Diabetic kidney disease in pediatric patients: A current review
2022-08-18CarmenMunteanIulianaMagdalenaStarceaClaudiaBanescu
lNTRODUCTlON
Diabetes mellitus (DM), a chronic metabolic condition, is characterized by complete or insufficient insulin production. The main form of DM in childhood and adolescence is type 1 DM (T1DM) compared to type 2 DM (T2DM), which is more frequent in adulthood. Within the last 20 years, DM prevalence increased significantly worldwide. In the last decades, we have also assisted in an ascending trend in the prevalence of T2DM in childhood and youth because of the outbreak in juvenile obesity prevalence[1]. T1DM and T2DM have similar symptoms upon diagnosis, and both include polyuria, polydipsia and polyphagia. While obesity and insulin resistance signs (acanthosis nigricans and polycystic ovarian syndrome) are typical hallmarks of T2DM, loss of weight may be present in both types of DM[1].
When they said that a visitor was asking for her, and then proceeded each one to tell breathlessly a different tale of wonder, in which she could only distinguish the words, oxen, gold, club, giant, lion, she thought they were all out of their minds
Both T1DM and T2DM, with lasting inadequate glycemic control, are associated with long-term vascular complications[2] and a significant increase in mortality, especially in those who develop kidney disease[3]. While DM represents the main worldwide cause of end-stage kidney disease in adults, this is uncommon during childhood[2,3].
Although specific kidney structural changes in DM patients, namely thickening of the glomerular basement membrane and mesangial expansion, appear soon after DM onset (1.5 years to 5.0 years), they are in a clinically silent phase[4]. These structural changes of diabetic kidney injury progress at different rates among T1DM patients, and this is more evident in T2DM cases[4]. Clinical and biological abnormalities (micro/macroalbuminuria) and glomerular filtration rate (GFR) decline will develop over a longer period (10 years to 25 years)[3]. This emphasizes that diabetic kidney disease (DKD) starts early. Therefore, an early diagnosis, intensive monitoring and therapeutic interventions are necessary.Albuminuria and changes in GFR, which are late biomarkers, are the most used tools to assess kidney involvement. Diagnostic strategies for early diagnosis of kidney involvement are necessary.
There are several reviews in the literature that contributed to the pathophysiology, diagnostics and therapeutic options for DKD in pediatric patients. In this work, the state-of-the-art novel biomarkers and methods to identify diabetic children and adolescents at risk of renal complications at an early stage as well as renoprotective strategies to delay the progression of kidney disease to end-stage kidney disease was carried out.
EPlDEMlOLOGY OF DM lN CHlLDREN
From 2002 to 2015 the Centers for Disease Control and Prevention reported a 4.8% increase
year for T1DM and a 1.9% increase
year for T1DM in youths aged < 20 years[5]. A very recent study,comprising six areas of the United States from 2001 to 2017, reported an important increase in estimated prevalence for both T1DM and T2DM (T1DM from 1.48 to 2.15
1000 youths < 19 years and T2DM from 0.34 to 0.67
1000 youths among those aged 10-19 years)[6]. Up-to-date research that included a large cohort of Hungarian children and teenagers during the period 2001 to 2016 (covering 16 years),showed that T1DM is still the most common type, and its prevalence is rising, with a significant male predominance (male/female ratio: 1.25). Also, there is a high prevalence of T2DM, affecting more females every year (female/male ratio: 2.86)[7]. A Danish study showed no increase in T2DM prevalence in children and adolescents[8], while in the United Kingdom a rising incidence and prevalence of T2DM have been observed in youths, especially in some ethnicities[9].
With these and like words the wolf comforted the Prince, and warned him specially17 not to touch the wall or let the horse touch it as he led it out, or he would fail in the same way as he had done with the bird
DM represents the main cause of end-stage renal disease (ESRD) worldwide in adults[14]. Diabetic nephropathy affects 20% (1 in 5) of adults with diabetes[15]. Within the pediatric population, a significant increase in the incidence of DKD was also observed, the prevalence rate being three times higher in 2013 compared to 2002 (1.16% to 3.44%)[16].
We are as happy as human beings can be, said the young couplefrom the depths of their hearts. They had indeed only one stephigher to mount on the ladder of happiness- they hoped that Godwould give them a child, a son like them in form and spirit. The happylittle one was to be welcomed with rejoicing, to be cared for withlove and tenderness, and enjoy every advantage of wealth and luxurythat a rich and influential family can give. So the days went bylike a joyous festival.
This is sustained by tubular hypertrophy observed in the immediate future of hyperglycemia. Also,an increase in tubular basement membrane thickening was found even among diabetic patients with normoalbuminuria. Tubular basement membrane is one of the location of the earliest structural changes.Therefore, it may represent a better severity marker of DKD than glomerular basement membrane alteration[22]. Pathological glomerular changes in DKD are typical and consist of glomerular basement membrane thickening, podocyte foot process widening, expansion of the mesangial matrix and loss of endothelial fenestrations[23].
I have, said she, the unhappiness of loving a Prince who is fickle, frivolous, proud, incapable70 of caring for anyone but himself, who has been spoilt by flattery, and, to crown all, who does not love me
Is that all? said the little tailor; that s child s play to me, so he dived into his wallet, brought out the cheese, and pressed it till the whey ran out
She lightly touches the tiny gold bracelet10 that she wears. It was a present from her mother and father, and she refuses to remove it from her wrist. It is engraved11 with her name on the side that is visible to others, but as in everything there are two sides, and only she knows the other is there. It is a single word engraved on the side of the bracelet that touches her skin and touches her heart: “Hope.” One small word that says so much about her life and what is now missing from it. She vaguely12 remembers hope -- what it felt like to hope for a college basketball scholarship or maybe a chance to dance professionally. Only now, she’s not sure she remembers hope as it was then -- a driving force, a fundamental part of her life. Now, hope is something that haunts her.
A 4-fold higher risk of kidney failure was found in a large cohort of youth with T2DM
those with T1DM[17]. Also, compared with the control group, those with youth-onset T2DM had a 16-fold higher risk of a kidney disorder, a 23-fold higher risk of severe renal injury and a 39-fold increased risk of ESRD[17]. A multicenter study reported that more than a quarter (28%) of T2DM youth aged under 20 years developed microalbuminuria[18].
PATHOPHYSlOLOGY
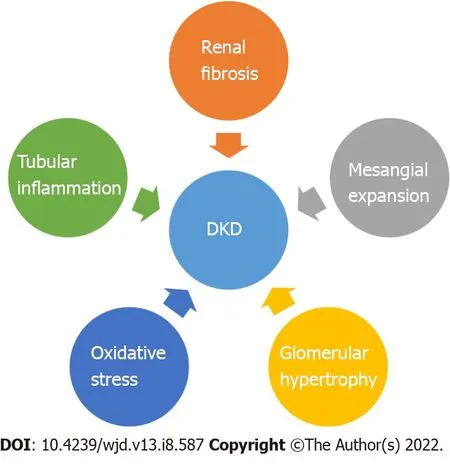
Chronic hyperglycemia leads to the occurrence of diabetic nephropathy, retinopathy and neuropathy as well as macrovascular complications (cardiovascular disease: Stroke, coronary artery disease, peripheral vascular disease)[1,19,20]. DKD recognizes four major pathogenic mechanisms: Glomerular damage,tubular injury, inflammation and oxidative stress[21] (Figure 1). In DKD patients there are important alterations in tubules as well as in the interstitium. These findings may pave the way, or they may appear concomitant with glomerular changes[22].
Contributing risk factors to this major increase in incidence are obesity, race, ethnicity, exposure to maternal obesity and diabetes as well as exposure to environmental contaminants[6]. There is an increased morbidity and mortality rate, mainly in T1DM and in those with early T2DM onset.According to Rhodes
[10], a considerably lower life expectancy (approximately 15 years) was observed in the diabetic group compared to the general population of children without diabetes[10]. A significantly shorter life expectancy was reported in children developing T1DM before 10 years of age(loss of 17.7 years for females
14.0 years for males) compared with those diagnosed at 25-30 years(loss of 10.0 years for females and 9.4 years for males)[11]. There is a double cardiovascular risk in pediatric diabetes that triggers early cardiovascular mortality and a four-fold higher mortality rate for all causes in youth[12]. In a nationwide Swedish study of patients with T1DM, age before 10 years at diabetes onset, was the most important risk factor for survival and cardiovascular disease (coronary heart disease and acute myocardial infarction) in their early adult years, especially in females (2-3-fold higher
males)[13].
