Phytohormone-triggered Transcriptional Changes Revealed β-Glucosidase as a Key Player for Polysaccharide Metabolism in Dendrobium officinale*
2022-09-22WANGZhiCaiZHAOMeiLiZHANGXiaoJieZHANGZhenLiangLISuZhenLIJianCUIHongQiuLIWuJiaoLIUYanChunWANGYuLILiQiangGULiLiWANGMeiNa
WANG Zhi-Cai,ZHAO Mei-Li,ZHANG Xiao-Jie,ZHANG Zhen-Liang,LI Su-Zhen,LI Jian,CUI Hong-Qiu,LI Wu-Jiao,LIU Yan-Chun,WANG Yu,LI Li-Qiang,GU Li-Li,WANG Mei-Na***
(1)Key Laboratory of National Forestry and Grassland Administration for Orchid Conservation and Utilization,Shenzhen 518114,China;2)Shenzhen Key Laboratory for Orchid Conservation and Utilization,The National Orchid Conservation Center of China and the Orchid Conservation&Research Center of Shenzhen,Shenzhen 518114,China;3)South China Limestone Plants Research Center,College of Forestry and Landscape Architecture,South China Agricultural University,Guangzhou 510642,China;4)Xinjiang Key Laboratory of Grassland Resources and Ecology,College of Grassland Sciences,Xinjiang Agricultural University,Urumqi 830052,China;5)Department of Urology,Shenzhen Second People’s Hospital,The First Affiliated Hospital of Shenzhen University,International Cancer Center,Shenzhen University School of Medicine,Shenzhen 518039,China;6)Guangdong Provincial Key Laboratory for Plant Epigenetics,Longhua Bioindustry and Innovation Research Institute,College of Life Sciences and Oceanography,Shenzhen University,Shenzhen 518060,China)
Abstract Objective Dendrobium officinale has long been used as an important medicinal herb in oriental medicine.Polysaccharide,flavonoid,and alkaloid are the major active ingredients,the production and accumulation of which are frequently affected by numerous environmental cues.Phytohormone supplemented in culture medium has facilitated the mass production of orchids.However,their mechanism of action on the production of active components in Dendrobium officinale is far from clear.Methods Here,major medicinal metabolites were comparatively analyzed in Dendrobium officinale seedlings exposed to the most commonly used phytohormones(NAA and/or 6-BA),and transcriptomes corresponding to the treatments were generated.Results Results showed that phytohormones greatly affected the accumulation of polysaccharide,alkaloid,and flavonoid,and triggered tremendous transcriptional changes.It was demonstrated that 6-BA induced more transcripts than NAA and that β-glucosidase(BGLU)expression was closely related to polysaccharide production.Further functional analysis revealed that factors including phytohormone category,concentration,treating duration,and seedling growth stages,can drastically affect the BGLU expression and the corresponding polysaccharide production,thus partially answered the key question at molecular level why the medicinal constituents are unstable in tissue culture derived Dendrobium plants.Conclusion Altogether,the present study clearly demonstrated that BGLU is a key regulator for polysaccharide production in Dendrobium officinale in response to phytohormone treatments.
Key words Dendrobium officinale,transcriptomic analysis,polysaccharide,β-glucosidase
TheDendrobiumis one of the largest genera in the Orchidaceae that contains more than 1 500 species around the world with high economic and scientific value[1].Dendrobium officinale(D.officinale),an endangered perennial epiphytic herb widely distributed in subtropical Asia[2],has been ranked as one of the most prizedDendrobiumspecies[3]exhibiting diverse therapeutic effects such as stomach nourishing,body fluids secretion,immunity enhancement,and cancer inhibition[4-5].The stem contains a variety of active compounds including polysaccharides,alkaloids,flavonoids,phenols,etc[6-7].D.officinaleis widely used both for medicine and dietary supplement in the form of juice,powder,capsules,and functional wine or tea according to the consumer’s preferences[8],leading to intensified agricultural production of this species.Currently,more than 6 000 ha of agricultural land is used for producing more than 20 000 tons of freshD.officinalein southern China[9].Thus,the quality ofD.officinaleproduction is crucial,which can be influenced by diverse genetic and environmental factors[10].Therefore,high-efficient,stable and non-toxic propagation methods were necessary for commercial production ofDendrobiumplants.Even though the improved technologies on tissue culture and micropropagation had been performed[11],current widely used large scale propagation by hybridization and medium induced germination resulted in homozygous individual deficiency,unstable phenotypical traits and medicinal quality of progenies[12-13].Yet,the underlining mechanism is far from clear.
