probable origin of the solutes,a Pearson correlation matrix was calculated,as shown in Table 4.The relationships are considered strong,moderate or weak when the correlation coefficient is greater than 0.7,between 0.7 and 0.5,or less than 0.5,respectively (Esmaeili et al.2018;Owoyemi et al.2019;Liu et al.2020).
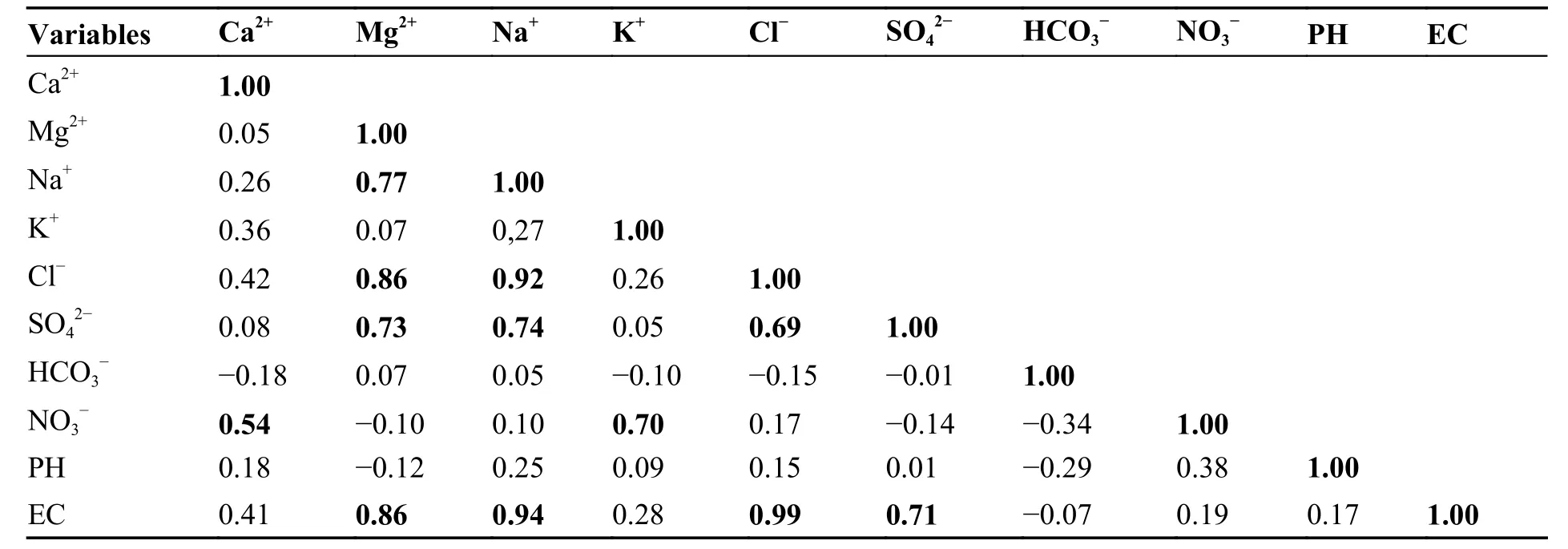
Table 4 Pearson correlation matrix of major physicochemical parameters in groundwater
The table shows a strong correlation between EC and Cl-(r=0.99),EC and Na+(r=0.94),EC and Mg2+(r=0.86) and EC and SO42-(r=0.71),revealing the contribution of these ions to the groundwater salinity.
Cl-and Na+shows a high positive correlation (r=0.92) that can be attributed to the same origin with identical geochemical and hydrodynamical conditions.The high correlation between the Cl-and Na+may be derived from the dissolution of halite.Though,the strong correlation between Mg and SO42-(r=0.73) and Mg2+and Cl-(r=0.86) may be from the weathering of minerals containing magnesium sulfate and magnesium chloride,respectively(Bharti et al.2017;Krupp,2005).Mg2+,SO42-,and Cl-don’t have any relationship with NO3-or K+.Therefore,these ions are not originated from anthropogenic activities.
However,a strong correlation exists between K+and NO3-(r=0.70) indicating a common sourcewhich is the leaching of the NPK fertilizers used in agriculture.A moderate correlation exists between Ca2+and NO3-(r=0.54) showing an anthropogenic source of a part of calcium dissolved in groundwater.Indeed,the use of calcium nitrate fertilizer in agriculture is very frequent (Makhlouf,2015),especially in the study area,which can explain this correlation.
