Is rosuvastatin protective against sepsis-associated encephalopathy? A secondary analysis of the SAILS trial
2022-09-01ShiyuanYuZengzhengGeJunXiangYanxiaGaoXinLuJosephHaroldWallineMubingQinHuadongZhuYiLi
Shi-yuan Yu ,Zeng-zheng Ge ,Jun Xiang ,Yan-xia Gao ,Xin Lu ,Joseph Harold Walline ,Mu-bing Qin,Huadong Zhu,Yi Li
1 Emergency Department,State Key Laboratory of Complex Severe and Rare Diseases,Peking Union Medical College Hospital,Chinese Academy of Medical Science and Peking Union Medical College,Beijing 100730,China
2 General Medicine Department of Jingnan Medical Center,General Hospital of PLA,Beijing 100039,China
3 Emergency Department,the First Affiliated Hospital of Zhengzhou University,Zhengzhou 450052,China
4 Department of Emergency Medicine,Penn State Health,Milton S.Hershey Medical Center,Hershey 17033,USA
BACKGROUND: Sepsis is a common cause of death in emergency departments and sepsisassociated encephalopathy (SAE) is a major complication.Rosuvastatin may play a neuroprotective role due to its protective effects on the vascular endothelium and its anti-inflammatory functions.Our study aimed to explore the potential protective function of rosuvastatin against SAE.METHODS: Sepsis patients without any neurological dysfunction on admission were prospectively enrolled in the “Rosuvastatin for Sepsis-Associated Acute Respiratory Distress Syndrome” study (SAILS trial,ClinicalTrials.gov number: NCT00979121).Patients were divided into rosuvastatin and placebo groups.This is a secondary analysis of the SAILS dataset.Baseline characteristics,therapy outcomes,and adverse drug events were compared between groups.RESULTS: A total of 86 patients were eligible for our study.Of these patients,51 were treated with rosuvastatin.There were significantly fewer cases of SAE in the rosuvastatin group than in the placebo group (32.1% vs.57.1%,P=0.028).However,creatine kinase levels were significantly higher in the rosuvastatin group than in the placebo group (233 [22-689] U/L vs.79 [12-206] U/L,P=0.034).CONCLUSION: Rosuvastatin appears to have a protective role against SAE but may result in a higher incidence of adverse events.
KEYWORDS: Rosuvastatin calcium;Sepsis-associated encephalopathy;Anti-inflammatory agents;Sepsis;Adverse reactions
INTRODUCTION
Sepsis is a life-threatening condition,defined as organ dysfunction caused by intense systemic inflammatory and coagulation cascade reactions due to severe infection(s).Sepsis-associated encephalopathy (SAE),a transient and reversible encephalopathy caused by infection outside of the brain,occurs in 25%-50% of sepsis patients.SAE is associated with high rates of long-term disability and mortality in sepsis patients.Therefore,early diagnosis and prevention of SAE may be critical for reducing the disability,mortality,and public health burden of sepsis.
Rosuvastatin is a 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor,which can control high cholesterol levels,and thereby prevente cerebrovascular diseases.In addition to its cholesterol control function,rosuvastatin has also shown to suppress pro-inflammatory cytokines such as interleukin-1 (IL-1),IL-6,and tumor necrosis factor-α (TNF-α).These three cytokines are theorized to play important roles in SAE initiation.Anstudy showed that statins could increase the expression of endothelial nitric oxide (NO) synthase,downregulate inducible NO synthase,and attenuate endothelial dysfunction in sepsis patients.Furthermore,statins have antioxidant and antiapoptotic functions,which contribute to inhibiting the inflammatory cascade during sepsis.
A 2010 meta-analysis demonstrated that statin drugs could reduce all-cause mortality in sepsis-induced acute respiratory distress syndrome (ARDS) patients.Recently,a series of studies were carried out to investigate the role of statins in different types of infections,with varying results.A randomized controlled trial (RCT) including 745 participants showed that rosuvastatin did not reduce the mortality rate in sepsis-associated ARDS patients.However,the effect of statins in SAE patients has not been studied in clinical trials.Therefore,our team performed a secondary analysis of clinical study data to investigate whether rosuvastatin had a protective role in SAE patients.We hypothesized that rosuvastatin would reduce the rate of SAE among sepsis patients.