We previously analyzed the functional mechanisms of active ingredients fromDendrobiumspecies[5,14-15],and the putative biosynthesis pathways for the active metabolites[10].Here,comprehensive changes in the seedlings ofD.officinalein response to phytohormones(NAA and/or 6-BA)treatments were compared using two RNA-seq analyses based on an Illumina NovaSeq 6000 platform,and the content of polysaccharide,flavonoid and alkaloid was determined accordingly.It was found that theBGLU(β-glucosidase)family members were frequently isolated.Particularly,BGLU2Lin theBGLUfamily is associated with the accumulation of polysaccharide.Short period of 6-BA application induced soluble polysaccharide production,while knockdown ofBGLU2Ldecreased the levels.This work provides new insights into the molecular mechanism ofD.officinaleseedlings under phytohormone treatments,which should be a useful resource to enhance the biosynthesis of active ingredients inD.officinaleand other orchids.
1 Materials and methods
1.1 Plant materials and treatments
D.officinaleseeds were germinated on halfstrength Murashige and Skoog(MS)medium with 3.0% sucrose,0.4% agar,and 20.0% potato(pH 5.8).Materials were grown in a culture room at(25±2)℃with a 12 h/12 h light/dark cycle(40 μmol/m2/S)and 60%~70% relative humidity.Two months later,protocorms were emerged from the seeds and then formed plantlets.For phytohormone feeding experiments in the first RNA sequencing,2-month-old uniform plantlets were sub-cultured on a medium described above,supplemented with distilled water,0.1 mg/L NAA,1.0 mg/L 6-BA,and 0.1 mg/L NAA+1.0 mg/L 6-BA,two biological replicates for each treatment.The plantlets were sampled after 3 weeks(w)of cultivation.For the second RNA sequencing,9-month-old uniform plantlets were transferred onto mediums supplemented with varied concentrations of 6-BA(0,0.5,1.0,and 2.0 mg/L)and sampled at different time points(2 w and 4 w).
1.2 Measurement of the content of polysaccharide,flavonoid,and alkaloid
Water-soluble polysaccharide was determined using plant soluble polysaccharide assay kit(Solarbio,Beijing,China).Flavonoid was measured using flavonoid assay kit(Solarbio,Beijing,China).Reducing polysaccharide was determined according to Avwiorokoet al.[16].Alkaloid was estimated following the method by Wanget al.[17].Phytohormone treated seedlings(0.5 g)were harvested,ground in liquid nitrogen,and extracted by extraction buffer.The isolated compounds were determined by using a spectrophotometer(BeckmanCoulter DU730).
1.3 cDNA library preparation and transcriptome sequencing
Total RNA was isolated from phytohormone treatedD.officinaleplantlets using the plant RNA isolation mini kit(BioTeke,Beijing,China)according to the manufacturer’s recommendation.DNA contamination was removed with recombinant RNasefree DNase I(TaKaRa,Tokyo,Japan)and then mRNA was enriched with Oligo(dT)magnetic beads(Thermo Fisher Scientific,MA,USA).The purified mRNA was cut into small pieces using fragmentation buffer and reverse-transcribed to produce the cDNA library using mRNA-seq sample preparation kit(Illumina,San Diego,CA,USA)as instructed by the manufacturer.The prepared libraries were sequenced in the 150-nt paired-end mode on an Illumina NovaSeq 6000 platform(BerryGenomics,Beijing,China).