3.4 Principal component analysis PCA
The results of the PCA are presented in Table 5.PCA factors can provide insight into the major mechanisms and sources of variation in the chemistry of the samples.The first three components extracted have eigenvalues greater than 1 and account for 79.19% of the total variance in the dataset (Trabelsi et Zouari,2019).To maximize the variance of the first three principal axes,the Varimax normalized rotation was applied.
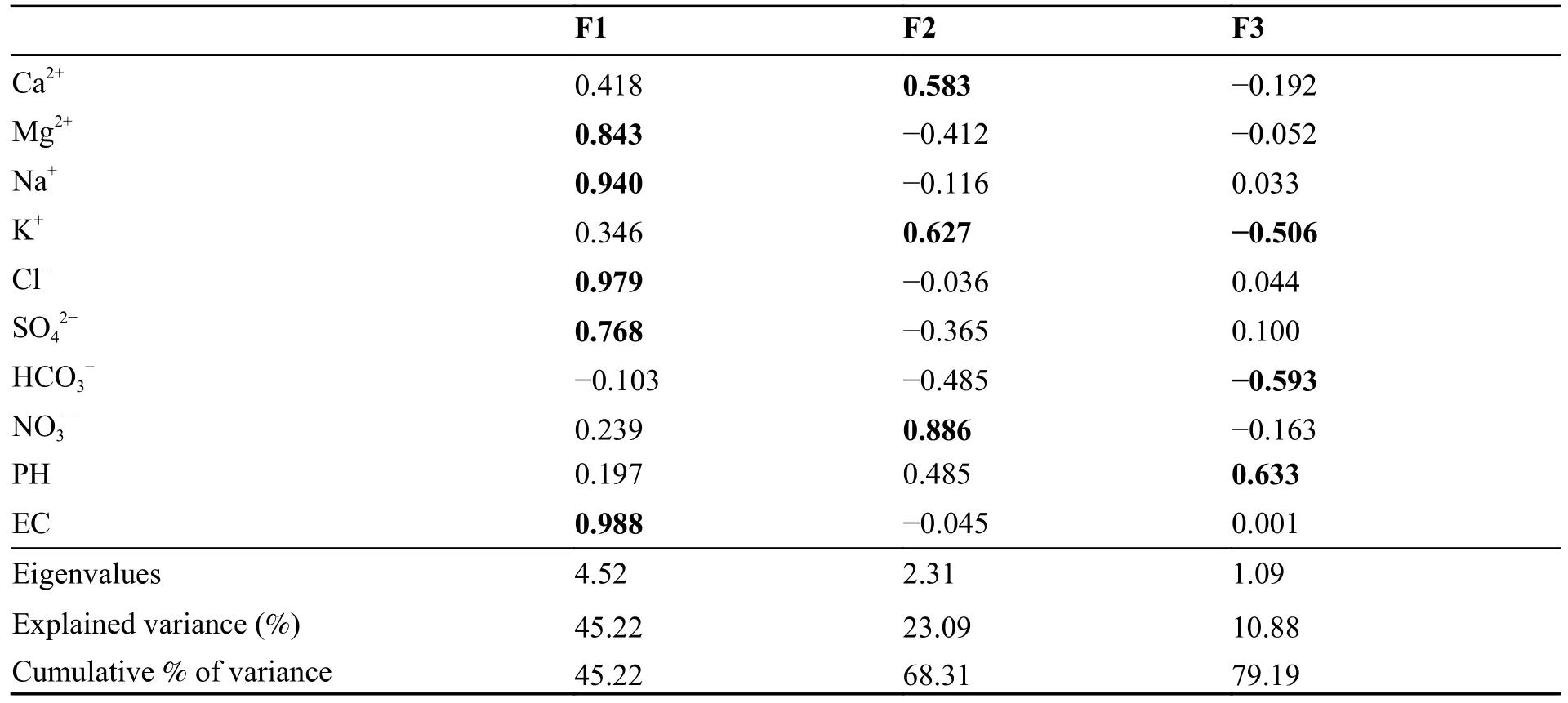
Table 5 Principal component loadings and eigenvalues of the factor analysis
F1 accounted for 45.22% of the variance strongly positively loaded with EC (0.99),Cl-(0.84),Na+(0.94),Mg2+(0.84) and SO42-(0.77).This group of variables associated with F1 indicates that salinization is the major process controlling the hydrochemical variability.Consequently,salinity augmentation is mainly related to the dissolution of minerals and weathering of rocks (Masoud,2014).