The crow nodded very thoughtfully and said, It might be! It might be! What! Do you think you have? cried the little girl, and she almost squeezed the crow to death as she kissed him
There is a greater risk for complication occurrence in youths with T2DM
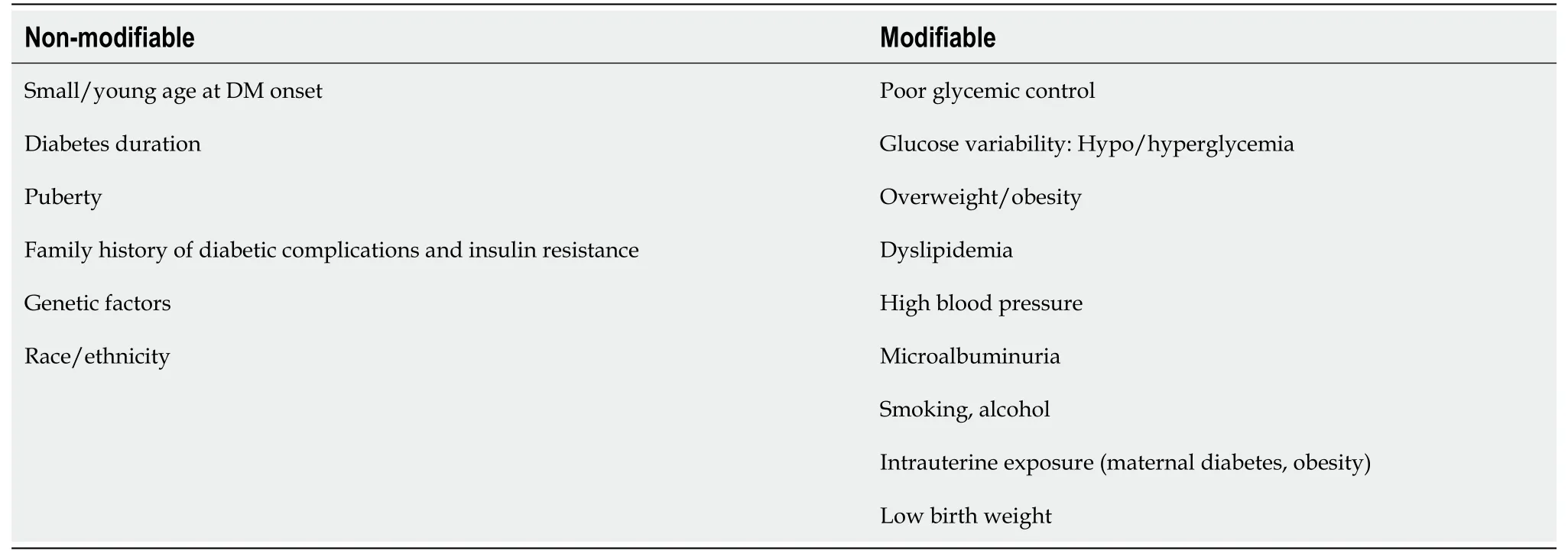
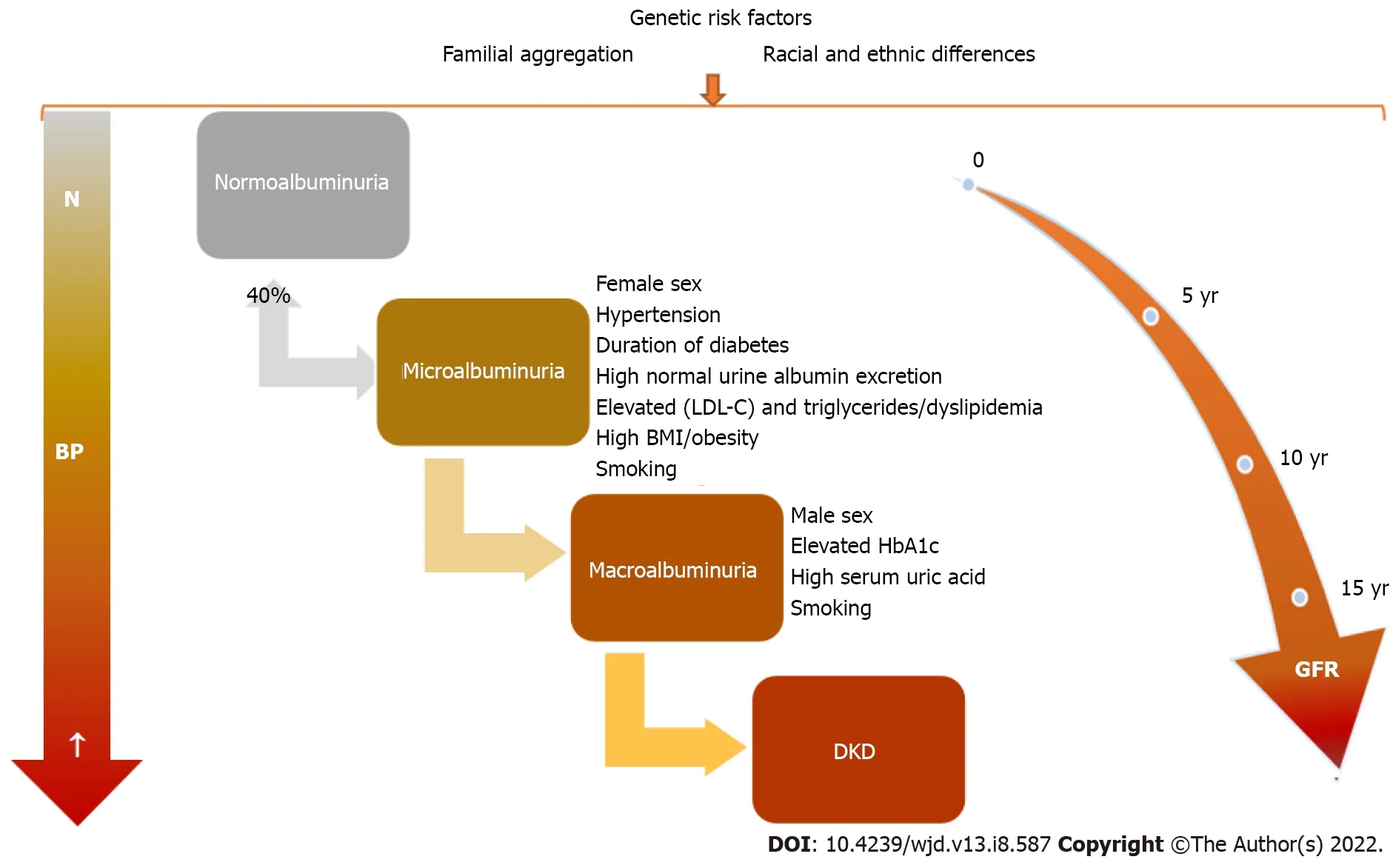
adults with T1DM and T2DM[1]. The main microvascular complication of diabetes is represented by DKD and later by diabetic nephropathy, which finally leads to ESRD. In time, with diabetes evolution, clinical and biological changes will be observed (Figure 2). DKD, one of the most important and frequent complications of DM,recognizes a wide spectrum of risk factors, some of which are modifiable. Therefore, DKD occurrence or evolution may be considerably influenced by strict control of these factors that are listed in Table 1.Children with T1DM may have damaged renal function at the disease onset as acute complications through acute kidney injury (AKI) and renal tubular damage as well as chronic complications by diabetic nephropathy development[24].