METHODS
Study population
This study is a secondary analysis of the Rosuvastatin for Sepsis-Associated Acute Respiratory Distress Syndrome(“SAILS” study,ClinicalTrials.gov number: NCT00979121).The SAILS study was an RCT with 745 patients,investigating the protective effect of rosuvastatin on sepsis.Patients were enrolled if their chest radiography showed pulmonary edema and acute respiratory failure.The research methodology of the RCT was described in the SAILS report.Access to the full database of the original RCT can be requested from the National Institutes of Health (NIH) (https://biolincc.nhlbi.nih.gov/studies/rocprimed/?q=primed).Our research team was authorized by the NIH,and we were able to download the full database from the NIH website.
Patient enrollment
Patients were enrolled in the original study if they were receiving positive-pressure mechanical ventilation through an endotracheal tube,had a ratio of the partial pressure of arterial oxygen (PaO) to the fraction of inspired oxygen(FiO) of 300 mmHg (1 mmHg=0.133 kPa) or less,and had bilateral infiltrates on chest radiography that were consistent with pulmonary edema,without evidence of left atrial hypertension.
Inclusion criteria: (1) patients over 18 years old;(2)patients diagnosed with sepsis (patients with an infection and sequential organ failure assessment [SOFA] score ≥2);(3)patients with a complete informed consent form.
Exclusion criteria: (1) pregnancy;(2) patients with any psychiatric disorders;(3) patients diagnosed with or suspected of having an intracranial infection;(4) patients with cerebral injuries;(5) patients with acute cerebral vascular disease;(6)patients with underlying intracranial diseases.
Treatment exposures
In the SAILS study,patients received a 40 mg loading dose of rosuvastatin within four hours of randomization.Maintenance doses of 20 mg rosuvastatin were administered daily at 10 a.m.(±4 h).For patients with a morning serum creatinine level of 2.8 mg/dL (250 μmol/L) or more and who were not receiving renal-replacement therapies,the daily dose was reduced to 10 mg.Rosuvastatin was administered until the third day after discharge from the intensive care unit(ICU),study day 28,hospital discharge,or death,whichever came first.Rosuvastatin administration was suspended for safety reasons if creatine kinase (CK) levels were >10 times the upper limit of the normal (ULN) or if alanine aminotransferase (ALT) or aspartate aminotransferase (AST)levels were >8 times ULN.
Study outcomes
In our study,SAE was defined by a decrease in Glasgow Coma Scale (GCS) or confusion assessment method for the intensive care unit (CAM-ICU) scores relative to those on admission (measured every 24 h),excluding central nervous system (CNS) infections,cerebrovascular accidents,CNS organic diseases,or patients undergoing cardiopulmonary cerebral resuscitation.
The primary outcome of this analysis was the rate of SAE.Secondary outcomes included: 28-d all-cause mortality,duration of hospital stay,length of ICU stay,and adverse events.Adverse events were described using the highest levels of CK,ALT,and AST during the patient’s hospitalization period (symptoms such as muscle pain were not available in the current dataset).
Statistical analysis
Baseline characteristics were compared between the rosuvastatin and placebo groups.Continuous data were described as means with standard deviation (SD),and categorical data were described using absolute numbers with percentages.All statistical calculations were performed using SPSS 20.0 (IBM Inc.,USA).Chi-square and-test tests were used for percentage and continuous statistics,respectively.Kaplan-Meier curves were generated with time since randomization in the SAILS study.-values <0.05 were considered statistically significant.
RESULTS
Baseline characteristics
A total of 86 patients from the SAILS study were included in our analysis.Figure 1 shows the study flow diagram.Fifty-one patients received rosuvastatin treatment,and 35 received placebo.The median age in the rosuvastatin group was 55 years and 56 years in the placebo group(=0.737).There were no significant differences between the rosuvastatin and placebo groups in SOFA scores (=0.061),Acute Physiology and Chronic Health Evaluation(APACHE) II scores (=0.243),or GCS scores (=0.515).The use of antibiotic,vasoactive,narcotic,and paralytic medications was comparable between the two groups.
