Skin Lesion Segmentation and Classification Using Conventional and Deep Learning Based Framework
2022-08-24AminaBibiMuhamamdAttiqueKhanMuhammadYounusJavedUsmanTariqByeongGwonKangYunyoungNamRehamMostafaandRashaSakr
Amina Bibi,Muhamamd Attique Khan,Muhammad Younus Javed,Usman Tariq,Byeong-Gwon Kang,Yunyoung Nam,*,Reham R.Mostafa and Rasha H.Sakr
1Department of Computer Science,HITEC University,Taxila,Pakistan
2College of Computer Engineering and Sciences,Prince Sattam Bin Abdulaziz University,Al-Khraj,Saudi Arabia
3Department of ICT Convergence,Soonchunhyang University,Asan,31538,Korea
4Information Systems Department,Faculty of Computers and Information Sciences,Mansoura University,Mansoura,35516,Egypt
5Computer Science Department,Faculty of Computers and Information Sciences,Mansoura University,Mansoura,35516,Egypt
Abstract:Background:In medical image analysis,the diagnosis of skin lesions remains a challenging task.Skin lesion is a common type of skin cancer that exists worldwide.Dermoscopy is one of the latest technologies used for the diagnosis of skin cancer.Challenges:Many computerized methods have been introduced in the literature to classify skin cancers.However,challenges remain such as imbalanced datasets,low contrast lesions,and the extraction of irrelevant or redundant features.Proposed Work:In this study,a new technique is proposed based on the conventional and deep learning framework.The proposed framework consists of two major tasks:lesion segmentation and classification.In the lesion segmentation task,contrast is initially improved by the fusion of two filtering techniques and then performed a color transformation to color lesion area color discrimination.Subsequently,the best channel is selected and the lesion map is computed,which is further converted into a binary form using a thresholding function.In the lesion classification task,two pre-trained CNN models were modified and trained using transfer learning.Deep features were extracted from both models and fused using canonical correlation analysis.During the fusion process,a few redundant features were also added,lowering classification accuracy.A new technique called maximum entropy score-based selection(MESbS)is proposed as a solution to this issue.The features selected through this approach are fed into a cubic support vector machine(C-SVM)for the final classification.Results:The experimental process was conducted on two datasets:ISIC 2017 and HAM10000.The ISIC 2017 dataset was used for the lesion segmentation task, whereas the HAM10000 dataset was used for the classification task.The achieved accuracy for both datasets was 95.6% and 96.7%,respectively, which was higher than the existing techniques.
Keywords: Skin cancer; lesion segmentation; deep learning; features fusion;classification
1 Introduction
Skin cancer is a popular research topic due to the high number of deaths and diagnosed cases [1].Cancer is a group of diseases characterized by unrestrained development and the spread of atypical cells.This may cause death if the expansion of irregular cells is not controlled.Skin carcinoma is an irregular expansion of skin cells that frequently appears on the skin when exposed to sunlight or ultraviolet rays.Skin cancer is a fatal disease that can be classified into two types:melanoma and benign (basal cell and squamous cell carcinoma).Benign is constantly retorting to treatment and hardly spreads to other skin tissues.Melanoma is a dangerous type of skin cancer that starts in the pigment cells.Skin cancer develops as a result of malignant lesions and accounts for approximately 75% of all deaths [2].
In the United States of America, 2021 cases are reported to be 207,390, of which 106,110 are noninvasive and 101,280 are invasive, including 62,260 men and 43,850 women.The estimated death count in 2021 in the USA is 7,180, including 4600 men and 2580 women (https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf).The number of cases reported in the United States of America in 2020 is 100,350, including 60,190 men and 40,160 women, with 6,850 deaths from melanoma, including 4,610 men and 2,240 women.Since 2019, the total number of skin cancer patients in the USA has been 192,310.The death count has been 7,230, including 4,740 men and 2,490 women.In 2020, it is estimated that over 16,221 novel cancer cases were analyzed in Australia, including 9,480 men and 6,741 women, with a death count of 1,375, including 891 men and 484 women (https://www.canceraustralia.gov.au/affected-cancer/cancer-types/melanoma/statistics).According to dermatologists, if a melanoma is not detected at a very early stage, it spreads to the entire body or nearby tissues.However, if detected early on, there is a good chance of survival [3].Melanoma has received a lot of attention from the research community because of its high mortality rate.
Dermatologists have previously used the ABCDE rule, a seven-point check list, laser technology, and a few other methods [4].However, these methods require an expert dermatologist.In addition, manual inspection and diagnosis of skin cancer using these methods is difficult,time-consuming, and expensive.Therefore, it is essential to develop a computerized method for automated skin cancer segmentation and classification [5].Dermoscopy is a new technology for the diagnosis of skin cancer [6].Through dermoscopy technology, RGB images of the skin are captured and later analyzed by experts.A computerized method consists of the following steps:preprocessing of dermoscopic images, segmentation of skin lesions, feature extraction, and finally classification [7].Preprocessing is the step in which low-contrast images are enhanced and artifacts such as hair and noise can be removed through different dermoscopic image techniques [8].This step follows the segmentation step in which the lesion region is segmented based on the shape and color of the lesion, and irregularity of the border [9].Many techniques for lesion segmentation have been introduced in the literature.Some focused on traditional techniques, and few used convolutional neural networks (CNNs).Feature extraction is the third step used to represent an image [10].In this step, image features are extracted such as color, texture, shape, and name.Color is an important feature in skin cancer classification [11].These different features are fused later to obtain the maximum image information [12].However, one major disadvantage is high computational time required to complete this step.Many researchers have implemented feature selection techniques to select the most valuable features.The main purpose of this approach is to obtain maximum accuracy with less computational time.In addition, this step is useful for the redundancy of irrelevant features for classification [13,14].The final step is to classify the features.Features are classified using different classifiers in a relevant category, such as benign or malignant [5].