Genetic aspects
DKD is a multifactorial disorder and is influenced by genetic susceptibility, epigenetics and environmental factors (such as lifestyle, diet and medication). Also, oxidative stress, metabolic disturbance,activation of the renin-angiotensin-aldosterone system and production of inflammatory factors are involved in the development and progression of DKD[25]. Genetic and epigenetic studies were performed to understand the pathogenesis of the DKD and to identify genes that confer susceptibility to disease. Genetic studies of DKD investigated mainly the association between genomic DNA variants(for example, single nucleotide polymorphisms, copy number variants,
) and clinical phenotypes of DKD in both T1DM and T2DM[26]. Epigenetic modifications (histone modifications and DNA methylation) may play a critical role in DKD as it was shown that histone acetylation and methylation are involved in the regulation of inflammation and fibrosis in DKD[27]. Epigenetics studies of DKD investigated the potentially inherited changes in gene expression that occur without changing the DNA nucleotide sequence.
Microvascular as well as macrovascular complications can lead to serious morbidity and mortality.Nephropathy (which is preceded by microalbuminuria), retinopathy and neuropathy represent diabetic microvascular complications[2,41]. According to the International Society for Pediatric and Adolescent Diabetes guidelines, annual microalbuminuria or urinary protein screening should start from the age of 11 years and after 2 years of diabetes evolution and then annually. It was demonstrated that persistent microalbuminuria predicts the progression to ESRD and is linked with an increased risk of macrovascular complications occurrence[41].
Genome-wide association studies identified about 30 genes associated with DKD (for example
)[25]. Epigenome-wide association studies identified several genes (for example
) that have epigenetic effects on DKD[25]. The data presented above provide further evidence for the contribution of genetic factors in DKD offering new perspectives in the discovery of new therapies for personalized medicine.
DlAGNOSlS
GFR abnormalities
Hyperfiltration, defined as an increase in GFR with more than 2 standard deviations than the mean GFR value, is related to an early increase in renal blood flow and high intraglomerular pressure[31]. In the first phases of DKD, hyperfiltration is observed in up to 40% of diabetic patients[32]. In both T1DM and T2DM, hyperfiltration has been linked to GFR loss[33,34]. Hyperfiltration was noticed more frequently in females
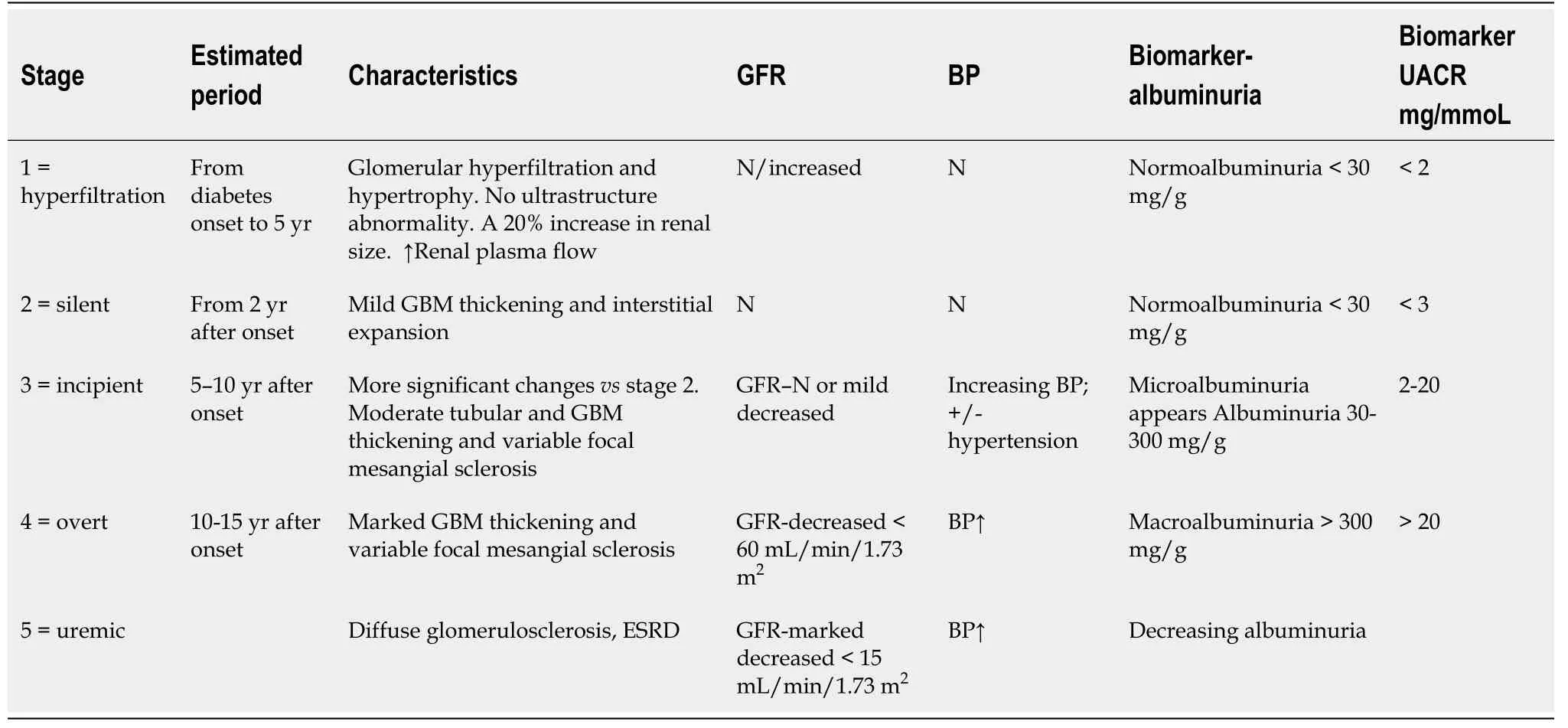
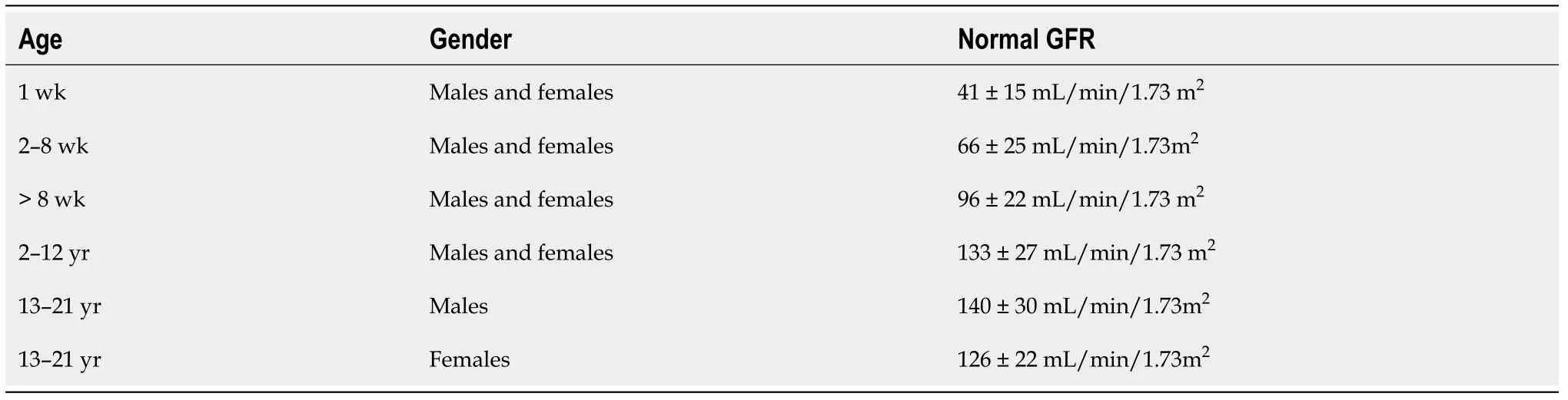
males in both T1DM and T2DM[32,35]. The estimated GFR (eGFR) in children and adolescents with T1DM or T2DM should be screened at diagnosis and then annually[36]. These ongoing changes help us to assess DKD stages, which are presented in Table 2[20,21,37]. Normal GFR values according to child age are listed in Table 3.
Seric and urinary biomarkers for DKD
Common markers for kidney injury are creatinine, albuminuria, cystatin C, neutrophil gelatinaseassociated lipocalin and alfa-1-microglobulin in plasma and urine. Kidney function in pediatrics is assessed mainly by eGFR according to updated/bedside Schwarts equation eGFR = k × height(cm)/serum creatinine (mg/dL), k = 0.413[38].