The second factor F2,accounted for 23.09% of the total variance,is found to be strongly positively loaded with NO3-(0.89),K+(0.63),and Ca2+(0.58)which are the principal indicators of contamination from agricultural activities.A negative loading is observed for HCO3-(-0.49) which comes from the carbonate dissolution.This result underlines two different types of recharge impacting the hydrocherochemistry of the groundwater,which are identified as 1) superficial recharge from effective rainfall and excess irrigation water indicated by the presence of nitrate,and 2) lateral recharge andvertical leakage from Jurassic carbonate formations marked by the omnipresence of HCO3-(Bekkoussa et al.2018).
The third factor accounting for 10.88% of the cumulative variance shows positive loading for pH(0.63) and negative loadings for HCO3-(-0.59)and K+(-0.5),highlighting the role of pH in the hydrochemical processes in the groundwater.For example,the production of NO3-through the nitrification processes is accompanied by the production of H+which are released in the solution and consequently impact the pH values (Trabelsi et Zouari,2019).In addition,the dissolution of carbonate minerals is controlled by the pH of the solution and vice versa.
According to the projection of individual samples on the factorial plan F1-F2,three poles were distinguished (Fig.4).Samples in Pole 1,i.e P8,P25,P26,P27,P30,P33…,have a negative correlation with factor F1 and F2 and show good quality water.These samples are characterized by relatively high HCO3-and low NO3-,TDS concentrations.Waters of this pole are impacted by recharge from the carbonate aquifer,marked by relatively high concentrations of HCO3-,good quality and preserved from anthropogenic effects.Pole 2 reflects the salinization domain with samples of P2,P4,P6 and P7 with high concentrations of TDS,Na+,Cl-,Mg2+,and SO42-.This group doesn’t show any anthropogenic contamination and the mean concentration of NO3-is 12.8 mg/L.Thus,Pole 2 indicates the natural processes of mineralization(water-rock interactions and evaporation) and the distribution of samples along the F1 axis means an increase in salinity linked to the natural effect.Samples noticeably contaminated by agricultural activities (use industrial fertilizers and livestock manure)fall in Pole 3.Concentrations of NO3-vary between 60 mg/L to 102 mg/L and represent the higher values in the study area.Therefore,samples with the strongest correlations with factor F2 are the most affected by human activity and vice versa.
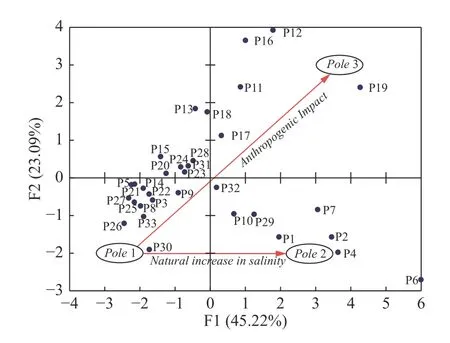
Fig.4 Factor scores (33 samples) of F1 and F2
3.4.1 Spatial distribution of factor scores F1 and F2
To examine the spatial variation of multivariate integrated physicochemical parameters and for a better interpretation and display of the PCA results in space,iso-factor maps were established (Abou Zakhem,2016;Abou Zakhem et al.2017;Loni et al.2014).
3.4.1.1 Distribution map of factor scores F1
Fig.5a showed that water samples with high positive F1 scores were located in the northeast and northwest of the Ghriss plain where mineral dissolution and evaporation effects play a major role in the mineralization process of groundwater (Bekkoussa et al.2013).All samples with a strong positive correlation with factor F1 have Ca-Cl,Na-Cl,or Mg-Cl type while samples with negative loadings have Ca-HCO3or Mg-HCO3type.The latter were mainly located in the center of the plain where recharge by the karst aquifer is dominant and the groundwater is characterized by good quality.
3.4.1.2 Distribution map of factor scores F2
The spatial distribution of F2 scores (Fig.5b) revealed that the majority of the samples which are strongly positively correlated with this factor were situated in the eastern part of the plain.The impact of anthropogenic activities such as intensive agricultural activity is visible in this part and noticeably marked with high values of NO3-(64.5 mg/L on average).Negative scores of F2 mean that groundwater is not or weakly influenced by anthropogenic activities.Furthermore,we can observe that areas suffering from anthropogenic contaminations (negatives scores) are much smaller than preserved zones (positive scores).
