Deuterated N-difluoromethylthiophthalimide: A stable, scalable reagent for radical and electrophilic deuteriodifluoromethylthiolations
2022-09-15ChunyngHuFngmingChenGuoPingLuWenBinYi
Chunyng Hu, Fngming Chen, Guo-Ping Lu,∗, Wen-Bin Yi,b,∗∗
a School of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
b Key Laboratory of Organofluorine Chemistry, Shanghai Institute Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
ABSTRACT A new, stable and scalable reagent for deuteriodifluoromethylthiolation (deuterated Ndifluoromethylthiophthalimide, PhthSCF2D) has been developed.This reagent can be applied for the photocatalytic radical deuteriodifluoromethylthiolation of various olefins and aldehydes (30 examples).Meanwhile, it can achieve the electrophilic deuteriodifluoromethylthiolation of a series of electrophilic substrates including electron-rich arenes, aryl/vinylboronicacids, alkynes, amines, thiols and β-ketoesters (22 examples).Some complex molecules can also be applied in both radical and electrophilic deuteriodifluoromethylthiolation using PhthSCF2D as the reagent.
Keywords:Deuteration Deuteriodifluoromethylthiolation Photocatalytic Electrophilic Radical
Deuteration technology has been widely applied in various fields, such as organic synthesis, drug metabolism, NMR analysis and drug development [1–8].The C-D bond is more stable than the C–H bond (6–9 times) owing to its larger atomic mass than H,so the deuteration of C–H bonds may greatly alter the metabolism and pharmacokinetic properties of drug candidates [9–14].Meanwhile, the potency and selectivity of these molecules can be retained since the deuteration of C–H bonds does not change its chemical structure, physical properties, or biological activity [9].Therefore, the exploration of novel and efficient routes for the incorporation of deuterium to organic molecules is significant and appealing [15–18].
On the other hand, the introduction of difluoromethylthio group (SCF2H) into organic compounds has recently attracted much attention [19–22], because SCF2H is a potential alternate as the lipophilic OH or NH surrogate, and some SCF2H-containing molecules display unique biological and pharmaceutical activity[23–27].Considering the importance of deuteration and difluoromethylthio group in drug candidates, it is highly desirable to construct SCF2D substituted molecules for drug discovery.
In 2016, Billard and coworkers introduced the first example on the synthesis of SCF2D-containing compounds through the reduction of the PhSO2CF2S group (Scheme 1a) [28].In 2020, our group described a one-pot deuteriodifluoromethylthiolation of alkyl electrophiles with thiourea and diethyl bromodifluoromethylphosphonate (Scheme 1b) [29].In 2021, Li’s group disclosed the C–H deuteriodifluoromethylation of indole derivatives with CF2DSO2Na(Scheme 1c) [30].Nevertheless, the substrate scope of all these methods is limited, moreover none of these SCF2D-reagents is commercial reagent owing to their complex synthesis process.
To solve these issues, we envisage to develop a stable and scalable reagent for deuteriodifluoromethylthiolation, which has good reactivity and can be applied to a variety of complex substrates.In 2015, Shen’s group disclosed a powerful reagent:Ndifluoromethylthiophthalimide (PhthSCF2H) that allowed the difluoromethylthiolation of a wide range of nucleophiles [22].In 2017,the same group has further optimized the preparation process of this reagent (Scheme 1d) [31].
Along this line, we have developed a new SCF2D-reagent deuteratedN-difluoromethylthiophthalimide (PhthSCF2D) through modifying the synthetic route of PhthSCF2H.This reagent has good stability, and its synthetic route is easy scalable (up to 28.7 g scale) (Scheme 2).Meanwhile, PhthSCF2D is not only an outstanding electrophilic SCF2D-reagent for aryl/vinyl boronic acids,alkynes, amines, thiols,β-ketoesters, and oxindoles and electronrich arenes, but also an excellent radical SCF2D-reagent for alkenes and aldehydes (Scheme 2).
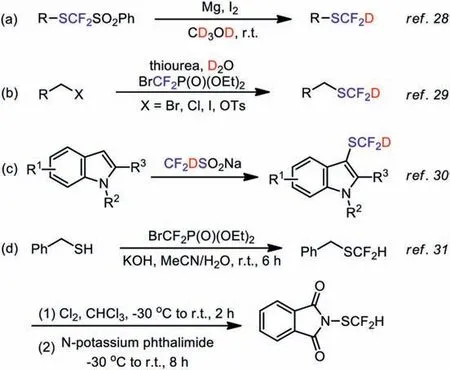
Scheme 1.(a-c) Recent advances in the deuteriodifluoromethylthiolation.(d) The synthesis route of N-difluoromethylthiophthalimide.