Although neither or them remembered the occasion, Diana first met her future husband when she was just a baby. It happened during the winter of 1961, when twelve-year-old Charles, Prince of Wales, was visiting his mother s Sandringham retreat() .
In a recent study, 11.5% of Romanian children with T1DM had DKD, manifested as transitory microalbuminuria (7.7%) and incipient diabetic nephropathy (3.8%)[39]. In another research study,T1DM patients were found to have microalbuminuria in 30% of cases, representing the most common microvascular complication. In T1DM children the occurrence of microvascular complications was correlated with metabolic control, higher glycated hemoglobin, albuminuria, systolic blood pressure(SBP), triglycerides and total cholesterol[40].
Candidate gene association studies, genome-wide association studies (GWAS) and epigenome-wide association studies were performed in DKD patients[28]. A large meta-analysis study conducted by Mooyaart[29] identified 24 genetic variants in 16 genes (
and
), which are the most likely to be associated with diabetic nephropathy[29]. Recently, Tziastoudi
[30] conducted a systematic review and meta-analysis of genetic association studies in diabetic nephropathy in order to elucidate the contribution of genetic background in the development of this disease and observed an association with the genes revealed by Mooyaart[29] and some additional genes (
cluster, interleukin genes (
),
)[30].
In T1DM pediatric patients, urine microalbumin to creatinine ratio (UACR) monitoring should start at puberty or 10 years of age (whichever is earlier), and when the child has had DM for 5 years this parameter should be checked annually. In T2DM the UACR should be checked at diagnosis and every year thereafter[36].
In medical practice, the common and still the “gold standard” marker for prediction and detection of diabetic kidney involvement in pediatric diabetes is represented by the microalbuminuria screening[21], even if it has a low specificity and sensitivity to detect early stages of DKD. Microalbuminuria screening should be done annually by timed overnight or 24-h urine collections (albumin excretion rate)or first-morning UACR[41].
Definitions of albuminuria and its abnormalities are based on the International Society for Pediatric and Adolescent Diabetes Clinical Practice Consensus Guidelines[37,41]. Normoalbuminuria is defined as a urine albumin level of ≤ 30 mg/L in all first-morning urine specimens, while microalbuminuria is characterized by the presence of an albumin limit of 30-300 mg or 20-200 μg/min in 24-h urine collection or a value of 30-300 mg/L in at least 2 of 3 first-morning urine specimens. Another parameter,namely UACR of 2.5-25.0 mg/mmol in males or 3.5-25.0 mg/mmol in females in at least 2 of 3 firstmorning urine specimens quantifies microalbuminuria. Macroalbuminuria is defined as the presence of> 300 mg/L albumins in at least two first-morning urine specimens[37,41].
There are some limitations in albuminuria value as a biomarker for the prediction and detection of DKD, as not all diabetic children with micro- or macroalbuminuria will present a decrease in kidney function. Also, there are a lot of factors that may influence albuminuria level, UACR and eGFR: Fever,infection, diet, hydration status, hemodynamics, stress, physical activity, periods and hyperglycemia.Furthermore, a significant proportion of cases with microalbuminuria (up to 40%) may return to normoalbuminuria with strict glycemic and blood pressure (BP) control. Therefore, microalbuminuria can be transitory[21].
Microalbuminuria incidence in children with T1DM spans between 3% to 30%[37]. A cross-sectional study that involved children with T1DM reported a 25.0% frequency for microalbuminuria, while macroalbuminuria was found in 3.5% of these cases. The results of the cited study revealed a significantly higher (3 times) prevalence of microalbuminuria in T2DM (68%) compared to T1DM (24%)patients[37]. This is of particular interest given that children with T1DM are already at risk for renal complications secondary to DKD over the long term. A recent study reported early occurrence of microalbuminuria within 2 years of diagnosis of DM in 3.5% (7 of 199) of patients, whereas in 2 of those with microalbuminuria it appeared within the 1
year of diagnosis (in 7 mo)[37].
Teenagers with T1DM are at risk for hyperfiltration and higher UACR (urinary albumin-to-creatinine ratio), which are biomarkers for early/ incipient nephropathy[35]. A recent meta-analysis found that almost 25% of T2DM patients have arterial hypertension, the male sex being more frequently affected,and that 1 in 4 or 5 children have albuminuria[58].
However, this was not all, King Gridelin insisted that the affairs of the kingdom should be explained to her, and that she should attend all the councils and give her opinion upon the matter in hand whenever it was asked of her, and this made her life such a burden to her that she implored20 Lolotte to take her away from a country where too much was required of an unhappy Princess
It is already known that patients with DM may present with kidney damage (decrease in GFR) but without micro- or macroalbuminuria[42]. Therefore, other biomarkers that precede albuminuria should be considered more reliable to predict renal lesions, especially in the early stages. However, most of these biomarkers still need validation in clinical practice[43].
As tubular damage occurs before the glomerular injury, tubular biomarkers are superior to the glomerular ones, namely microalbuminuria. Therefore, they may serve for early detection of DKD in both T1DM and T2DM[44]. Tubulointerstitial damage may be suggested by the urinary albumin-tocreatinine to total protein-to-creatinine ratio of 0.40, with high sensitivity and specificity[45].
In patients without glomerular involvement, low-molecular-weight (LMW) proteinuria or nonalbumin proteinuria represents an adequate marker of tubular dysfunction[46]. Urinary LMW proteins are absorbed in the proximal tubules so healthy individuals excrete up to 20 mg of LMW proteins/d in urine[46]. Alpha1 microglobulin, beta-2 microglobulins, immunoglobulin light chains, retinol-binding protein, cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-β-D-glucosaminidase,kidney injury molecule 1 and liver-type fatty acid-binding protein,
are included in the LMW protein group[46]. In the early period of diabetes, an increase in urinary tubular biomarkers suggests that kidney injury is present[47].
A recent study showed the association of proximal tubule (alpha-1 microglobulin and kidney injury molecule 1) and podocyte (nephrin, vascular endothelial growth factor) damage biomarkers in T2DM even in the normoalbuminuric stage, indicating they may serve for early DKD diagnosis[47].
Urinary NGAL level increases before the onset of microalbuminuria in the very early phase of the kidney disease[48]. Alongside urinary biomarkers of tubular health (NGAL), the oxidative stress biomarker (pentosidine) may be used in the early detection of diabetic nephropathy before the microalbuminuric phase, as they correlate with albumin excretion and loss of nocturnal dipping of SBP and mean arterial BP[49].
Talent and beauty He gives to many. Wealth is commonplace, fame not rare. But peace of mind - that is His final guerdon(,) of approval, the fondest insignia(,) of His love, He bestows12(,) it charily13. Most men are never blessed with it; others wait all their lives- yes, far into advanced age - for this gift to descend14 upon them.
Klotho, a transmembrane protein, is composed of a large extracellular and a small intracellular domain. Klotho is highly expressed in the renal tissue, especially in the distal tubules. The extracellular domain is cleaved by membrane proteases and discharged into the bloodstream, urine and cerebrospinal fluid as soluble klotho (s-klotho)[50,51]. A faster decline in eGFR was observed in DKD patients with low levels of serum s-klotho concentrations[52], which was opposite to the results of another study where s-klotho levels did not correlate with eGFR[50]. Bob
[50] found a direct correlation of s-klotho levels with the rate of eGFR decline and with the serum levels of tubular injury marker kidney injury molecule 1[50]. A recent study found an inverse correlation between the klotho and glycated hemoglobin levels in T1DM children suggesting its possible role in chronic complications of diabetes occurrence[53].
That wasn t Sonali s last singing prayer. When the Golden State Warriors24 awarded a check to the Beaven family at a fundraiser in their honor, guess who sang to thousands of people in their stadium? When asked how she was able to sing in front of so many people, Sonali said, I wasn t afraid because Daddy was singing with me.