1.4 Transcriptome data processing and analysis
The raw fastq format reads obtained from the sequencing were purified by filtering out low-quality reads.Next,the clean reads were mapped against the primary CDS from theD.officinalegenome database[18]for quantification of the gene abundance as read counts using Hisat2(version 2.0.6)software.The expression levels of genes were presented as the fragments per kilobase of transcript per million map reads(FPKM)using Cufflinks(version 2.2.2)software.EdgeR was used to compare differently expressed genes(DEGs)between groups.Genes were identified significantly differentially expressed with thePadjusted(padj)<0.05 and|log2fold change|>2 as the thresholds.KEGG(Kyoto encyclopedia of genes and genomes)enrichment analysis of DEGs were performed using KOBAS v2.0 software.Heatmap was used to generate heatmaps of DEGs.
1.5 Quantitative real-time PCR(qRT-PCR)validation
Eight genes of interest involved in glycolysis/gluconeogenesis(PDCandGALM),starch and sucrose metabolism(TPS,OTSB,andBGLU2L),and flavonoid biosynthesis(CHS,DFR,andCYP75B1)were randomly selected for further differential expression confirmation of the RNA-seq data by qRTPCR.Total RNA was extracted fromD.officinaleplantlets after 3 w of phytohormone treatment using TRIzol reagent(Thermo Fisher Scientific).Total RNA(1.0 μg)was reverse-transcribed into cDNA using PrimeScriptTMRT reagent kit with gDNA Eraser(Takara,Japan).qRT-PCR analysis was performed with SYBR green premix kit(Toyobo,Japan)in the ABI PRISM 7500 Fluorescent Quantitative PCR System(Thermo Fisher Scientific).Actinwas used as the internal control for normalization of expression levels;other genes and the primers were listed in Table S1.2-ΔΔCtmethod was used to calculate relative gene expression.
1.6 RNAi-induced gene silencing of BGLU2L in D.officinale leaves
The expression ofBGLU2Lwas suppressed by RNA interference(RNAi)method.Briefly,a 468-bp trigger fragment was PCR amplified from cDNA template ofD.officinaleseedlings by primers,5'CTTACGCTTGATGCTTTTAG 3'(forward),5'CATCCTCCATACTCATCTTG 3'(reverse),and cloned into pCambia1300 plasmid in both the sense and antisense orientations[19].Agrobacterium carrying the plasmid was infiltrated intoD.officinaleleaves treated with or without 1.0 mg/L 6-BA for 3 w.Polysaccharide was measured after 72 h of infiltration.
2 Results
2.1 Phytohormones affected bioactive compounds production
Considering that polysaccharides are the main medicinal components inD.officinale,and that flavonoids are the second most abundant metabolites,we measured the content of them after 0.1 mg/L NAA and/or 1.0 mg/L 6-BA treatment of 2-month-old seedlings for 3 w.The results showed that NAA and 6-BA had no observable effects on the growth of the plantlets(Figure S1a),but significantly increased the water-soluble polysaccharides content from 130.176 mg/g(water-treated control)to 184.158 mg/g and 171.379 mg/g,respectively(Figure 1a).About two-fold increase was further observed when combining the two phytohormones,indicating of their synergistic effects on soluble polysaccharides production.Likewise,the reducing polysaccharides content at control was 35.500 mg/g,which was much lower than NAA and 6-BA treated plantlets,with 48.167 mg/g and 46.167 mg/g,respectively(Figure 1b).In a similar manner,NAA and 6-BA synergistically enhanced the accumulation of reducing polysaccharides(58.500 mg/g,Figure 1b).Moreover,the total flavonoid content in the samples treated separately with NAA or 6-BA was comparable with that of the non-treated control(16.172 mg/g),but markedly increased when the two phytohormones applied together(22.964 mg/g,Figure 1c).The results clearly demonstrated that the relative long-term treatment of NAA and 6-BA promoted polysaccharide and flavonoid production.