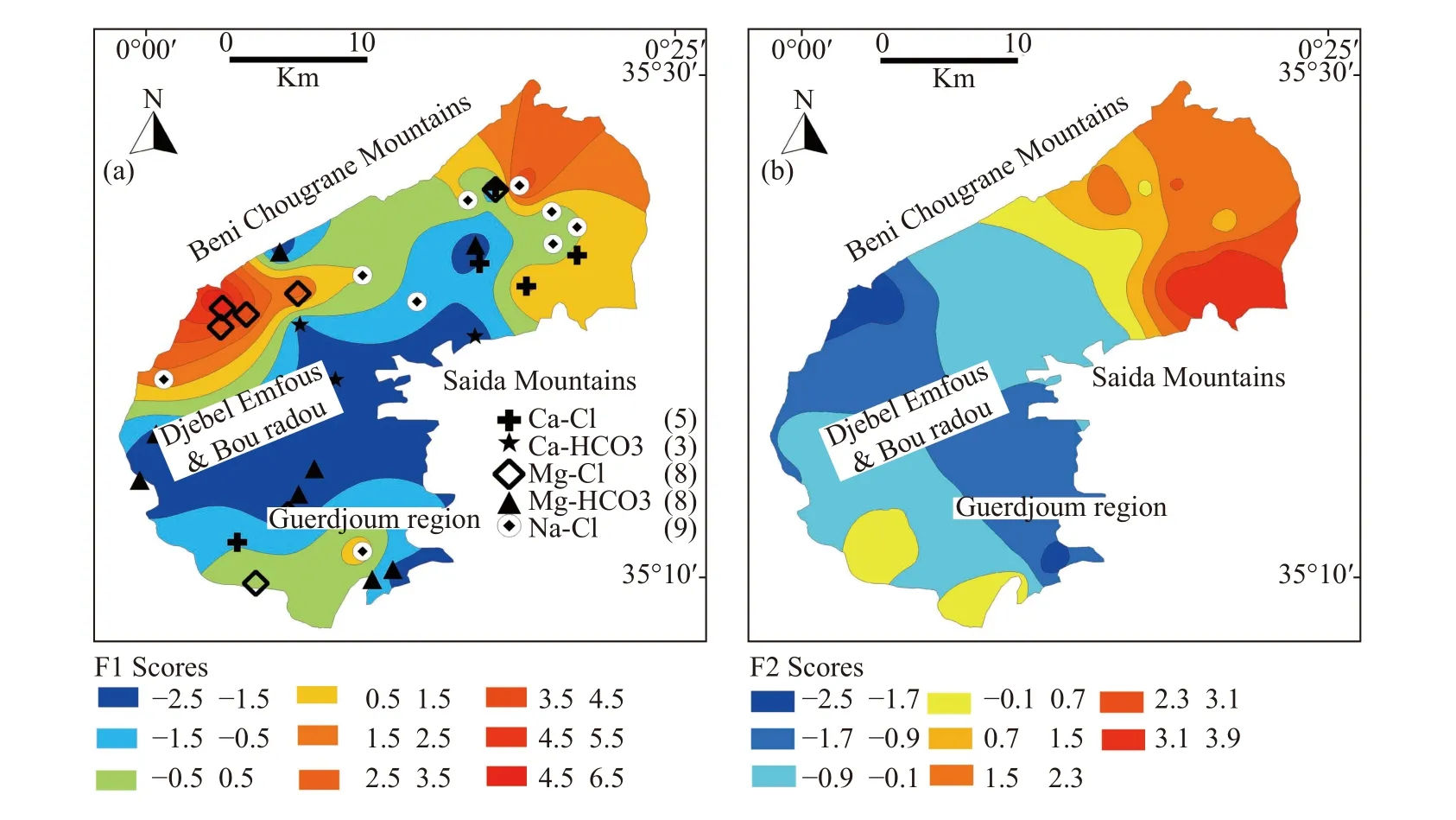
Fig.5 Distribution map of factor scores a) F1 and b) F2
3.5 Relationships among major ions
The study of the binary graphs of the major elements,allows us to reveal the anticipated relationship between the water chemistry and the lithology of the basin.The Gibbs diagram plots TDS vs.the ratio Na+/ (Na++Ca2+) and Cl-/(Cl-+HCO3-) and provides various mechanisms that controls the chemistry of the waters of a region such as: Atmospheric precipitation,rock weathering,evaporation and fractional crystallization (Ruiz-Pico et al.2019).As shown in Fig.6,the Na+/ (Na++Ca2+)and Cl-/(Cl-+HCO3-) ratios of most groundwater samples were lower than 0.6 and the sampling points were mainly distributed in the weathering zone of the rock,indicating that chemical weathering is the major and dominant mechanism controlling the water chemistry of the plio-quaternary aquifer in Ghriss plain (Toumi et al.2015).The few samples approaching the evaporation dominance zone are likely to be impacted by evaporites and/or evaporation effect (Ma et al.2017;Jampani et al.2020).In addition,some sample points have a ratio of Na+/ (Na++Ca2+) exceeding 0.5,indicating that the studied aquifer may be affected by cation exchange (Liu et al.2021b).