More recently, deep learning models have been shown to significantly contribute to medical image analysis for both segmentation and classification [15,16].In deep learning, CNNs are used for classification as they are composed of several hidden layers such as convolutional, pooling,batch normalization, ReLU, and fully connected layers [17,18].CV studies have introduced many techniques for the segmentation and classification of skin lesions.Afza et al.[19] presented a hierarchical framework for skin lesion segmentation and classification.They began with a preprocessing step to enhance the quality of images before running a segmentation algorithm.Later, the ResNet50 model was fine-tuned, and features were extracted.The extracted features are refined using the grasshopper optimization algorithm, which is classified using the Naïve Bayes algorithm.The experimental process was conducted on three dermoscopy datasets, and improved accuracy was achieved.Zhang et al.[20] presented an intelligent framework for multiclass skin lesion classification.In this method, the skin lesions were initially segmented using MASK RCNN.In the classification phase, they proposed 24 layered CNN model.Three datasets were used for the experimentation of the segmentation phase and the HAM10000 dataset was used for classification.On these datasets, the accuracy of the proposed method was improved.Akram et al.[21] presented a CAD system for skin lesion localization.They applied a de-correlation operation at the initial step and then passed it to the MASK RCNN for lesion segmentation.In the next step, the DenseNet201 pre-trained model is modified, and features are extracted from the two layers.The extracted features were fused and further refined using a selection block.The experimental process was conducted on dermoscopy datasets, and improved performance was achieved.Alom et al.[22]introduced a deep learning architecture for the segmentation of skin lesions.In this model, the best features are initially selected to better represent the lesion region, and then inception RCNN was applied for the final lesion classification.Dermoscopy datasets were employed for evaluation and achieved improved accuracy.Thomas et al.[23] applied interpretable CNN models for the classification of skin cancers.In this method, the outer padding was applied in the first step and then iterated through overlapping tiles.The next step segments the lesion, which later crops for the final segmentation.Al-Masni et al.[24] presented a two-stage deep learning framework for skin lesion segmentation and classification.The segmentation was performed using a fully resolved CNN (FrCNN), and four pre-trained networks were considered for the final classification.Sikkandar et al.[25] presented a computerized method for the segmentation and classification of skin lesions using traditional techniques.The authors combined the performance of the GrabCut and Neuro Fuzzy (NF) classifier for the final classification.In the preprocessing step, top-hat filtering and in-painting techniques were applied.In the later step, the GrabCut algorithm was applied to the segmentation task.In the feature extraction phase, deep learning features are extracted and finally classified using the NF classifier.A mutual bootstrap method was also presented in [26] for skin lesion classification.
The methods discussed above have some limitations that affect the performance of skin lesion segmentation and classification.The following are the major issues:i) the presence of hair bubbles and irrelevant areas not required for detecting accurate skin lesions; ii) low contrast skin lesions are a factor for inaccurate lesion segmentation; iii) knowledge of useful feature extraction for the accurate classification of skin lesion types; iv) presence of irrelevant features that mislead correct classification; v) manual inspection of skin lesions is time consuming, and vi) accuracy is always dependent on an expert.In this work, we proposed a new computerized method by amalgamating traditional and deep learning methods.The proposed method includes contrast enhancement of dermoscopic images, segmentation of skin lesions, deep learning feature extraction and fusion,selection of the best features, and classification.Our major contributions are as follows:
·A contrast enhancement approach was implemented based on the fusion of the haze reduction approach and fast local Laplacian filters.The fusion process followed the HSV color transformation.
·The best channel is selected based on the probability value, and then a saliency map is constructed, which is later converted into a binary form using a threshold function.
·Two modified pre-trained models, MobileNet V2 and VGG16, were trained on dermoscopic datasets using transfer learning.Later, the features were extracted from the dense layers.
·Canonical correlation-based features were fused and later refined using the maximum entropy score-based selection(MESbS).
The remainder of this article is organized as follows:the proposed methodology is presented in Section 2, the results are detailed in Section 3.Finally, the conclusions are presented in Section 4.
2 Proposed Methodology
The proposed method comprises two main tasks:lesion segmentation and classification.For lesion segmentation, a hybrid contrast enhancement technique was proposed, and the best channel was selected based on the histogram.Subsequently, an activation function was proposed to construct a saliency map.In the later stage, the threshold function is applied to convert the image into binary form, which is then mapped onto the original image for final detection.For classification,two pre-trained models were modified and trained through transfer learning.The features were extracted from both models and fused using canonical correlation analysis (CCA).Subsequently,the fused vector was further refined using the highest entropy score.Finally, multiple classifiers were used for the classification of selected features.Several datasets were used for the experimental process, and the results were obtained in visual and numeric form.The detailed architecture of the proposed methodology is illustrated in Fig.1.
2.1 Lesion Segmentation Task
As shown in Fig.1, the proposed method performs two tasks:lesion segmentation and classification.The lesion segmentation task is described in this section.Here, a hybrid method was initially proposed for contrast enhancement of the original dermoscopy images.Then, an HSV color transformation was applied and the best channel was selected based on the activation function.Subsequently, a lesion map was constructed based on the selected channel.The resultant lesion map was finally converted into binary form based on a threshold function.The details of each step are as follows:
Hybrid Contrast Enhancement:The first step was the hybrid contrast enhancement.Here,the image quality is enhanced and bubbles are removed.For this purpose, two techniques were implemented, and the resultant information was fused into one image.First, a haze reduction technique was implemented to clear the boundaries of the lesion region.AssumeU(x) is the input image,S(x) is the hazy image, andY(x) is the medium of transmission.Seff(x) is the image affected by haze and is represented as follows:


Figure 1:Proposed parallel architecture of skin lesion segmentation and classification
This image is affected by reflected light represented as follows:

Here,Ωrepresents a local patch with its origin atX.After this method, an estimation of transmissionY(x) is required before proceeding.Second, a fast local Laplacian filter was implemented to smooth the image and emphasize the edges.The local Laplacian can be defined as follows:

where,Z(I) is a sample function, wherehis the reference value,γcontrols the amount of increasing and decreasing value,αcontrols the dynamic range compression and expansion, andwzdefines the threshold function.

whererrepresents distribution time that how much time the process will run anduis the weighted function which is 1.

wheretrepresents number of iterations have been performed,A4represents the 4-neighborhood oft, andPshows the input image.Mathematically, it is defined as follows:
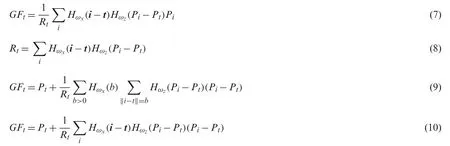
whereHωxandHωzare the Gaussian kernels.After that HSV color transformation is applied.HSV consists of three channels such as hue, saturation, and value.Through this transformation,the image is refined in terms of lesion colors.The visual results of this transformation are showing in Fig.2b.From this figure, we extracted the Hue channel for lesion map construction.Mathematically, this channel is defined as follows:

where,R′=R/255,G′=G/255,B′=B/255,Amax=max(R′,G′,B′), and ∇=Amax-Amin.In the next step, an activation function was constructed based on the multiplication function after which a lesion map was constructed, this was later converted into a binary form using a threshold function.Mathematically, the activation and threshold functions are defined as follows:


Figure 2:Proposed lesion segmentation task results.(a) Original image; (b) Enhanced image; (c)Binary segmented image; (d) Mapped image; (e) Localized image
Here, 0.4 is computed based on the mean value of all computed pixels of H.The visual results of the threshold function are shown in Fig.2.In this figure, the binary images are shown in (c),whereas the lesion mapped and final localized images are illustrated in (d) and (e), respectively.The final localized images are compared with the ground truth images for the final evaluation process.The numerical segmentation results are presented in Tab.1.The ISIC 2017 dataset was used for the experimental process, and an average dice rate of 95.6% was achieved.For each image, three parameters were computed:dice, Jaccard distance, and Jaccard index.

Table 1:Sample numerical results of lesion segmentation task
2.2 Skin Lesion Classification
During this phase, skin lesions are classified into relevant categories such as melanoma, bkl,and others.For classification, the features were extracted from the input images.Feature extraction is an important step in pattern recognition, and many descriptors have been extracted from the literature.More recently, deep learning has shown success in the classification of medical infections [27,28].A CNN is a deep learning method used for feature extraction [29].A simple CNN model consists of many layers, such as a convolutional layer, ReLU layer, pooling, normalization,fully connected, and softmax.
VGG16—VGG-16 contains N number of fully connected layers, where N = 1, 2, 3....ThePNunits are in the Nth layer forNST= 224,NX= 224, andNXH= 3.The dataset is represented byγand the training sample is shown by∈γ.Eachis a real number .

where r(.) represents the activation function ReLu6.ST expresses the no of rows, X symbolizes the number of columns, and XH symbolizes number of channels.Represents the bias vector andn(1)express the weights of the first level which is defined as below:

The output of the first layer is used as the input of the second layer and so on.This is shown in the mathematical form below:

wheren(2)∈RN(2)×N(1)andn(2)∈RN(2)×N(1).Soφ(z)represents the last fully connected layer that is used for high level feature extraction.Mathematically expression of last layer is shown as below:

Visually, the architecture of VGG16 is showing in Fig.3.

Figure 3:Architecture of VGG-16 CNN model
In Fig.3, the original architecture includes a total of 16 layers; the first 13 layers are convolutional and the final three are fully connected.The output was generated using softmax.In this study, we modified the VGG-16 pre-trained CNN model for skin cancer classification.For this purpose, the last layer was removed, and a new layer that included seven classes of skin carcinoma was added.These classes are known as the target labels.Transfer learning was then applied to transfer the knowledge of the original model to the target model and obtain a new customized CNN model.This model can be used for feature extraction.The modified architecture of the VGG-16 model is shown in Fig.4.
MobileNetV2—MobileNet V2 is a CNN model designed specifically for portable and resourceconstrained circumstances.It is founded on an upturned residual structure in which the connections of the residual structure are linked to the bottleneck layers [30].There are 153 layers in MobileNet V2, and the size of the input layer ish×w×k, whereh=224,w=224, andkrepresents the channels, three of which are in the first layer.There are two types of residual blocks in MobileNet V2, with strides 1 and 2.These blocks had three types of layers and were used for downsizing.The first layer is 1×1 convolutions with ReLU6, where ReLU6 is an activation function.It is min (max(x, 0), 6).The second layer is a depthwise convolution used to crop the unnecessary information, and the third layer is a 1×1 convolution, but without nonlinearity.Each layer has batch normalization and activation functions, but the third layer has only batch normalization because the output of this layer has less dimension, and by using ReLU6, the performance will decrease [31].The convolutional block of MobileNet V2 is shown in Fig.5.In the basic architecture of MobileNet V2, there is a convolution layer with 32 filters, followed by 19 residual bottleneck layers.The detailed architecture is presented in Tab.2.