Fig.1 Determination of metabolites accumulation and associated gene expression in response to long-term phytohormone treatments in D.officinale seedlings
2.2 Phytohormones triggered transcriptional changes
To further investigate the mechanisms underlining the differential accumulation of bioactive ingredients upon phytohormone application,8 cDNA libraries using materials mentioned above were prepared from two independent biological replicates and sequenced.The raw-and processed-data were deposited in GEO(accession:GSE184090),and the differentially expressed genes were listed in Table S3.The quality score at the Q30 levels(sequence error rate<1‰)were from 91.72% to 95.29% and the average GC content was ranged from 45.45% to 46.23%(Table S4).These results suggest that the sequencing is of high quality.In total,243 133 073 Illumina pared-end clean reads(about 30.4 million reads per sample)were obtained,90% of which were uniquely mapped to theD.officinalereference genome(23 068 genes)(Table S4).Of these genes,13 961 were annotated in the GO database,and 8 536 were annotated in the KEGG database by using topGO and KOBAS,respectively.
A total of 1 363 DEGs were identified;6-BA stimulated more transcript changes than NAA(Figure 1d,Table S5).Because that KEGG assignments provide alternative functional annotations for biochemical pathway related genes,the DEGs were further mapped to terms in KEGG pathway database to identify enriched genes associated with metabolic pathways under phytohormone treatments.The top 20 pathways withP<0.05 were significantly enriched.Notably,the most enriched DEGs were observed in the pathway of plant hormone transduction,flavonoid biosynthesis,biosynthesis of secondary metabolites,starch and sucrose metabolism,and alkaloid biosynthesis(Figure S1b).Data sets from different comparison groups were analyzed by a Venn diagram,resulting in 60 overlapping DEGs compared with the water-treated control(Figure 1e).Among these,genes possibly involved in polysaccharide metabolism includingBGLU2L,XTH22,XTH23,andCSLA9were up-regulated by phytohormone treatment(Figure 1f).Consistent with the increased accumulation of flavonoid,one cytochrome P450 family memberCYP93A1was significantly upregulated by phytohormone application(Figure 1f).Additionally,the expression of genes associated with alkaloid biosynthesis includingSTR1were stimulated in response to phytohormones(Figure 1f).
To experimentally validate the RNA-seq data,eight genes from active ingredient metabolism pathway(PDCandGALMfrom glycolysis/gluconeogenesis;TPS,OTSB,andBGLU2Lfrom starch and sucrose metabolism;CHS,DFR,andCYP75B1from flavonoid biosynthesis)were randomly selected for qRT-PCR analysis.Resultsshowed that expression of the eight genes was basically consistent with the expression profile in the RNA-seq datasets(Figure 2),which further confirmed the reliability of the sequencing data.
2.3 Transcriptome profiling revealed BGLUs as key players for polysaccharide production
It appeared that 6-BA has stimulated more transcript changes than NAA as revealed previously,6-BA application was thus further investigated.To verify if the responses is less dependent on developmental stages,9-month-old plantlets were subjected to varied concentrations of 6-BA treatments(0,0.5,1.0,and 2.0 mg/L)for short-term(2 w)or long-term(4 w)periods.Consistent with our previous results,application of 6-BA had nonobservable effects on the growth of seedlings(Figure S2a,b),but significantly increased the alkaloid levels(Figure 3a).The polysaccharide content was slightly decreased under short period of 6-BA treatments,but somehow increased with prolonged application(Figure 3b).Consensus results also showed that the content of flavonoid was increased with an ascended concentrations of 6-BA,especially when comparing the levels of 2-w against that of 4-w treatments(Figure S2c).To reconfirm the RNA-seq data as well as to gain more detailed information about different developmental stages ofD.officinaleseedlings in response to varied concentrations of phytohormone,the 6-BA-treated samples mentioned above were subsequently applied to a second RNA-sequencing analysis(Table S6).As expected this time,more DEGs were obtained under varied concentrations of 6-BA treatments(Figure 3c).The overlapping DEGs were further analyzed by Venn diagrams(Figure 3d,e),revealing genes associated with biosynthesis of alkaloid(CYP76B6andTR1),polysaccharide(BGLU2L,BGLU12,andBGLU41),and flavonoid(CYP75B1andF3H)in response to different 6-BA treatments(Figure 3f).It is worthy noting that theBGLUfamily members includingBGLU2Lwere frequently isolated in the data sets,suggesting of its importance in polysaccharide metabolism.What’s more fascinating is that the expression pattern ofBGLUs,down-regulated first and upregulated as prolonged treating,was in good agreement with the polysaccharide levels.