Other baseline characteristics were also comparable between the rosuvastatin and placebo groups.The complete baseline characteristics are shown in Table 1.
Patient outcomes
The SAE rate was lower in the rosuvastatin group than that in the placebo group (17/51 [33.3%] vs.20/35 [57.1%],=0.028).The SAE incidence rates are shown in Figure 2.Of the 37 SAE patients,18 were identified based on decreased GCS scores,and 19 were identified based on positive CAMICU.There were no patient deaths in either group during the 7-d observation period.There were no significant differences between the rosuvastatin and placebo groups in 28-d allcause mortality (27.5% vs.11.4%,=0.106),length of hospital stay (11.2±9.0 d vs.11.7±9.7 d,=0.811),or length of ICU stay (5.5±4.4 d vs.5.7±3.7 d,=0.815).

Figure 1. Flow diagram.CAM-ICU: confusion assessment method for the intensive care unit.
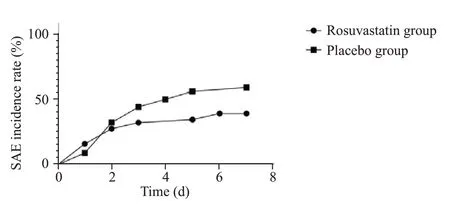
Figure 2. Sepsis-associated encephalopathy (SAE) incidence curve.
Safety outcomes
In our analysis,the CK level was significantly higher in the rosuvastatin group than that in the placebo group (=0.034).There were no significant differences between the rosuvastatin and placebo groups in ALT (=0.747) or AST levels (=1.000).The primary and safety outcomes are shown in Table 2.
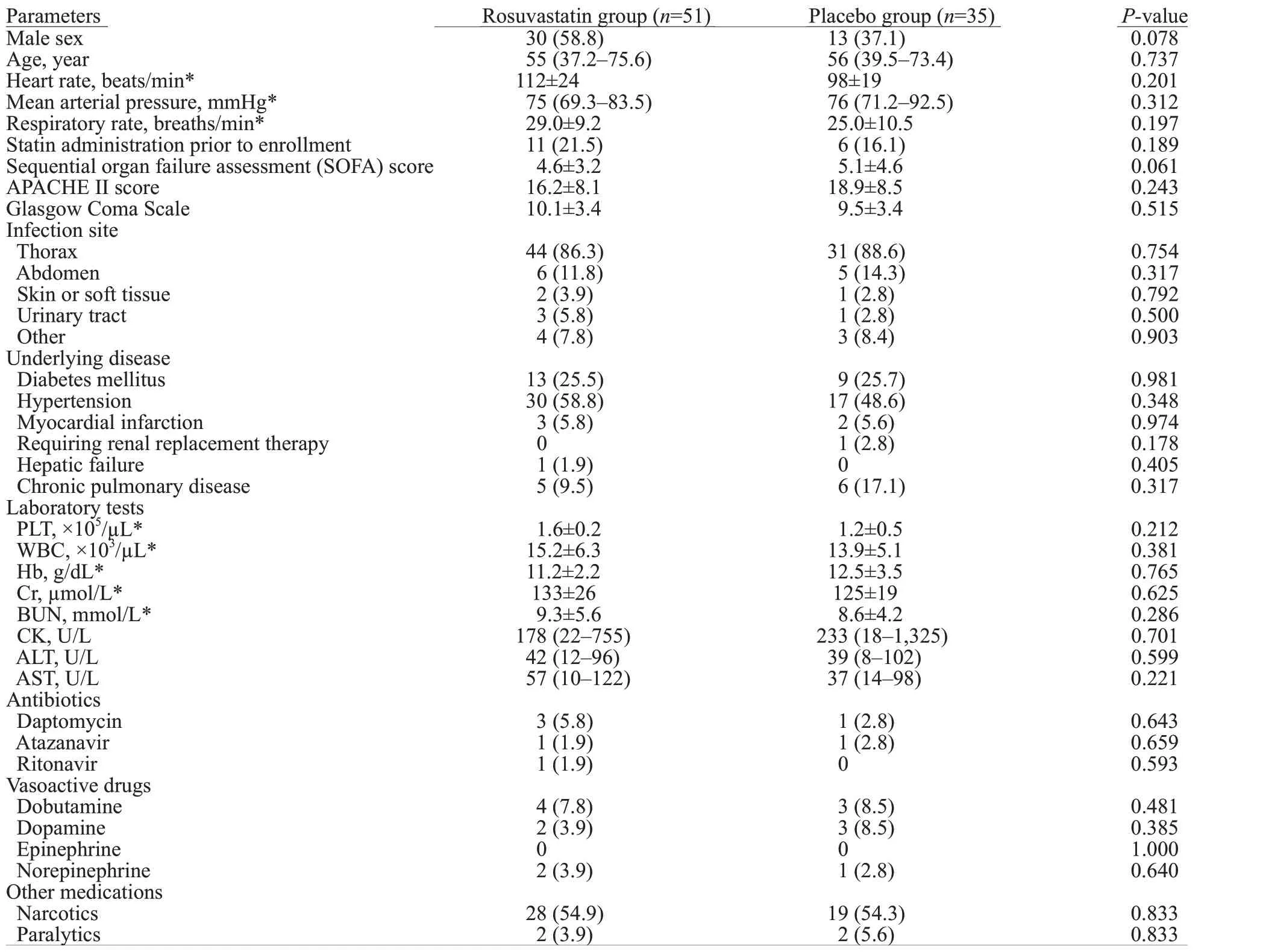
Table 1. Baseline characteristics of rosuvastatin and placebo groups

Table 2. Outcomes in the rosuvastatin and placebo groups
DISCUSSION
In this study,we compared SAE incidence in sepsis patients treated with rosuvastatin or placebo by performing a secondary analysis of a prospective RCT.We found that the rosuvastatin group had a significantly lower SAE incidence and a significant higher risk of myopathy.Similar to the original SAILS trial,our study showed that rosuvastatin treatment was not associated with reduced lengths of hospital or ICU stays.
A review showed a beneficial effect of statins in patients suffering from severe infections.A 2006 retrospective study showed that regardless of infection site,administration of statins at the time of bacteremia diagnosis was associated with a lower all-cause mortality rate,and continued administration of statins further reduced mortality.