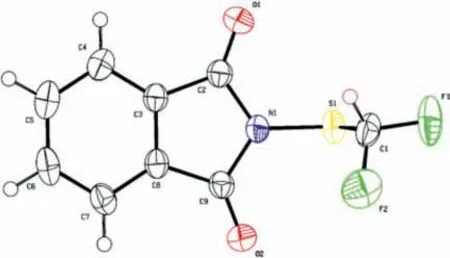
Fig.1.ORTEP diagrams of PhthSCF2D.
BrCF2P(O)(OEt)2is most common source of difluorocarbene to synthesize “SCF2H” with different sulfur sources [31–37], so BrCF2P(O)(OEt)2is selected as the “CF2” source for the synthesis of BnSCF2D.According to Shen’s synthetic route for PhthSCF2H(Scheme 1d) [31], we only replaced H2O by D2O initially, but the deuteration rate of the obtained BnSCF2D was poor (34% D).It is likely that there is still a lot of water in solvents and other raw materials.In order to eliminate this issue, anhydrous ether was employed as the solvent, and sodium hydride was applied to remove the trace of water in the system.A high yield of BnSCF2D(95%) with an excellent deuteration rate (97%) could be obtained through the modified approach.Then we further improved Shen’s method (Scheme 1d) [31].Good yield (85%) of PhthSCF2D with a high level of deuterium incorporation (97% D) could be afforded under milder temperature (Scheme 2).
The structural features of reagent 1 are fully confirmed by Xray analysis of its single crystal (Fig.1 and Table S1 in Supporting information).PhthSCF2D is a white crystalline solid with a melting point of 116–117°C.The reagent can be stored for a long time(more than 3 months) in a dry environment.It is stable in weakly polar solvents such as DCE, EA, DCM, toluene, THF, but it has poor stability in strong polar solvents such as DMF, water and alcohol solvents.
Guided by the concept of green chemistry [38–43], we choose photocatalysis to realize the application of 1.Visible-light photocatalytic radical reactions are a powerful tool for the construction of organic compounds [44–47].Initially, we investigated visible-light-promoted SCF2D of styrenes with PhthSCF2D inspired by Glorius’s work [48].After screen of solvents, bases, phase transfer catalyst and photocatalysts, the combination of MeCN,K2CO3and nBu4NBr fac-Ir(ppy)3, was the best option (Table S2 in Supporting information).Then, the substrate scope of this protocol was studied (Scheme 3).Styrenes with electron-donating groups in 1-position afforded good to excellent yield of desired products (3a-3g).Only moderate yields of SCF2D-products were obtained (3h-3o), when styrenes with electron-donating groups or weak electron-withdrawing groups in benzene ring were employed.
In general, bothE- andZ-isomers were observed, and the stereoselectivity was negligible.E- andZ-isomers have different chemical shifts and coupling constants (J) of alkenyl hydrogen [48], soE- orZ-isomers of 3 can be identified by1H NMR, and the ratio ofE/Zis judged by integral ratio of alkenyl hydrogen.In the cases of 3a and 3d, only single isomers were obtained, which may be due to steric hindrance (3a: Phenyl has greater steric hindrance thant–butyl) and hydrogen bonding between N and D (3d).
No reaction took place in the cases of styrenes with electronwithdrawing groups in 1-position or strong electron-withdrawing groups in benzene ring (such as 4-nitrostyrene).(E)-Prop-1-en-1-ylbenzene could also react with this reagent to yield final product (3p, 83%).All substrates maintained high level of deuteration rate after the reaction (>96% D).In addition, we also carried out a gram-scale reaction to obtain product 3b in a good yield (1.7 g,89%).The olefins converted with the drug Fenofibrate are synthesized as a raw material in the protocol, and a moderate yield of 3q(74%) was obtained (Scheme 4) [49–51].