Early stage prediction and recognition of DKD before microalbuminuria occurrence have a pivotal role in providing timely management. In this process, the assessment of more sensitive and specific biomarkers is essential. A new study showed that serum cystatin C may be used as a biomarker for DKD at an early stage in T1DM children with disease duration not exceeding 5 years before albuminuria detection[21]. The same study found a significantly lower eGFR-cystatin C value in diabetic children compared to controls. Also, significantly higher urinary cyclophilin A (CypA) and urinary CypA/creatinine ratios were found in T1DM children with microalbuminuria compared to the control group or normoalbuminuric subjects[21].
Salem
[21] observed a better diagnostic value with the highest sensitivity (93.5%), specificity(71.4%) and accuracy (86.7%) to predict microalbuminuria in T1DM children by the combined use of serum cystatin C and urinary CypA than that of urinary CypA alone[21]. CypA, an endogenous cytosolic protein, is expressed mainly by the proximal tubular epithelial cells. A kidney injury is followed by an increase in urinary CypA concentration[21]. CypA level proved an encouraging biomarker for the early stage of diabetic nephropathy in adults with T2DM, and it correlates with the progression of diabetic nephropathy[54-56]. Novel biomarkers (Table 4) were proposed as early predictors of DKD[21,43].
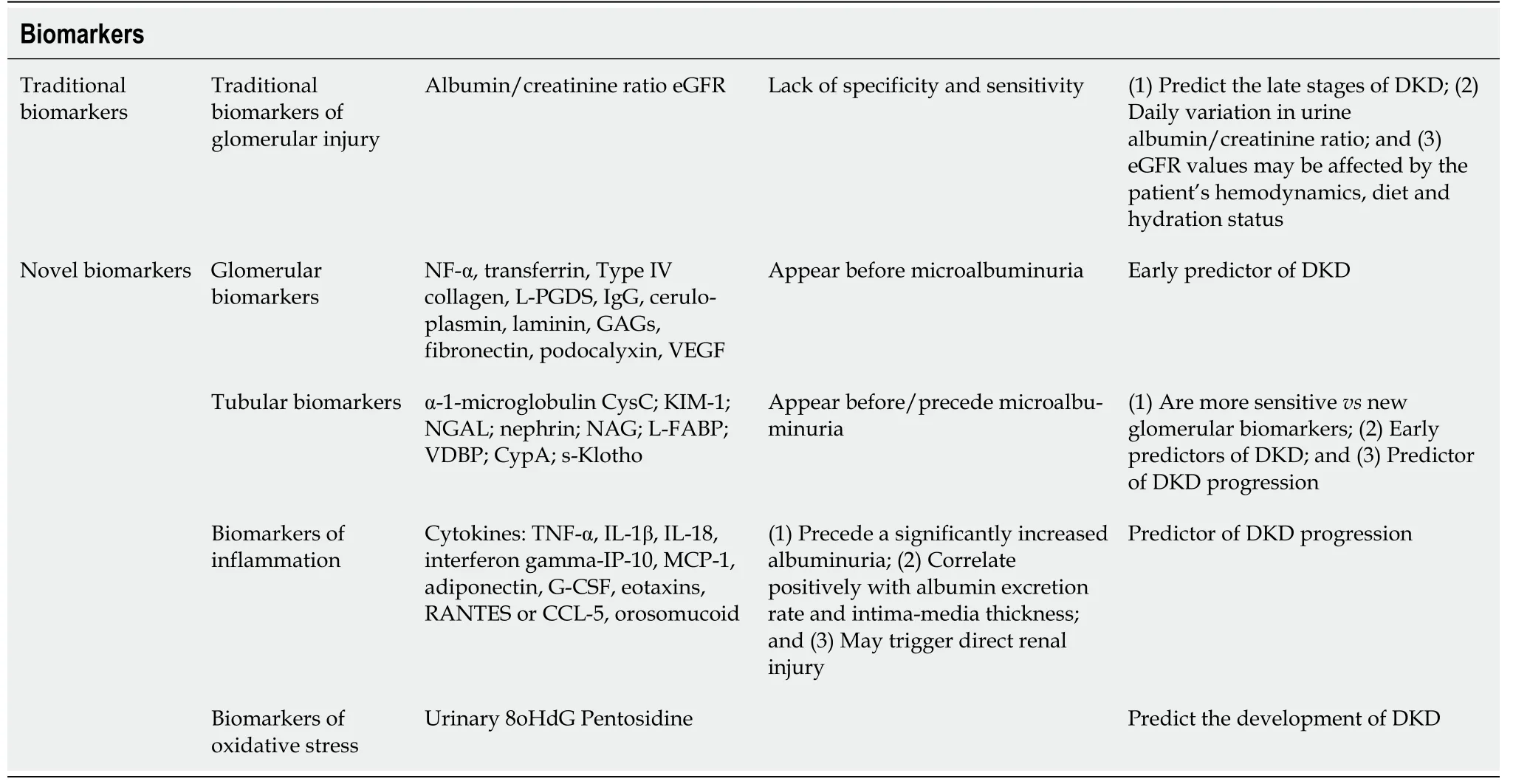
Urinary biomarkers in DKD are crucial as they can indicate the site of injury within the nephron(structural biomarkers) as well as the loss of/reduced function of the nephron (functional biomarkers)and the main pathophysiological pathways (pathophysiological biomarkers)[57]. The proposed functional and/or structural tubular biomarkers might be valuable in the timely detection of DKD[57].
BP in diabetic children
Another important sign of diabetes-related nephropathy is BP measurement. In pediatric T2DM the guidelines recommend BP and UACR evaluation at diagnosis and annually thereafter[58].
That night, I gave each of the children three pieces of stationery with envelopes. At the top of each page were the words, “What I love about my sister Mia,” “What I love about my brother Kris,” “What I love about my sister Lisa” and “What I love about my brother Erik.” The kids were 16, 14, 10 and 8, and it took some convincing on my part to assure them that they could find just one thing they liked about each other. As they wrote in privacy, I went to my bedroom and wrapped their few store-bought gifts.
An important and modifiable risk factor for the development of DKD is hypertension[59]. Arterial hypertension is an important and frequent risk factor for the appearance of cardiovascular disease in T1DM patients. High BP triggers the development and progression of microvascular complications,namely nephropathy, and retinopathy.
Ambulatory blood pressure measurement is superior to office BP measurements in predicting future cardiovascular events and targeting organ damage[60]. In their study, Shalaby and Shalaby[60] showed an abnormal BP profile for systolic and diastolic BP, with significant loss of nocturnal dipping. A significantly higher frequency of non-dipping patterns was observed in T1DM patients with microalbu-minuria[60].
A recent study that comprises 3529 children and adolescents with T1DM revealed impaired BP regulation with elevated systolic BP, nocturnal diastolic BP, mean arterial pressure and diastolic dipping but lower nocturnal systolic dipping[61]. Lurbe
[62] showed that an increase in nocturnal SBP precedes microalbuminuria occurrence within T1DM children[62].
The non-dipper pattern for SBD is one of the earliest abnormalities in the BP profile detected for children with T1DM. Also, non-dipping status has been associated with kidney damage (renal morphological changes) and hyperfiltration in adolescents with T1DM[63]. Also, the non-dipping status seems to be an early predictor of later nephropathy[63].
In a recent study, Hursh
[24] showed that more than 64% of children hospitalized for DKD developed AKI. The same authors showed that a decreased serum bicarbonate level (< 10 mEq/L) and an increased heart rate are associated with a higher risk of severe AKI[24]. Higher morbidity and mortality rate is encountered in diabetic children that developed AKI along with a higher risk of chronic kidney disease, a finding that is particularly important in these patients who are already at risk for DKD[24].
Mamilly
[49] found an increased urinary NGAL/creatinine (a marker of tubular injury) and pentosidine/creatinine (a marker of oxidative stress) in subjects with T1DM compared to controls even in the absence of microalbuminuria[49]. The same study reported a high incidence of abnormal BP dipping, which is important because dipping abnormalities may serve as a predictor for vascular complications, especially kidney injury in diabetic individuals[49]. The same study proved that urine NGAL correlates with loss of nocturnal dipping of SBP[49].