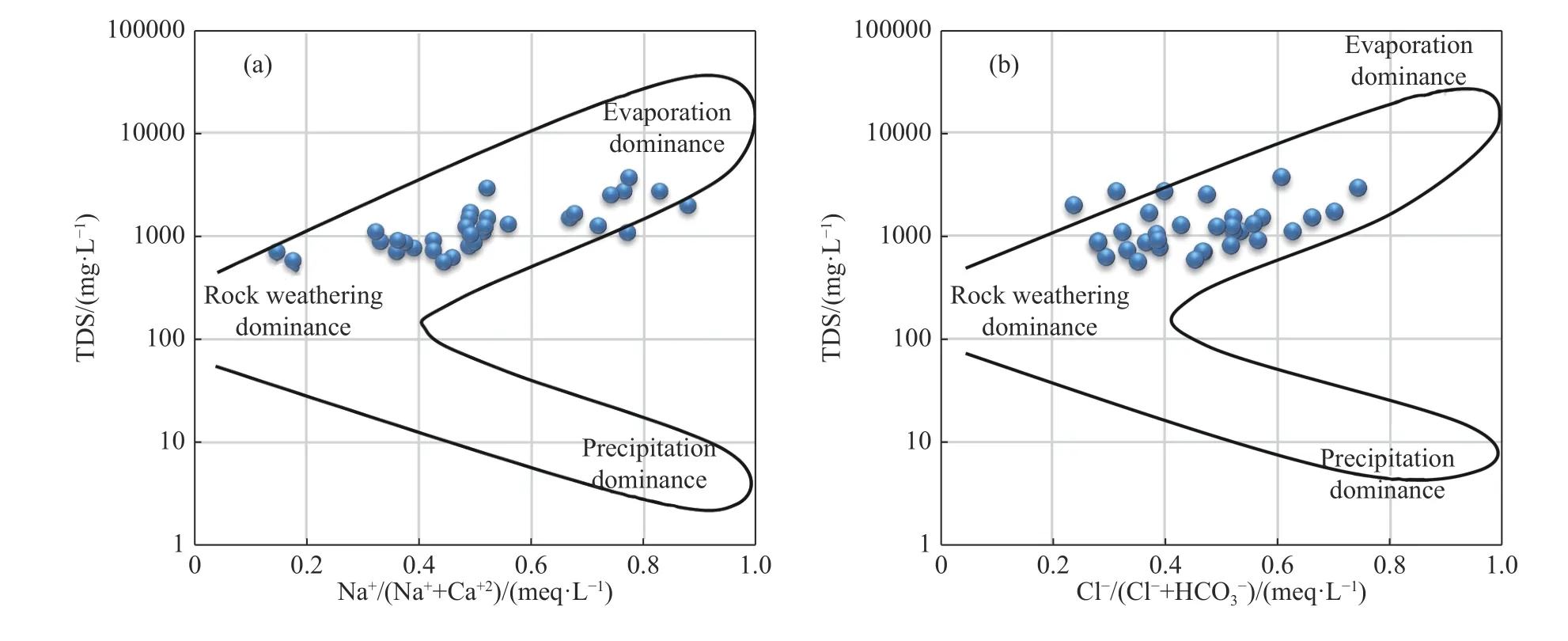
Fig.6 Gibbs plots illustrating main mechanisms impacting groundwater composition in the superficial aquifer in Ghriss plain
To determine the probable origin of the major elements involved in the geochemical process controlling the groundwater chemistry,the main bivariate graphs are established and illustrated in Fig.7 (Yang et al.2016).The results show a proportional relationship between the concentration of Cl-and TDS.The Cl ion is a very soluble element and is difficult to be absorbed by plants and soil surface particles.Evaporation is one of the main factors behind the high concentration of Cl-in semi-arid areas,which can be used as an indicator to detect the mineralization degree of groundwater(Yang et al.2016).The positive correlation between Cl-and TDS in Fig.7a explains the important role of evaporation in the salinization of water.To further confirm the significance of evaporation,the plot of Na+/Cl-against EC was analyzed(Fig.7b).The plot reveals that the Na+/Cl-ratio remains constant with increasing EC,indicating an evaporation dominant environment (Loni et al.2014;Zaidi et al.2016).In natural waters,the presence of Na+and Cl-is attributed to the dissolution of halite and the strong affinity of Na+with Cl-should reflect in their ionic relationship with a 1:1 line indicating their origin from halite dissolution.However,sodium chloride can have other origins such as anthropogenic activities.The plot of Na+against Cl-shows a deficit of Na+content,which may be explained by the existence of cation exchange (Fig.7c) caused by pollution indicators.When gypsum is dissolved in groundwater,theoretically the same amount of Ca2+and SO42-will be produced.Therefore,the ratio of Ca2+to SO42-in groundwater can be used to determine whether the main source of Ca2+and is gypsum(Wang et al.2021).In this study,samples are very deviated from the 1:1 line in the plot of SO42-vs Ca2+(Fig.7d),indicating that gypsum is not the principal source of Ca2+and SO42-in this area.The dissolution of dolomite and calcite are an itant source of Ca,Mg,and HCO3ions in the groundwater.If the dissolution of calcite or dolodissolution of calcite or dolomite is the sole source of Ca2+,the samples should be plotted in the zone between the lines 1:1 and 2:1 (Li et al.2016),which is the case in this study (Fig.7e).Furthermore,Fig.7f shows that majority of the samples fall below the equiline,indicating that carbonate weathering was the primary process involved in the evolution of groundwater (Boateng et al.2016).This confirms the plio-quaternary superficial aquifer was recharged by the karstic aquifer in this hydrogeological system.