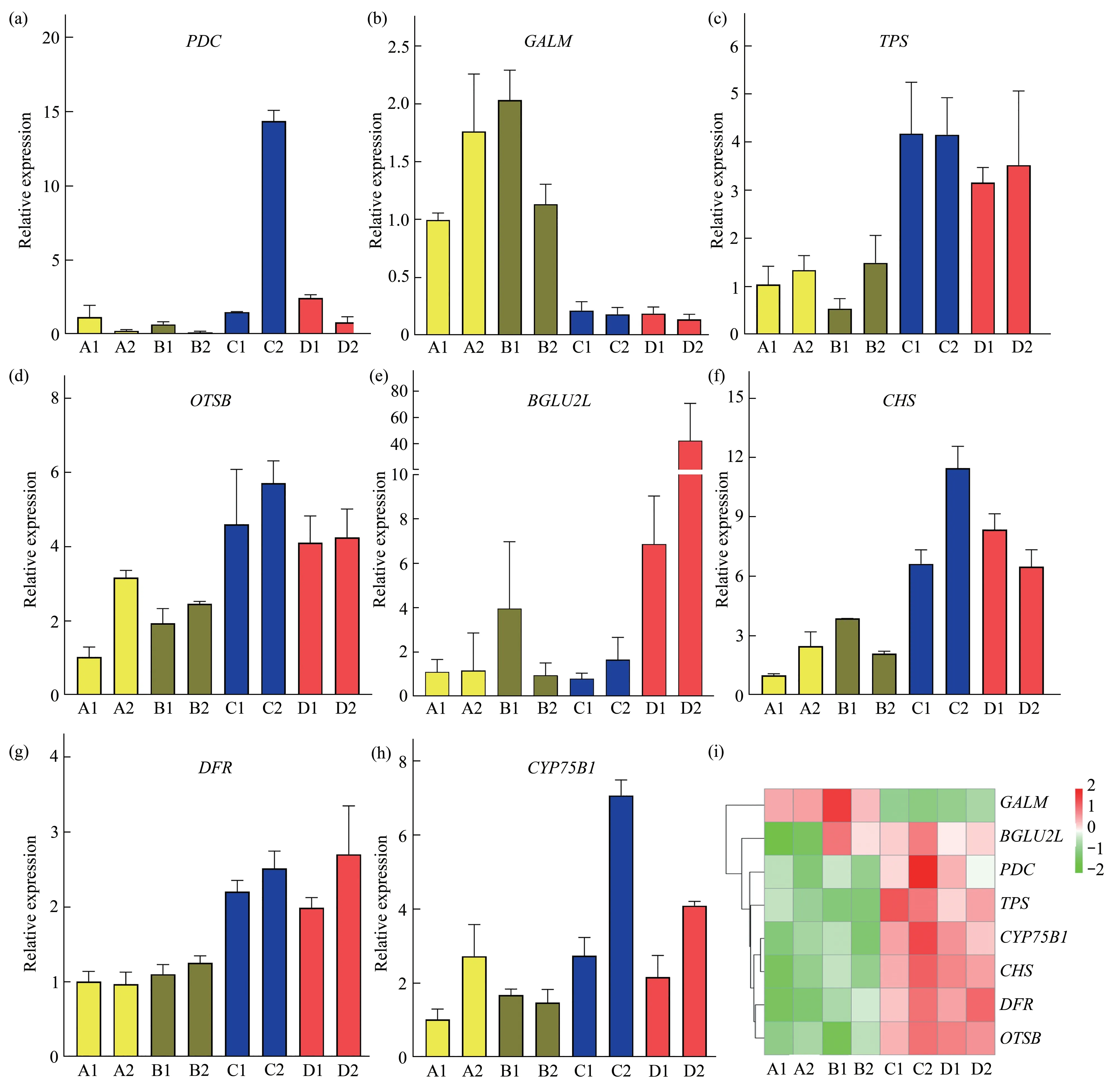
Fig.2 qRT-PCR validated the randomly selected genes involved in metabolites production in response to phytohormone treatments

Fig.3 Determination of metabolites accumulation and associated gene expression in response to varied concentrations of 6-BA treatments in D.officinale seedlings
2.4 BGLU2L in BGLU family modulated polysaccharide production in D.officinale
The consensus pattern ofBGLUs expression with the polysaccharide level prompted us to further investigate their tight association.Firstly,a phylogenetic tree for BGLUs ofD.officinale(DcBGLUs)was constructed based on protein sequences that available online(accession numbers were detailed in Table S7).The DcBGLUs were grouped into 4 sub-clusters(Figure S3a).For clarity,the 14 DcBGLUs were combined with 47 BGLUs fromArabidopsisthaliana(AtBGLUs) for phylogenetic analysis.Most of the DcBGLUs were grouped into cluster III and V,without any of them assigned to cluster I(Figure 4).Considering thatBGLU2Lwas repetitively isolated as DEGs in the two RNA-seq data,it was selected as a representative for further functional confirmation.The expression ofBGLU2Lwas silenced(BGLU2L-kd)viaRNAi(Figure 5a),leading