The work went merrily on, and they talked gaily16, and the young man told his friends of the payment promised him by the ogress if he had done her bidding
Based on these data, ambulatory blood pressure measurement represents the gold standard to assess BP regulation and should be used in all diabetic patients for timely therapeutic intervention to prevent renal and cardiovascular diabetic complications later in life.
PROPHYLACTlC AND THERAPEUTlC STRATEGlES FOR DKD
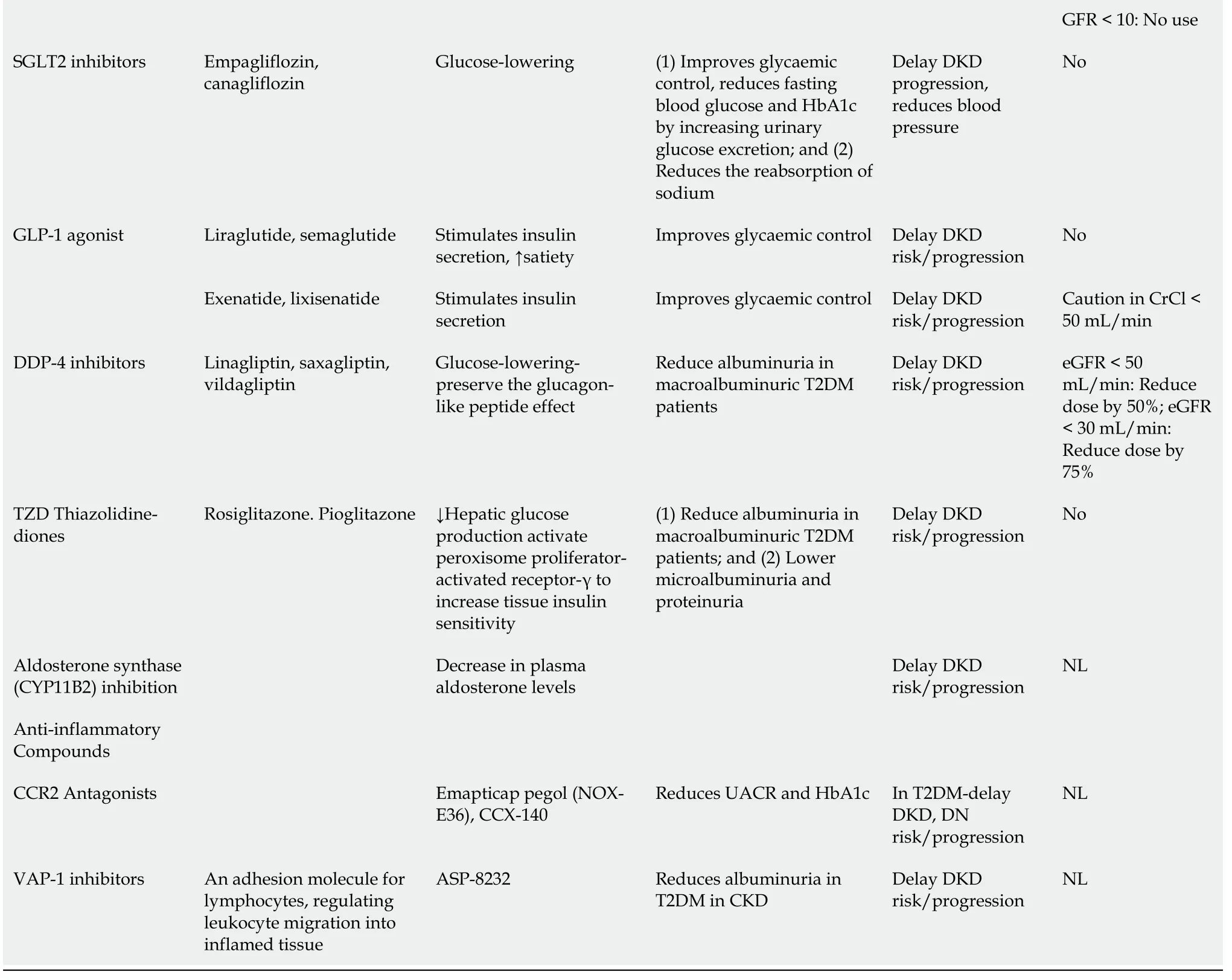
The well-known strategies, namely rigorous glycemic control, strict BP control and modulation of obesity, still represent the most important tools to prevent and slow down the progression of diabetic nephropathy/the deterioration of renal function. These therapies proved to be effective mainly by targeting the modifiable risk factors for diabetic nephropathy, which are listed in Table 1.
A recent systematic review confirmed that early high doses of vitamin D supplementation in combination with renin-angiotensin-aldosterone system blockers may slow the onset or progression ofDKD[64]. Standard and some novel proposed therapies in early-stage or late-stage development of diabetic nephropathy are presented in Table 5[20,64,65].

CONCLUSlON
DKD, the most significant and frequent burden of this metabolic disorder, is still discovered late as microalbuminuria is the most used biomarker for predicting kidney involvement. Novel biomarkers are valuable tools in the detection of kidney damage in the early phases as well as reliable predictors for DKD progression. Therefore, effective therapies may be proposed. Early stage prediction and recognition of DKD in children and adolescents before microalbuminuria occurrence have a pivotal role in preventing the development of and/or progression to irreversible kidney damage and to provide timely management and appropriate treatment by using conventional and novel therapies that may slow the onset or progression of DKD.
FOOTNOTES
All authors contributed equally to this work; Muntean C and Banescu C contributed to conception and design of the work, interpreting the relevant literature and drafting the manuscript; Muntean C,Banescu C and Starcea IM performed the research of the literature; Muntean C and Starcea IM made critical revisions of the manuscript; all authors have read and approved the final version of the manuscript.
All the authors report no relevant conflicts of interest for this article.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Romania
Carmen Muntean 0000-0002-8056-1339.
Fan JR
Filipodia
Fan JR
1 Zhao L, Long T, Hui A, Zhao R, Long S, Peng W. Type 2 Diabetes Mellitus in Children and Adolescents: Early Prevention and Non-Drug Therapy.
2017; 7: 121-141 [DOI: 10.4236/jdm.2017.73010]
2 Stoian A, Bacârea A, Moţăţăianu A, Stoian M, Gliga F, Bacârea V, Duicu C, Bănescu C. Vascular Endothelial Growth Factor Insertion/Deletion gene polymorphism in patients with type 2 diabetes and diabetic peripheral polyneuropathy.
2014; 22: 165-172 [DOI: 10.2478/rrlm-2014-0023]
3 Afkarian M. Diabetic kidney disease in children and adolescents.
2015; 30: 65-74; quiz 70 [PMID:24643739 DOI: 10.1007/s00467-014-2796-5]
4 Marshall CB. Rethinking glomerular basement membrane thickening in diabetic nephropathy: adaptive or pathogenic?
2016; 311: F831-F843 [PMID: 27582102 DOI: 10.1152/ajprenal.00313.2016]
5 Divers J, Mayer-Davis EJ, Lawrence JM, Isom S, Dabelea D, Dolan L, Imperatore G, Marcovina S, Pettitt DJ, Pihoker C,Hamman RF, Saydah S, Wagenknecht LE. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths - Selected Counties and Indian Reservations, United States, 2002-2015.
2020; 69: 161-165 [PMID:32053581 DOI: 10.15585/mmwr.mm6906a3]
6 Lawrence JM, Divers J, Isom S, Saydah S, Imperatore G, Pihoker C, Marcovina SM, Mayer-Davis EJ, Hamman RF,Dolan L, Dabelea D, Pettitt DJ, Liese AD; SEARCH for Diabetes in Youth Study Group. Trends in Prevalence of Type 1 and Type 2 Diabetes in Children and Adolescents in the US, 2001-2017.
2021; 326: 717-727 [PMID: 34427600 DOI: 10.1001/jama.2021.11165]
7 Barkai L, Kiss Z, Rokszin G, Abonyi-Tóth Z, Jermendy G, Wittmann I, Kempler P. Changes in the incidence and prevalence of type 1 and type 2 diabetes among 2 million children and adolescents in Hungary between 2001 and 2016 - a nationwide population-based study.