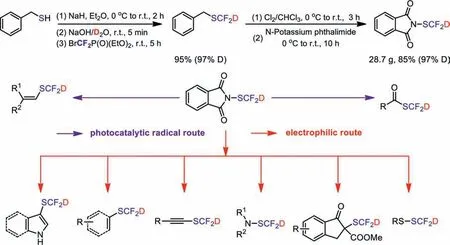
Scheme 2.The synthesis of PhthSCF2D and its applications for radical and electrophilic deuteriodifluoromethylthiolations.
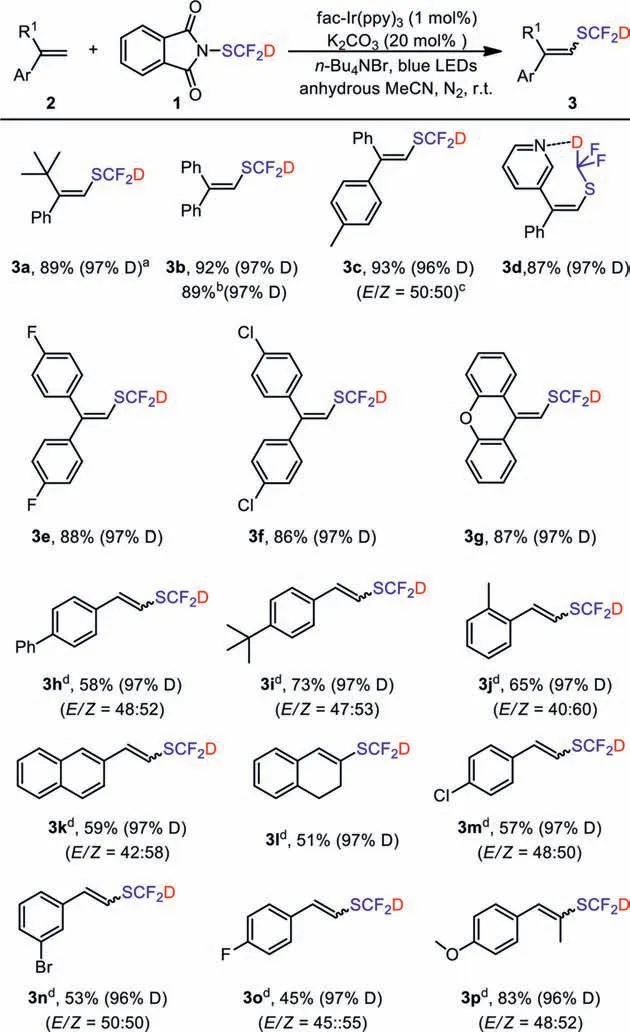
Scheme 3.The photocatalytic deuterated difluoromethylation of various alkenes.Conditions: 2 (0.5 mmol), 1 (1.25 equiv.), nBu4NBr (1 equiv.), fac-Ir(ppy)3 (1 mol%),K2CO3 (0.2 equiv.), anhydrous MeCN (4 mL), 10 W blue LEDs, room temperature,12 h, isolated yields.a The deuteration rate was determined by 19F NMR.b Yield was obtained at gram scale (1.7 g).c The E/Z ratio was determined by 1H NMR.d The reaction time was extended to 36 h.
This photocatalytic system can be also applied to the reaction of aldehydes with 1 with a slight modification (Table S3 in Supporting informaiton).Only a trace amount of 5a was produced under standard conditions.The yield of 5a reached 23% in the absence ofnBu4NBr.Finally, when Ir[dF(CF3)(ppy)]2(dtbbpy)PF6was employed instead of fac-Ir(ppy)3[52,53], and its amount was increased from 1 mol% to 2.5 mol%, an excellent yield of 93% was afforded.
With the optimized reaction conditions in hand, we then investigated the scope of the transformation with respect to the aldehydes (Scheme 5).In general, benzaldehydes with electron-donating or electron-withdrawing groups were converted smoothly into desired products in good to excellent yields (5a-5f, 5i).Hydroxyl substituted benzaldehydes were less reactive (5g, 5h), so longer reaction time was required.Meanwhile, aliphatic aldehydes can be also applied in the protocol successfully (5j, 5k).In the case of the heterocyclic aldehyde, a moderate yield was obtained (5l, 66%).The introduction of SCF2D into a complex aldehyde (4m) were also performed, and the desired product 5m was synthesized with a moderate yield (49%) (Scheme 6) [54].