The protective effect of statins was also recently highlighted in severe sepsis cases.Almog et alconducted a prospective cohort study in 2004 and found that prior statin therapy was associated with a lower rate of severe sepsis.Statin also showed a long-term protective effect in bacteraemic patients,reducing the 180-d mortality rate.These results were consistent with our findings that statins have a beneficial effect on SAE patients.However,the original SAILS trial did not include a dose-effect study.
Another prospective cohort study with 470 ICU patients showed that statin treatment increased the number of delirium-free ICU days;however,the delirium incidence rate was not significantly different between the groups(40% in the statin group vs.33% in the control group).In agreement with our findings,this study also reported a reduced SAE rate (32.1% in the statin group vs.57.1% in the placebo group).A multicenter prospective cohort study,including 763 ICU patients (263 of which were sepsis or ARDS patients),showed that in-hospital statin administration was associated with a reduced delirium rate while statin use prior to hospitalization did not show a benefit.
However,there are also some studies challenging the use of statins in patients with sepsis.Another secondary analysis from the SAILS trial reported contradictory results showing that statins could not attenuate delirium in ICU.Nonetheless,this study recruited patients whose ICU-CAM might be absent in some days.The patients’ mental status could hardly be evaluated in these ICU-CAM absent days;thus,we excluded patients whose ICU-CAM was incomplete.Additionally,GCS criteria for delirium were not included in the previous study,leading to different results from our study.A retrospective cohort study of 438 sepsis patients reported worse outcomes in the statin group,with a significantly higher in-hospital mortality rate.A population based study also showed that compared with atorvastatin and simvastatin,rosuvastatin had inferior effectiveness in reducing mortality.Specifically,statins did not attenuate lung injury during sepsis,and the ARDS rate was not significantly reduced in sepsis patients when statins were administered.In our study,we found that rosuvastatin treatment was protective against SAE,and the mortality rate was not significantly elevated in patients receiving rosuvastatin.