2020; 16: 34-41 [PMID: 32051703 DOI: 10.5114/aoms.2019.88406]
8 Oester IM, Kloppenborg JT, Olsen BS, Johannesen J. Type 2 diabetes mellitus in Danish children and adolescents in 2014.
2016; 17: 368-373 [PMID: 26111830 DOI: 10.1111/pedi.12291]
9 Candler TP, Mahmoud O, Lynn RM, Majbar AA, Barrett TG, Shield JPH. Continuing rise of Type 2 diabetes incidence in children and young people in the UK.
2018; 35: 737-744 [PMID: 29460341 DOI: 10.1111/dme.13609]
10 Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus.
2012; 29: 453-463 [PMID: 22150528 DOI:10.1111/j.1464-5491.2011.03542.x]
11 Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O,Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus.
1993; 329: 977-986 [PMID: 8366922 DOI:10.1056/NEJM199309303291401]
12 Pastore I, Bolla AM, Montefusco L, Lunati ME, Rossi A, Assi E, Zuccotti GV, Fiorina P. The Impact of Diabetes Mellitus on Cardiovascular Risk Onset in Children and Adolescents.
2020; 21 [PMID: 32664699 DOI:10.3390/ijms21144928]
13 Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson AM, Eliasson B, Gudbjörnsdottir S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, registerbased cohort study.
2018; 392: 477-486 [PMID: 30129464 DOI: 10.1016/S0140-6736(18)31506-X]
14 Narres M, Claessen H, Droste S, Kvitkina T, Koch M, Kuss O, Icks A. The Incidence of End-Stage Renal Disease in the Diabetic (Compared to the Non-Diabetic) Population: A Systematic Review.
2016; 11: e0147329 [PMID:26812415 DOI: 10.1371/journal.pone.0147329]
15 Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R,Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Trends in Prevalence of Chronic Kidney Disease in the United States.
2016; 165: 473-481 [PMID: 27479614 DOI:10.7326/M16-0273]
16 Li L, Jick S, Breitenstein S, Michel A. Prevalence of Diabetes and Diabetic Nephropathy in a Large U.S. Commercially Insured Pediatric Population, 2002-2013.
2016; 39: 278-284 [PMID: 26681728 DOI: 10.2337/dc15-1710]
17 Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes.
2012; 35: 1265-1271 [PMID: 22432116 DOI: 10.2337/dc11-2312]
18 Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, Imperatore G, Williams DE, Bell RA, Wadwa RP, Palla SL, Liu LL,Kershnar A, Daniels SR, Linder B. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study.
2006; 29: 1891-1896 [PMID: 16873798 DOI:10.2337/dc06-0310]
19 Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease.
2018; 117: 662-675 [PMID: 29486908 DOI: 10.1016/j.jfma.2018.02.007]
20 Uwaezuoke SN, Ayuk AC. Diabetic Kidney Disease in Childhood and Adolescence: Conventional and Novel Renoprotective Strategies.
2020; 8: 68-77 [DOI: 10.33590/emjnephrol/20-00077]
21 Salem NA, El Helaly RM, Ali IM, Ebrahim HAA, Alayooti MM, El Domiaty HA, Aboelenin HM. Urinary Cyclophilin A and serum Cystatin C as biomarkers for diabetic nephropathy in children with type 1 diabetes.
2020; 21:846-855 [PMID: 32304131 DOI: 10.1111/pedi.13019]
22 Fu H, Liu S, Bastacky SI, Wang X, Tian XJ, Zhou D. Diabetic kidney diseases revisited: A new perspective for a new era.
2019; 30: 250-263 [PMID: 31767176 DOI: 10.1016/j.molmet.2019.10.005]
23 Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease.
2014; 124:2333-2340 [PMID: 24892707 DOI: 10.1172/JCI72271]
24 Hursh BE, Ronsley R, Islam N, Mammen C, Panagiotopoulos C. Acute Kidney Injury in Children With Type 1 Diabetes Hospitalized for Diabetic Ketoacidosis.
2017; 171: e170020 [PMID: 28288246 DOI:10.1001/jamapediatrics.2017.0020]
25 Gu HF. Genetic and Epigenetic Studies in Diabetic Kidney Disease.
2019; 10: 507 [PMID: 31231424 DOI:10.3389/fgene.2019.00507]
26 Florez JC. Genetics of Diabetic Kidney Disease.
2016; 36: 474-480 [PMID: 27987549 DOI:10.1016/j.semnephrol.2016.09.012]
27 Lu HC, Dai WN, He LY. Epigenetic Histone Modifications in the Pathogenesis of Diabetic Kidney Disease.
2021; 14: 329-344 [PMID: 33519221 DOI: 10.2147/DMSO.S288500]
28 Liu R, Lee K, He JC. Genetics and Epigenetics of Diabetic Nephropathy.
2015; 1: 42-51 [PMID:27536664 DOI: 10.1159/000381796]
29 Mooyaart AL. Genetic associations in diabetic nephropathy.
2014; 18: 197-200 [PMID: 24129556 DOI:10.1007/s10157-013-0874-9]
30 Tziastoudi M, Stefanidis I, Zintzaras E. The genetic map of diabetic nephropathy: evidence from a systematic review and meta-analysis of genetic association studies.
2020; 13: 768-781 [PMID: 33123356 DOI: 10.1093/ckj/sfaa077]
31 Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, Joles JA. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment.
2017; 28: 1023-1039[PMID: 28143897 DOI: 10.1681/ASN.2016060666]
32 Bjornstad P, Nehus E, El Ghormli L, Bacha F, Libman IM, McKay S, Willi SM, Laffel L, Arslanian S, Nadeau KJ;TODAY Study Group. Insulin Sensitivity and Diabetic Kidney Disease in Children and Adolescents With Type 2 Diabetes:An Observational Analysis of Data From the TODAY Clinical Trial.
2018; 71: 65-74 [PMID: 29157731 DOI: 10.1053/j.ajkd.2017.07.015]
33 Bjornstad P, Cherney DZ, Snell-Bergeon JK, Pyle L, Rewers M, Johnson RJ, Maahs DM. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with Type 1 diabetes.
2015; 30: 1706-1711[PMID: 26050268 DOI: 10.1093/ndt/gfv121]
34 Ruggenenti P, Porrini EL, Gaspari F, Motterlini N, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Parvanova A, Iliev I, Dodesini AR, Trevisan R, Bossi A, Zaletel J, Remuzzi G; GFR Study Investigators. Glomerular hyperfiltration and renal disease progression in type 2 diabetes.
2012; 35: 2061-2068 [PMID: 22773704 DOI: 10.2337/dc11-2189]
35 Lovshin JA, Škrtić M, Bjornstad P, Moineddin R, Daneman D, Dunger D, Reich HN, Mahmud F, Scholey J, Cherney DZI,Sochett E. Hyperfiltration, urinary albumin excretion, and ambulatory blood pressure in adolescents with Type 1 diabetes mellitus.
2018; 314: F667-F674 [PMID: 29357443 DOI: 10.1152/ajprenal.00400.2017]
36 Lopez LN, Wang W, Loomba L, Afkarian M, Butani L. Diabetic kidney disease in children and adolescents: an update.
2021 [PMID: 34913986 DOI: 10.1007/s00467-021-05347-7]
37 Zabeen B, Nahar J, Islam N, Azad K, Donaghue K. Risk Factors Associated with Microalbuminuria in Children and Adolescents with Diabetes in Bangladesh.
2018; 22: 85-88 [PMID: 29535943 DOI:10.4103/ijem.IJEM_269_17]
38 Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD.
2009; 20: 629-637 [PMID: 19158356 DOI: 10.1681/ASN.2008030287]
39 Szabo CE, Man OI, Istrate A, Kiss E, Catana A, Creț V, Șerban RS, Pop IV. Role of Adiponectin and Tumor Necrosis Factor-Alpha in the Pathogenesis and Evolution of Type 1 Diabetes Mellitus in Children and Adolescents.
2020; 10 [PMID: 33202729 DOI: 10.3390/diagnostics10110945]
40 El-Samahy MH, Adly AA, Ismail EA, Salah NY. Regulatory T cells with CD62L or TNFR2 expression in young type 1 diabetic patients: relation to inflammation, glycemic control and micro-vascular complications.