In order to verify its reactivity for nucleophilic substrates,six types of nucleophilic substrates including electron-rich heteroarenes, aryl/vinyl boronic acids, alkynes, amines, thiols,βketoesters are implemented (Scheme 7).By comparing the reactivity of PhthSCF2D and PhthSCF2H with different substrates,there is almost no difference in the reactivity of these two reagents for different substrates (Table S4 in Supporting information).For electron-rich heteroarenes, PhthSCF2D exhibited excellent reactivity to gain good to excellent yields of the final products (7a-7d).Reactions of aryl or vinyl boronic acids also took place smoothly to give the corresponding SCF2D-prodcuts(7e-7h).Satisfactory yields could be provided in all the cases of aryl, aliphatic primary and secondary amines (7i-7l).Meanwhile, deuter-iodifluoromethylthiolations of alkynes and mercaptans were also implemented, and excellent yields were obtained(7m-7p).Adaptive application ofβ-ketoesters was also successful(7q-7t).The deuteration rate of the substrates remains almost unchanged, reaching 96% or 97%.To further highlight the potential of PhthSCF2D, this electrophilic method was carried out to introduce SCF2D into two drugs, and high yields of desired prodcut were obtained (7u, 7v) with the deuteration rate of 97% (Scheme 8).
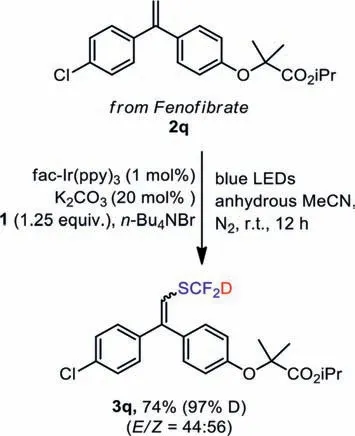
Scheme 4.The deuterated difluoromethylation of 2q.
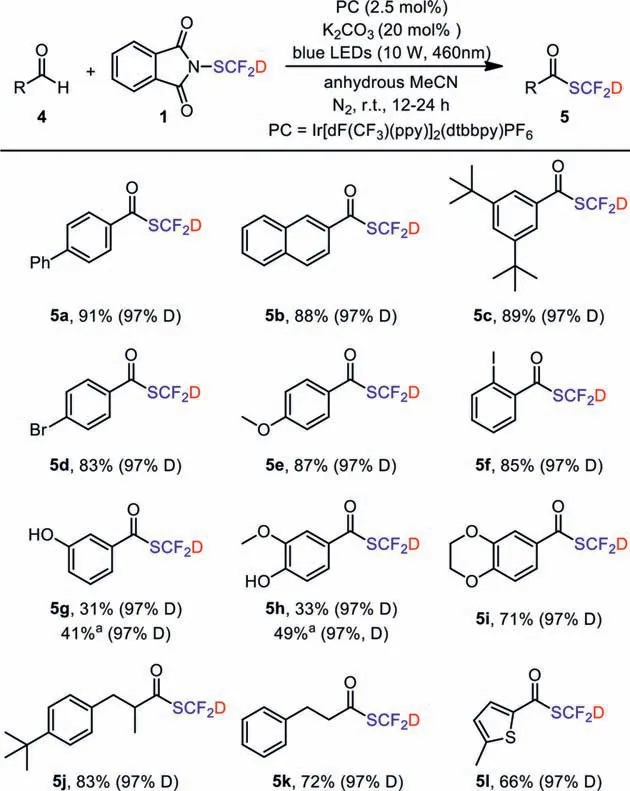
Scheme 5.Substrate scope for the photocatalytic deuterated difluoromethylation of aldehydes.Reaction conditions: 4 (0.5 mmol), 1 (1.25 equiv.),Ir[dF(CF3)(ppy)]2(dtbbpy)PF6 (2.5 mol%), K2CO3 (0.2 equiv.), anhydrous MeCN(4 mL), 10 W blue LEDs, r.t., 12 h, isolated yields.a The reaction time was 24 h.
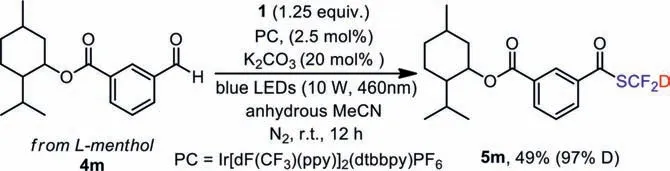
Scheme 6.The photocatalytic deuterated difluoromethylation of 4m.