Figure 4:Fine-tuned VGG-16 model for lesion classification

Figure 5:MobileNetV2 convolutional blocks [31]
In the original architecture, there were 153 layers.The output is generated in the last layer.In our work, we used the MobileNet-V2 pre-trained CNN model for skin cancer classification.For this purpose, the original architecture was fine-tuned and the last layer was removed.Subsequently,a new layer was added that includes seven skin classes.These classes are known as the target labels.Subsequently, transfer learning (TL) was used to transfer the knowledge of the original model to the target model and obtain a new customized CNN model.The TL process is discussed in the next section.After the training process, features are extracted from the feature layer(convolutional layer).

Table 2:Architecture of mobileNetV2 [31]
2.2.1 Transfer Learning for Feature Extraction
Transfer learning is a technique that transfers information from a pre-trained model to a modified CNN model for a new task.The primary objective was to obtain the result for the target problem with better performance [32].Given a source domain Dsand target domain as DT,the learning task isTs,andTt.Transfer learning assists the learning of the target predictive functionF(t) in the target domain with knowledge in the source domain and learning task, whereDs≠DtandTs≠Tt.Fig.6 illustrates the TL process for the modified VGG16 model for skin lesion feature extraction.Fig.6 illustrates that the source data ØDare from the Imagenet dataset,the source model is VGG16, represented by ØModand the source labels ØLare 1000.The target dataψτare the HAM10000 dataset, the target model is modified VGG16, and the target labels are seven, represented byψL.Through TL, the weights, and parameters of the VGG16 model are transferred to the modified VGG16 model, whereas the following condition holds.

Similarly, this process was performed for the modified MobileNet V2 CNN model.In this study, the MobileNet V2 model was used as the source model and the modified MobileNet V2 model was used as the target model (Fig.6).After training both modified models, the deep learning features were extracted from the FC7 layer (modified VGG16) and convolutional layer(modified MobileNet V2).The extracted feature vector sizes of both vectors wereN×4096 andN×1056, respectively.
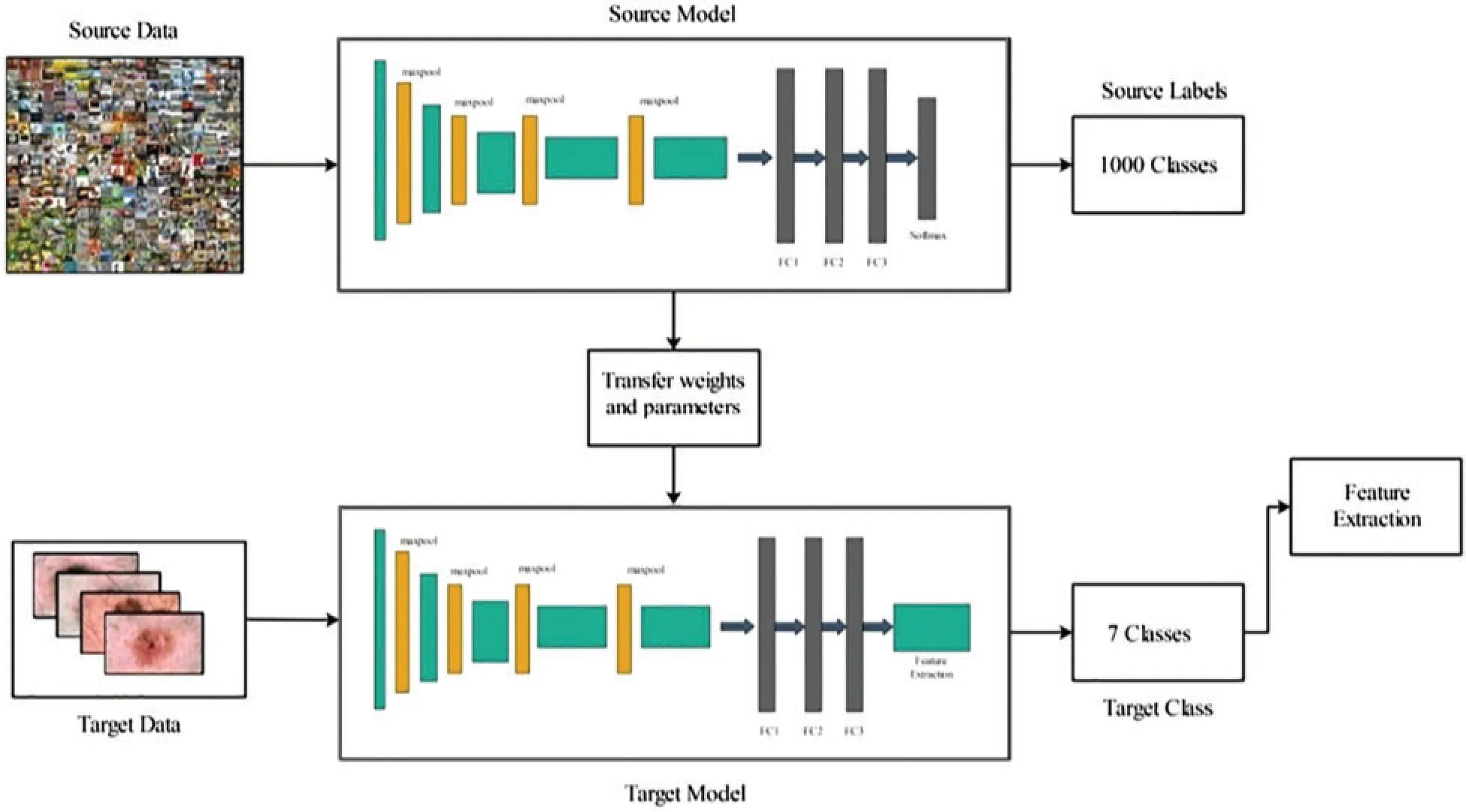
Figure 6:Transfer learning process for VGG16 CNN model
2.2.2 Features Fusion and Selection
Feature fusion is an important research area and many techniques have been introduced for the fusion of two or more feature vectors [33].The most useful fusion techniques are serial-based,parallel, and correlation-based approaches.In this study, we used the CCA approach [34] for the fusion of both extracted feature vectors.Using CCA, a fused vector is obtained withN×1750 dimensions.However, after the fusion process, we determined that some features had repeated and had to be removed from the final vector.For this purpose, a new method called the MESbS has been proposed.In this approach, initially, the entropy vector is computed using the fused feature vector (column-based).Then, the entropy vector is sorted in descending order.Subsequently, we computed the mean value of the entropy vector and used this value as a threshold function for selecting the best features.Mathematically, this process is defined as follows:

where,EnFVrepresents the entropy feature vector,μis the mean entropy value, andFuncis the final threshold function.Through this function, the featureFv(i) values greater than the mean value are considered for the final selection, and the remaining features are discarded.Lastly,the final selected features are classified using a multiclass SVM classifier with a one-against-all method.
3 Experimental Results and Discussion
3.1 Experimental Setup
This section presents the experimental process for the proposed classification process.The HAM10000 dataset [35] was used.This dataset consists of approximately 10,000 dermoscopic images in RGB format.A total of seven skin lesion classes, Bkl, Bcc, Vasc, Akiec, Nevi, Mel,and Df.This dataset is highly imbalanced because of the high variation in the number of sample images in each class.Many classifiers are used to compare the accuracy of the proposed method on a cubic SVM.To train the classifiers, a 70:30 approach was used.This ratio indicates that 70% of the images were considered for the training process and 30% for the testing process.The recall rate (TPR), precision rate (PPV), FNR, AUC, accuracy, and time were calculated for each classifier in the evaluation process.All experiments were conducted in MATLAB 2020b on a system with an Intel(R) Core(TM) i5-7200u CPU running at 2.50 and 2.7 GHz, with 16 GB RAM, and an 8 GB graphics card.
3.2 Proposed Classification Results
This section presents the proposed classification results in a numerical and confusion matrix.The results were obtained using four different experiments.The first experiment extracted features from the modified VGG16 CNN model and used them in the experimental process.The results are presented in Tab.3.In this table, it can be observed that the cubic SVM showed a better accuracy of 78.2%, whereas the computational time of this classifier was approximately 468 s.The minimum computational time of this experiment was 83.230 s for the Fine KNN classifier.The recall rate of the cubic SVM was 78.2%.