SAE is a CNS dysfunction caused by an inflammatory cascade.Rosuvastatin is widely used in cardiovascular and cerebrovascular disease patients to lower blood cholesterol levels and the rates of disease recurrence.In recent decades,statins such as rosuvastatin have been indicated to have pleiotropic pharmacologic effects,including anti-inflammatory and immunomodulatory functions.In particular,their immunomodulatory effects may contribute to neuroprotectionfor sepsis patients suffering from inflammation.
Up-regulation of IL-1 and IL-6 is associated with reduced expression of inter-endothelial junction proteins,leading to blood-brain barrier (BBB) disruption,which may also cause SAE in sepsis patients.Statins can reduce the expression of pro-inflammatory factors such as IL-1,IL-6,IL-10,IL-17,IL-18,intracellular adhesion molecule(ICAM),and TNF-α,which are associated with the degree of sepsis severity in animal models.These mechanisms are modified through prenylation of proteins that are important in antigen processing,affecting Th1/Th2 cell balance and Treg/Th7 balance.Similar outcomes were also shown in burn patients treated with statins.Moreover,statins inhibit the prenylation of Rac and Rho proteins,thereby upregulating the expression of endothelium-derived nitric oxide synthetase (eNOS).In turn,eNOS increases NO expression,maintaining endothelial cell function and attenuating BBB damage in sepsis patients,thereby reducing the rate of SAE among sepsis patients through an anti-inflammatory pathway.In an animal model,atorvastatin and rosuvastatin increased the abundance of gastrointestinal microbial,which is associated with anti-inflammatory factors.However,the exact pharmacological mechanism of statin-mediated immunomodulation has not yet been elucidated.
Although rare,statin-associated adverse events include myopathy,hepatic function damage,rhabdomyolysis,nausea and vomiting.Myopathy is a major adverse effect of statins,which is associated with mitochondrial dysfunction and decreases in protein prenylation.The original SAILS study selected rosuvastatin due to its fewer drug interactions compared with other statins.Unfortunately,in our current study,these symptoms were not included in the original dataset.We could only assess adverse events by comparing the levels of CK,AST,and ALT between the rosuvastatin and placebo groups.Our study showed significantly increased CK levels in the rosuvastatin group,which could lead to a higher risk of myopathy.
Our study still has several limitations.Bilirubin levels are an important laboratory test for evaluating hepatic function,which may be affected by rosuvastatin.However,bilirubin levels were not tested in the SAILS study.Although the original study recruited mechanical ventilated patients,and the baseline PaCO/PaOwas 39.9±9.9 mmHg/170±71 mmHg in the satin group and 40.7±11.7 mmHg/170±67 mmHg in the placebo group,pulmonary encephalopathy and other types of metabolic encephalopathy could not be ruled out,which were difficult to be distinguished from SAE.Baseline drug administration details,including the type of narcotics and antibiotics,could interfere with the SAE outcomes;however,the information was not fully reported in the original study.Statin treatment outcomes may also vary among sepsis cases caused by different types of pathogens.Therefore,further large sample,multiple center study is still needed to prove the association between statin and SAE outcomes.
CONCLUSIONS
Rosuvastatin may have a protective effect against SAE in sepsis patients,but it may be associated with a higher rate of muscle damage.However,the sample of our study was relatively small,and the SOFA score was almost comparable between the two groups.Therefore,a future RCT of rosuvastatin dosage effect may help confirm our findings and identify treatment recommendations.
ACKNOWLEDGEMENTS
We would like to thank the SAILS investigators from the National Heart,Lung,and Blood Institute ARDS Clinical Trials Network for their data and insights on this project.
This research received funding from the CAMS Innovation Fund for Medical Sciences (CIFMS) (2020-I2MC&T-B-014;2021-I2M-1-020).
The study was approved by the ethics committee of Peking Union Medical College Hospital.
The authors declare that they have no competing interests.
YL and HDZ formulated the research concept.SYY and ZZG collected and analyzed the data.SYY,JX and YXG prepared the manuscript.JHW,MBQ and YL revised the manuscript.All authors approved the final version of the manuscript.SYY,ZZG and JX contributed equally to this study.