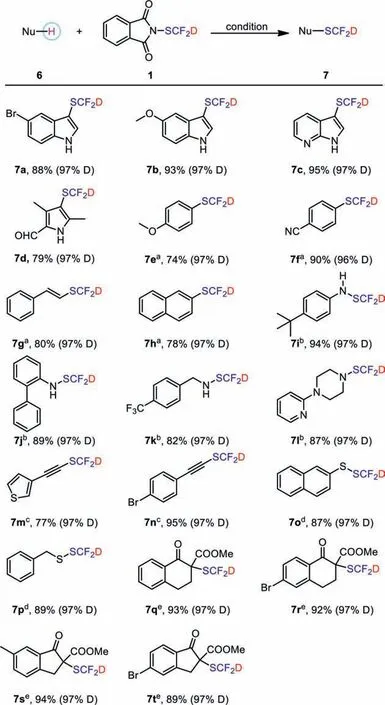
Scheme 7.The photocatalytic deuterated difluoromethylation of various nucleophilic substrates.Reaction conditions: heteroarene (0.5 mmol), 1 (1.2 equiv.),Me3SiCl (1.5 equiv.), DCE (3.0 mL), 80 °C, 16 h, isolated yields.a Reaction conditions: boronic acid (0.7 mmol), 1 (1.2 equiv.), Li2CO3 (0.35 equiv.), CuI (5 mol%),2,2′-bipyridine (bpy) (5 mol%), diglyme (5.0 mL), 60 °C, 15 h.b Reaction conditions:amines (0.7 mmol), 1 (1.1 mmol), toluene (4.0 mL), 80 °C, 20 h.c Reaction conditions: alkyne (0.6 mmol), 1 (1.3 equiv.), Li2CO3 (0.5 equiv.), copper(I) thiophene-2-carboxylate (5.0 mol%), 2,2′-bipyridine (bpy) (5.0 mol%), diglyme (4.0 mL), 60 °C,15 h.d Reaction conditions: thiols (0.7 mmol), 1 (1.1 equiv.), DCE (4.0 mL), 80 °C,20 h.e Reaction conditions: β-ketoester (0.7 mmol), 1 (1.2 equiv.), K2CO3 (1.1 equiv.), DCM (4.0 mL), r.t., 24 h.
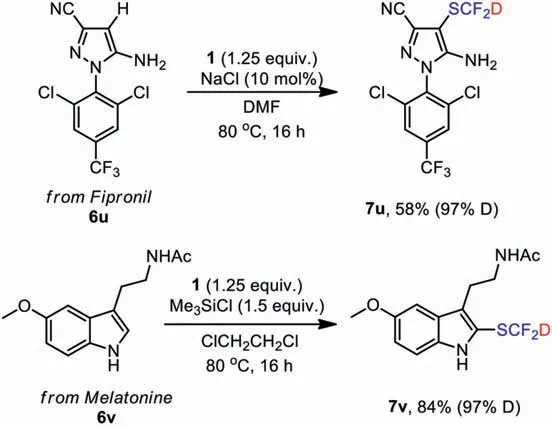
Scheme 8.The electrophilic deuterated difluoromethylation of 6u and 6v.
In summary, we have disclosed a new approach for the synthesis of deuteratedN-difluoromethylthiophthalimide (PhthSCF2D)that is a novel, stable and scalable SCF2D-reagent.This reagent exhibits excellent reactivity in the deuteriodifluoromethylthiolation(SCF2D) of a wide range of substrates including alkenes, aldehydes, electron-rich arenes, aryl/vinyl boronic acids, alkynes, amines,thiols andβ-ketoesters (52 examples).All the SCF2D-products have high level deuteration rates (>96% D).This SCF2D-reagent can be also applied in some complex molecules by photocatalytic radical or electrophilic routes.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (Nos.21776138, 22078161), the Fundamental Research Funds for the Central Universities (Nos.30920021124, 30918011314), the Natural Science Foundation of Jiangsu Province (No.BK20180476), the Postdoctoral Science Foundation Funded Project (No.2019M661848), the Priority Academic Program Development of Jiangsu Higher Education Institutions,and the Center for Advanced Materials and Technology in Nanjing University of Science and Technology for their financial support.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.013.
杂志排行
Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