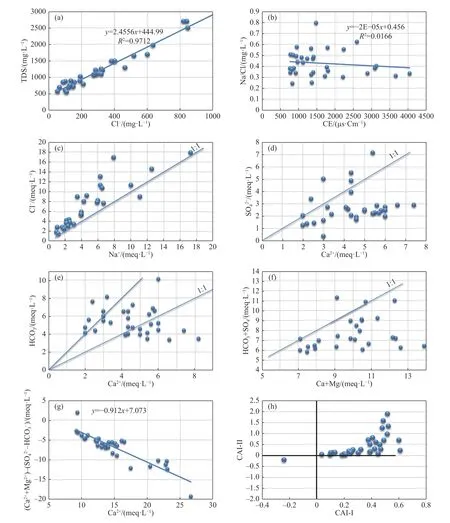
Fig.7 Relationships between major ions
The clays can release Ca2+or Mg2+ions after fixing the Na+or K+.This cation exchange is proved by the linear relationship between (Ca2++Mg2+) -(SO42-+HCO3-) and (Na++K+-Cl-)(McLean et al.2000;García et al.2001).Fig.7g shows a decrease in Na++K+related to an increase in Ca2++Mg2+.All data plot close to the 1:1 line indicates that cation exchange occurs throughout the study area.In the absence of this hydrogeochemical phenomenon,all data should be close to the origin.Furthermore,the chloro-alkaline index(CAI) provides valuable quantitative information about the presence of either direct or reverse cation exchange (Zaidi et al.2016;Liu et al.2020).Negative CAI-I and CAI-II indicates direct cation exchange between Ca2+/Mg2+and Na+/K+in the aquifer,whereas positive CAI-I and CAI-II suggests an reverse ion exchange between them (Ismail et al.2020).(Fig.7h) shows that the majority of data points have positive CAI,which indicates the dominance of reverse cation exchange.
In order to identify the influence of the waterrock interactions of the three major rock types (silicate,carbonate,and evaporite) on the hydrochemical characteristics of the region,bivariate plots of the ion ratios among Ca2+,Mg2+,Na+,and HCO3-were constructed (Liu et al.2020).As shown in Fig.8a and 8b,the data points mainly fall within both the carbonate zone and the silicate zone,indicating that the groundwater hydrochemistry were affected,at the same time,by carbonate rocks and silicate rocks.