to decreased accumulation of reducing polysaccharide(Figure 5b)and soluble polysaccharide(Figure 5c)in transiently transformed 2-year-oldD.officinaleleaves.In order to reassure the responsiveness ofBGLU2Lexpression to phytohormone as mentioned above,9-month-old seedlings were treated with or without 1.0 mg/L 6-BA for 3 w before RNAi transient transformation.Resultsshowed that knock-down ofBGLU2L(Figure 5d)suppressed the accumulation of reducing polysaccharide(Figure 5e)and the soluble polysaccharide(Figure 5f),which was well corroborated with the previous results obtained by the second RNAseq.Application of 6-BA did not lead to observable phenotypic changes(Figure S3b),but did inhibit reducing polysaccharide production.A slight increase of soluble polysaccharide upon 6-BA application was also noticed(Figure 5f),which might due to compensatory effects between the reducing and soluble polysaccharide because of their interconvertible nature.These results reconsolidated the vital role ofBGLUs in polysaccharide metabolism.
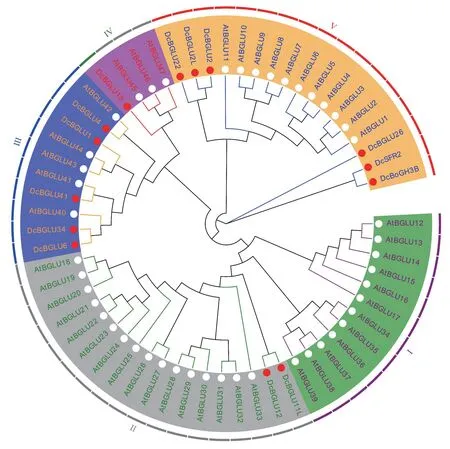
Fig.4 A molecular phylogenetic tree representing relationships among BGLU proteins from D.officinale and Arabidopsis thaliana