Table 3:Classification results using modified VGG16 CNN model
In the second experiment, features were extracted from the modified MobileNet V2 CNN model and used in the experimental process.The results presented in Tab.4 show that the best accuracy of 82.1% was achieved on the cubic SVM.This classifier performed better than the other classifiers listed in this table.The computational time of the cubic SVM was approximately 91 s,whereas the minimum noted time was 20 s for the linear discriminate classifier.The recall rate of the cubic SVM was 82.1%.This table illustrates that the correct prediction accuracy of each class is better than that of the confusion matrix of the modified VGG16 (Tab.4).In addition,the accuracy of this experiment was improved compared to Experiment 1.

Table 4:Classification results using modified mobileNetV2 model
In this experiment, we fused the features of both models using CCA.The results are presented in Tab.5 which shows that the maximum accuracy achieved is 82.8% on the cubic SVM.The other calculated evolution measures include a recall rate of 82.1%, a precision rate of 82.97%, an FNR of 17.03%, and an AUC value of 0.97.The computational time of this classifier is 988.07 s.The recall rate of the cubic SVM is 82.81%, as shown in Fig.7.The minimum time required for this experiment was approximately 245 s.From Tab.5, it can be observed that the accuracy of all classifiers increases slightly, however, the execution time increases significantly.This indicates that there are many redundant features included in the fused vector, which degrades the classification accuracy.

Table 5:Classification result of fused models

Figure 7:Confusion matrix of cubic SVM for fused features of both models
In this experiment, features were selected based on the MESbS approach; the results are detailed in Tab.6.In Tab.6, it can be observed that the top-attained accuracy is 96.7% on cubic SVM, whereas the additional calculated measures have a recall rate of 88.31%, a precision rate of 94.48%, an FNR of 5.52%, and an AUC value of 0.98.The computational time is 51.771 s,which is significantly minimized compared to Experiments 1 and 3.The recall rate of the cubic SVM was 88.31%, as shown in Fig.8.From Fig.8, it can be observed that the correct prediction accuracy of each skin lesion class is considerably higher than that of the first three experiments.In addition, the overall computational time of this experiment decreased.Hence, based on the results, we can demonstrate that the proposed method outperforms the proposed framework.A fair comparison was also conducted with the recent techniques, given in Tab.7, which shows the proposed framework outclass for multiclass lesion classification.

Table 6:Skin lesion classification results using proposed framework

Figure 8:Confusion matrix of cubic SVM using proposed framework

Table 7:Comparison of the proposed method with recent techniques
4 Conclusion
A conventional and deep learning-based framework is proposed in this study for skin lesion segmentation and classification using dermoscopy images.Two tasks were performed.In the first task, conventional techniques-based skin lesions were segmented.The contrast of lesions was improved for accurate lesion map creation.The accurate lesion map creation process improves segmentation accuracy.The segmentation performance was evaluated on the ISIC 2017 dataset and achieved an accuracy of 95.6%.In the classification tasks, VGG16 and MobileNet V2 CNN models were fine-tuned and trained through TL on dermoscopic images.These models performed better according to recent studies in the medical image processing field.The features were extracted from these fine-tuned trained CNN models and fused using the CCA approach.The main purpose of fusion in this study was to increase image information.However, some redundant features were also added during the fusion process.The redundant features have an impact on classification accuracy.Therefore, we propose MESbS, a novel feature selection method.This method selects the features and classifies them using the C-SVM classifier.The results of our experiments demonstrate better accuracy than the existing techniques.We conclude that the lesion contrast enhancement step improves segmentation accuracy.In addition, the selection of the best features increases classification accuracy and minimizes execution time.Future studies will focus on the CNN for lesion segmentation and provide segmented lesions to modified models for useful feature extraction.
Funding Statement:This research was supported by Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (MOTIE) (P0012724, The Competency Development Program for Industry Specialist) and the Soonchunhyang University Research Fund.
Conflicts of Interest:The authors declare that they have no conflicts of interest to report regarding the present study.
杂志排行
Computers Materials&Continua的其它文章
- Hybrid Renewable Energy Resources Management for Optimal Energy Operation in Nano-Grid
- HELP-WSN-A Novel Adaptive Multi-Tier Hybrid Intelligent Framework for QoS Aware WSN-IoT Networks
- Plant Disease Diagnosis and Image Classification Using Deep Learning
- Structure Preserving Algorithm for Fractional Order Mathematical Model of COVID-19
- Cost Estimate and Input Energy of Floor Systems in Low Seismic Regions
- Numerical Analysis of Laterally Loaded Long Piles in Cohesionless Soil