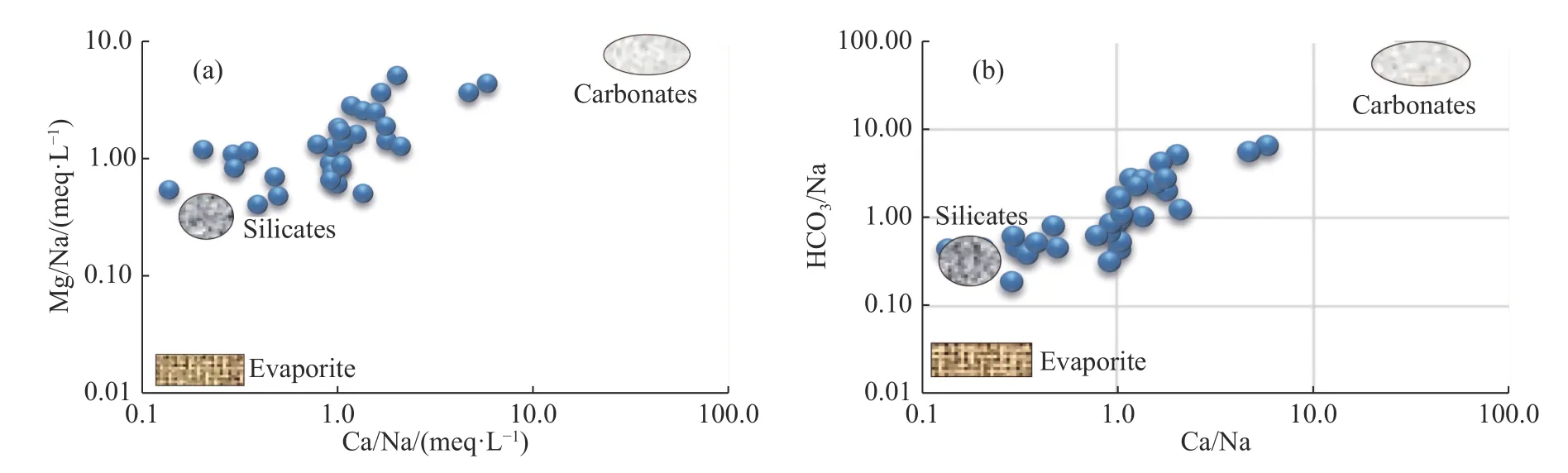
Fig.8 Bivariate plots of (a) (Ca2+/Na2+) vs (Mg2+/Na2+) and (b) (Ca2+/Na2+) vs (HCO3-/Na2+)
3.5.1 The anthropogenic impacts
Anthropogenic activities result in significant degradation of groundwater quality through the world.Anthropogenic factors affecting groundwater chemistry include agriculture,such as the use of fertilizers,manures and pesticides,animal husbandry activities,pollution due to industrial effluents and domestic sewage,mining,and recreational activities.Groundwater is loaded with Cl-,SO42-,and NO3-from these activities (Khatri et Tyagi,2015).While Cl-and SO42-can originate from natural sources,such as the dissolution of evaporites (halite and gypsum) or sulfide mineral oxidation,NO3-is mainly attributable to agricultural activities and domestic sewage.Higher ratios of NO3-/Na+and Cl-/Na+indicate polluted groundwater.However,as shown in Fig.9a,all water samples show low Cl-/Na+and NO3-/Na+ratios,with many ratios close to the halite zone.Most water samples in this study fall below the 1:1 line for NO3-/Na+versus Cl-/Na+,indicating that anthropogenic activities have a minor impact on the chemistry of Ghriss aquifer compared to other mechanisms (Fan et al.2014;Kou et al.2019).In addition,Fig.9b shows that industrial activities also contribute to the groundwater degradation in the study area.
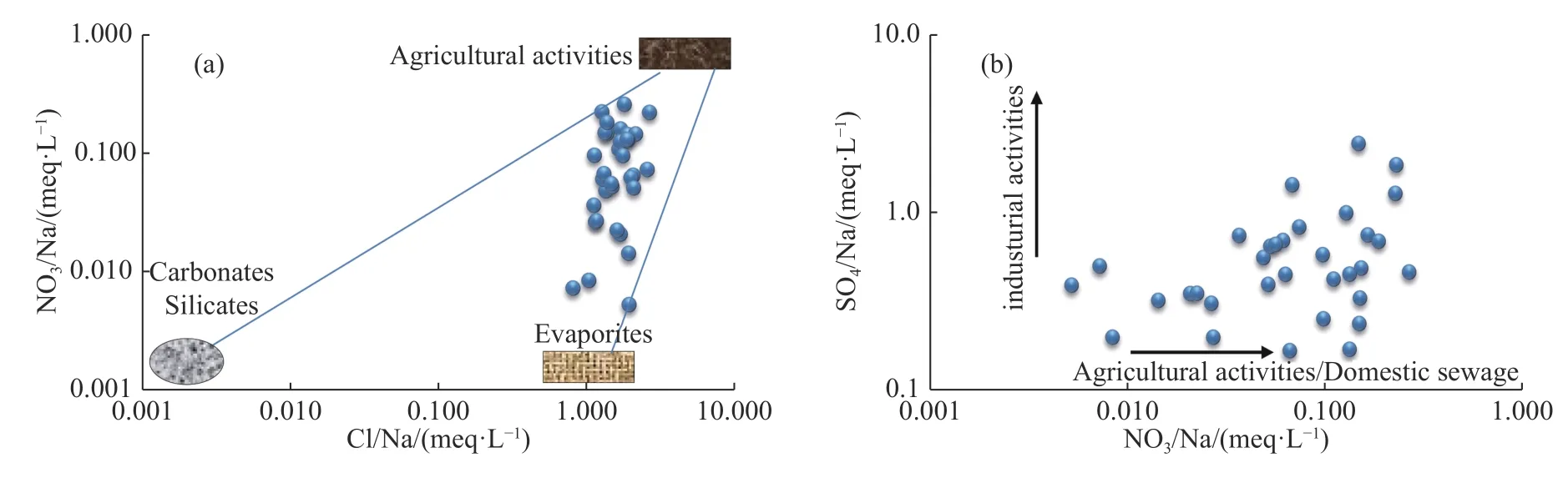
Fig.9 Plots of (a) Cl-/Na+ versus NO3-/Na+ and (b) NO3-/Na+ versus SO42-/Na+
4 Conclusions
The present study presents an approach of using multivariate statistics and classical hydrochemical tools to characterize the groundwater resource in a semi-arid region situated in northwest Algeria.