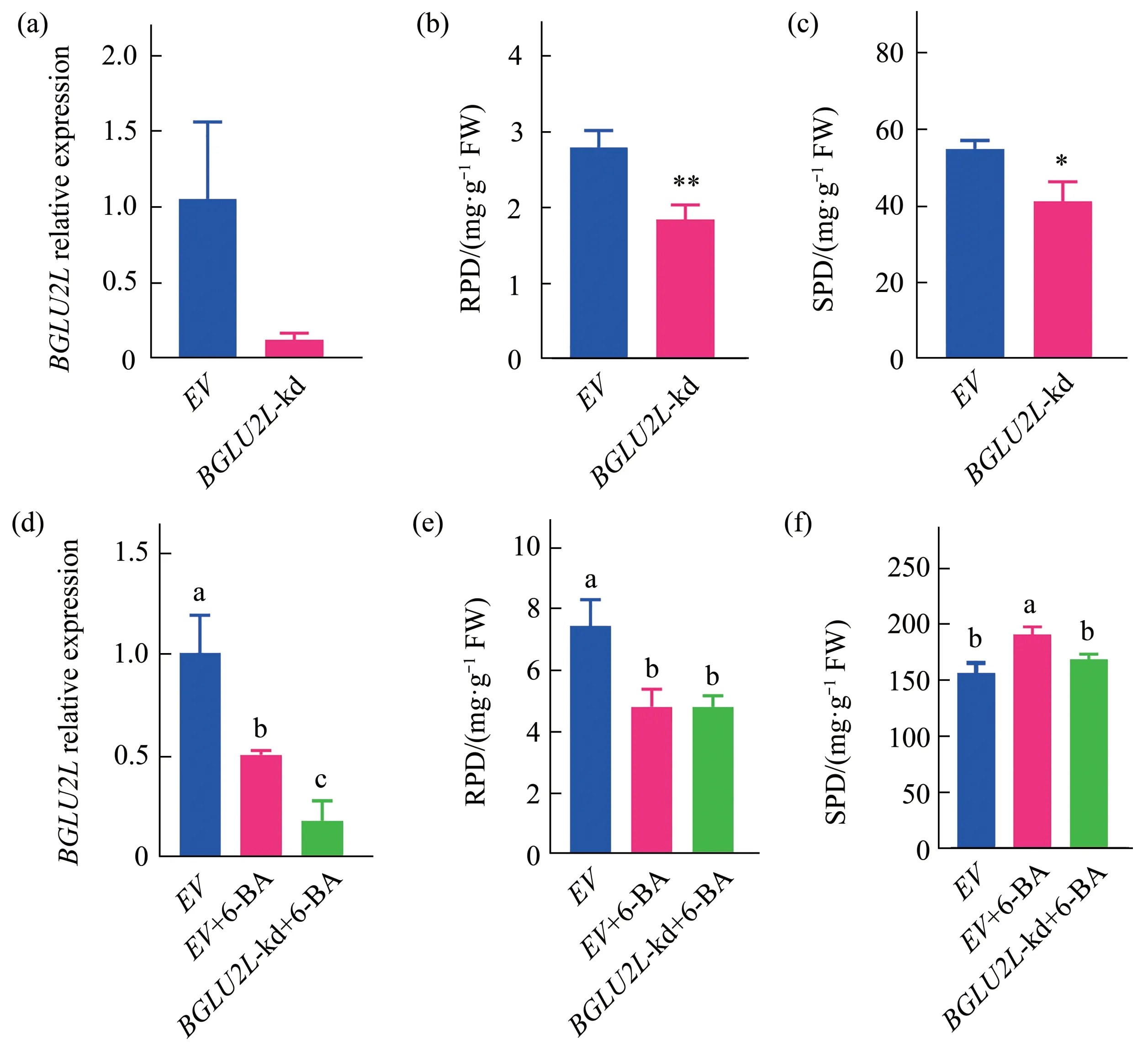
Fig.5 Verification of BGLU2L as a key player in polysaccharide production
3 Discussion
D.officinaleis an extremely important medicinal orchid with polysaccharides,flavonoids,and alkaloids as the major effective components[18].However,wild resources ofD.officinaleare rare and endangered because of low rate of propagation in nature,habitat destruction,and overexploitation[20].Therefore,alternative methods for mass production ofD.officinaleplants are urgently needed.To this end,extensively exploited tissue culture techniques for the rapid and large scale micropropagation of orchids through phytohormone-induced protocorm-like bodies(PLB)have facilitated the successful culture ofD.officinale in vitro[21].Even though phytohormones in the culture medium have been demonstrated to stimulate a variety of biological processes in various plants,an in-depth assessment of drug yielding potential and the underling mechanism is sporadic.The present study investigated the impact of NAA and 6-BA,two of the most frequently used plant growth regulators for orchid tissue culture,on the bioactive metabolite biosynthetic capacity inD.officinaleplantlets micropropagatedin vitro.