2015; 29: 120-126 [PMID: 25113439 DOI: 10.1016/j.jdiacomp.2014.07.004]
41 International Diabetes Federation, 2011. ISPAD. [cited 10 March 2022]. Available from:https://cdn.ymaws.com/www.ispad.org/resource/resmgr/Docs/idf-ispad_guidelines_2011_0.pdf
42 Currie G, McKay G, Delles C. Biomarkers in diabetic nephropathy: Present and future.
2014; 5: 763-776[PMID: 25512779 DOI: 10.4239/wjd.v5.i6.763]
43 Uwaezuoke SN. The role of novel biomarkers in predicting diabetic nephropathy: a review.
2017; 10: 221-231 [PMID: 28860837 DOI: 10.2147/IJNRD.S143186]
44 Uwaezuoke SN, Muoneke VU, Mbanefo NR. Tubular Biomarkers as Diagnostic Tools in Diabetic Kidney Disease: A Review of Published Evidence.
2018; 4 [DOI: 10.16966/2380-5498.156]
45 Smith ER, Cai MM, McMahon LP, Wright DA, Holt SG. The value of simultaneous measurements of urinary albumin and total protein in proteinuric patients.
2012; 27: 1534-1541 [PMID: 22193048 DOI:10.1093/ndt/gfr708]
46 Thethi TK, Batuman V. Challenging the conventional wisdom on diabetic nephropathy: Is microalbuminuria the earliest event?
2019; 33: 191-192 [PMID: 30651179 DOI: 10.1016/j.jdiacomp.2018.12.006]
47 Petrica L, Vlad A, Gluhovschi G, Gadalean F, Dumitrascu V, Gluhovschi C, Velciov S, Bob F, Vlad D, Popescu R, Milas O, Ursoniu S. Proximal tubule dysfunction is associated with podocyte damage biomarkers nephrin and vascular endothelial growth factor in type 2 diabetes mellitus patients: a cross-sectional study.
2014; 9: e112538 [PMID:25397960 DOI: 10.1371/journal.pone.0112538]
48 Yürük Yıldırım Z, Nayır A, Yılmaz A, Gedikbaşı A, Bundak R. Neutrophil Gelatinase-Associated Lipocalin as an Early Sign of Diabetic Kidney Injury in Children.
2015; 7: 274-279 [PMID: 26777038 DOI:10.4274/jcrpe.2002]
49 Mamilly L, Mastrandrea LD, Mosquera Vasquez C, Klamer B, Kallash M, Aldughiem A. Evidence of Early Diabetic Nephropathy in Pediatric Type 1 Diabetes.
2021; 12: 669954 [PMID: 33995287 DOI:10.3389/fendo.2021.669954]
50 Bob F, Schiller A, Timar R, Lighezan D, Schiller O, Timar B, Bujor CG, Munteanu M, Gadalean F, Mihaescu A, Grosu I,Hategan A, Chisavu L, Pusztai AM, Covic A. Rapid decline of kidney function in diabetic kidney disease is associated with high soluble Klotho levels.
2019; 39: 250-257 [PMID: 30396700 DOI: 10.1016/j.nefro.2018.08.004]
51 Kim JH, Hwang KH, Park KS, Kong ID, Cha SK. Biological Role of Anti-aging Protein Klotho.
2015; 5:1-6 [PMID: 26528423 DOI: 10.15280/jlm.2015.5.1.1]
52 Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, Poster D, Wüthrich RP, Russmann S, Serra AL. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study.
2013; 28: 352-359 [PMID: 23129826 DOI: 10.1093/ndt/gfs460]
53 Zubkiewicz-Kucharska A, Wikiera B, Noczyńska A. Soluble Klotho Is Decreased in Children With Type 1 Diabetes and Correlated With Metabolic Control.
2021; 12: 709564 [PMID: 34603200 DOI:10.3389/fendo.2021.709564]
54 El-Saeed GK, El-Deen WAS, Montasr BA, Omar TA, Mohamed DS. Urinary podocalyxin and cyclophilin A: markers for early detection of type 2 diabetic nephropathy.
2019; 32: 996-1003 [DOI: 10.4103/mmj.mmj_223_18]
55 Harun H, Lunesia R, Azmi S. Correlation between urinary Cyclophilin A and urinary albumin levels on diabetic kidney disease.
2019; 2: 10-16 [DOI: 10.32867/inakidney.v2i2.29]
56 Amer HMA, Sabry IM, Bekhet MMM, Mohammed RNS. The role of urinary cyclophilin A as a new marker for diabetic nephropathy.
2018; 70: 1431-1439 [DOI: 10.12816/0044664]
57 Zeni L, Norden AGW, Cancarini G, Unwin RJ. A more tubulocentric view of diabetic kidney disease.
2017; 30:701-717 [PMID: 28840540 DOI: 10.1007/s40620-017-0423-9]
58 Cioana M, Deng J, Hou M, Nadarajah A, Qiu Y, Chen SSJ, Rivas A, Banfield L, Chanchlani R, Dart A, Wicklow B,Alfaraidi H, Alotaibi A, Thabane L, Samaan MC. Prevalence of Hypertension and Albuminuria in Pediatric Type 2 Diabetes: A Systematic Review and Meta-analysis.
2021; 4: e216069 [PMID: 33929524 DOI:10.1001/jamanetworkopen.2021.6069]
59 Rohani F, Hooman N, Moradi S, Mobarra M, Najafizadeh M, Tatarpoor P. The Prevalence of Pre-hypertension in Children with Type 1 Diabetes Mellitus.
2014; 5: S44-S49 [PMID: 24791191]
60 Shalaby NM, Shalaby NM. Study of ambulatory blood pressure in diabetic children: prediction of early renal insult.
2015; 11: 1531-1537 [PMID: 26491340 DOI: 10.2147/TCRM.S87751]
61 Dost A, Bechtold-Dalla Pozza S, Bollow E, Kovacic R, Vogel P, Feldhahn L, Schwab KO, Holl RW; Initiative DPV. Blood pressure regulation determined by ambulatory blood pressure profiles in children and adolescents with type 1 diabetes mellitus: Impact on diabetic complications.
2017; 18: 874-882 [PMID: 28117539 DOI:10.1111/pedi.12502]
62 Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes.
2002; 347: 797-805 [PMID: 12226150 DOI:10.1056/NEJMoa013410]
63 Torbjörnsdotter TB, Jaremko GA, Berg UB. Nondipping and its relation to glomerulopathy and hyperfiltration in adolescents with type 1 diabetes.
2004; 27: 510-516 [PMID: 14747237 DOI: 10.2337/diacare.27.2.510]
64 Uwaezuoke SN. Vitamin D Analogs Can Retard the Onset or Progression of Diabetic Kidney Disease: A Systematic Review.
2021; 2: 763844 [DOI: 10.3389/fcdhc.2021.763844]
65 Idzerda NMA, Pena MJ, de Zeeuw D, Heerspink HJL. Future and Novel Compounds in the Treatment of Diabetic Nephropathy.
2019 [DOI: 10.1007/978-3-319-93521-8_29]
66 KDOQI. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease.
2007; 49: S12-154 [PMID: 17276798 DOI: 10.1053/j.ajkd.2006.12.005]
67 Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, Balk E, Lau J, Levin A, Kausz AT, Eknoyan G, Levey AS; National Kidney Foundation's Kidney Disease Outcomes Quality Initiative. National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents:evaluation, classification, and stratification.
2003; 111: 1416-1421 [PMID: 12777562 DOI:10.1542/peds.111.6.1416]
杂志排行
World Journal of Diabetes的其它文章
- Loss of skeletal muscle mass is not specific to type 2 diabetes
- Association of rs1137101 with hypertension and type 2 diabetes mellitus of Mongolian and Han Chinese
- Metformin toxicity: A meta-summary of case reports
- In vivo evaluation and mechanism prediction of anti-diabetic foot ulcer based on component analysis of Ruyi Jinhuang powder
- Clopidogrel delays and can reverse diabetic nephropathy pathogenesis in type 2 diabetic db/db mice
- lmproved systemic half-life of glucagon-like peptide-1-loaded carbonate apatite nanoparticles in rats