The waters are highly mineralized and predominantly charged with anion Cl-and cation Mg2+.Though,three different types of hydrochemical facies are detected,with their sources from the dissolution of evaporites and other rocks,leaking recharge by deep aquifers and even a mixing of the formers.The water quality is noticeably degraded with high concentrations of Cl-and TDS.Correlation analysis shows significant correlations between Cl-and Na-,Mg2+and SO42-,Mg2+and Cl-,K+and NO3-,indicating such ions in the groundwater are likely from the same sources.Based on PCA with a variance of 79%,three factors have been identified: (1) salinization process related to the minerals dissolution and rock weathering;(2)contamination caused by agricultural activities identified by high concentrations of NO3-and K+;(3) the role of pH in the hydrochemical processes in the groundwater hydrochemistry.In addition,according to the projection of individual factors on the factorial plan F1-F2,three water types were distinguished: (1) waters impacted by recharge from the carbonate aquifer with good water quality and preserved from anthropogenic effects;(2)waters marked by natural salinization process(water-rock interaction and evaporation) with high concentrations of TDS,Na+,Cl-,Mg2+and SO42-and (3) waters contaminated by agricultural activities from use of fertilizers and livestock manure.The spatial distribution of first two principal (PCA)factors shows that samples correlated with the first factor representing the natural mineralization process are situated in the northeast and northwest of the Ghriss basin,where mineral dissolution and evaporation effects play a major role.Whereas samples strongly correlated with the second factor representing the anthropogenic contamination are mostly situated in the eastern part of the Basin.The bivariate plots reveal that the origin of Na+and Clin the Ghriss Plio-quaternary aquifer was from the dissolution of halite,while the main source of Ca2+,Mg2+and HCO3-was from lateral recharge and vertical leakage of the Jurassic carbonate formations.Furthermore,the results indicate that reverse cation exchange dominated during which Na+/K+were exchanged with Ca2+/Mg2+in the aquifer.Thus,the chemical signature of the groundwater has been acquired mainly through different mechanisms,such as rock-water interaction (noticeably carbonate and silicate),evaporites dissolution (halite in particular),mixture of processes and cation exchange.Due to intense agricultural activity and increasing population density,the anthropogenic impact on groundwater in the study area cannot be neglected.However,its effect on the overall water chemistry remains minor compared to natural processes.
Groundwater resource management should focus on the preservation of water quality and incite farmers to rationalize the use of fertilizers in their farming processes.Population and water consumers especially in the rural areas must be advised when water quality breaks the standards in order to protect public’s health.
Acknowledgements
The authors sincerely acknowledge the necessary facilities extended for physico-chemical analyses by the laboratory of the National Hydric Resources Agency (NHRA) in Algeria.
杂志排行
地下水科学与工程(英文版)的其它文章
- Evaluation of groundwater resource potential by using water balance model: A case of Upper Gilgel Gibe Watershed,Ethiopia
- Transformation of ammonium nitrogen and response characteristics of nitrifying functional genes in tannery sludge contaminated soil
- Carbon,nitrogen and phosphorus coupling relationships and their influencing factors in the critical zone of Dongting Lake wetlands,China
- Temporal variations of reference evapotranspiration and controlling factors: Implications for climatic drought in karst areas
- Determination of total sulfur in geothermal water by inductively coupled plasma-atomic emission spectrometry
- Using time series analysis to assess tidal effect on coastal groundwater level in Southern Laizhou Bay,China