Cytochrome P450 superfamily members are associated with metabolism of alkaloid.The present study detected up-regulation ofCYP76B6upon increased concentrations of 6-BA application,which is in good agreement with the accumulation of alkaloid in the corresponding samples.On the other hand,flavonoids are synthesized mainly through phenylpropanoid and polyketide pathways,using malonyl-CoA and p-coumaroyl-CoA as the starting materials[22].CHS condensation and DFR catalysis are involved in most of flavonoids biosynthesis[23].This study revealed that NAA and 6-BA increased accumulation of flavonoid and up-regulatedCHSandDFRexpression as well in 2-month-oldD.officinalesedlings.
Glucosylation could increase the solubility and stability of compounds produced in plant metabolism,thus more suitable for storage in the vacuole or other organelles[24].PlantBGLUsexhibit diverse biological functions in response to developmental and environmental cues.We and others previously sequenced the genome ofD.officinale[18,25],and the gene expression atlas is publicly available,which facilitated the functional genomic studies.However,members of theBGLUfamily inD.officinalehave hardly been investigated.In the present study,a group ofBGLUsincludingBGLU2L,BGLU12,andBGLU41were stimulated by 6-BA in 9-month-oldD.officinaleseedlings.Phylogenetic analysis revealed a close relatedness betweenD.officinaleandArabidopsis thaliana[18].In this study,DcBGLU18 was grouped into cluster IV with AtBGLU45 and AtBGLU46,both of which function in lignification[26],indicating of its possible role in lignin precursor hydrolysis inD.officinale.DcBGLU2,DcBGLU2L,and DcBGLU22 were assigned into cluster V with AtBGLU6 and AtBGLU10,which act as glucosyltransferases inArabidopsis[27],implying of their similar functions inD.officinale.It is noticed that even though the expression was closely associated with polysaccharide production,BGLUis extremely susceptible to environmental cues including phytohormone category, treatment duration,concentration,and seedling developmental stages,thus partially unraveled the molecular mechanism under the unstable production of medicinal compounds in tissue-culture derivedDendrobiumplants.Altogether,our results presented clear evidences definingBGLUas a key player in polysaccharide metabolism possibly through its glucosyltransferase activity,yet the detailed chemical catalytic mechanism and other functions are still to be explored.
4 Conclusion
DEGs associated with the putative polysaccharide,flavonoid and alkaloid biosynthetic pathway inD.officinaleseedlings in response to phytohormones were analyzed.The expression of DEGs was in good agreement with the content of corresponding active ingredients,especiallyBGLU2Lin DEGs was involved in the accumulation of polysaccharide,thus unraveled the underlying mechanisms of altered compound accumulation under phytohormone treatments.The dataset presented paves the way for manipulating bioactive ingredients production through phytohormones in the tissue culture system of orchids.
SupplementaryAvailable online(http://www.pibb.
ac.cn or http://www.cnki.net).
PIBB_20210352_Figure S1.jpg
PIBB_20210352_Figure S2.jpg
PIBB_20210352_Figure S3.jpg
PIBB_20210352_Table S1.pdf
PIBB_20210352_Table S2.pdf
PIBB_20210352_Table S3.xlsx
PIBB_20210352_Table S4.pdf
PIBB_20210352_Table S5.xlsx
PIBB_20210352_Table S6.xlsx
PIBB_20210352_Table S7.pdf
