Development and validation of a nomogram for predicting overall survival in cirrhotic patients with acute kidney injury
2022-08-13YiPengWanAnJiangWangWangZhangHangZhangGenHuaPengXuanZhu
Yi-Peng Wan,An-Jiang Wang, Wang Zhang, Hang Zhang, Gen-Hua Peng, Xuan Zhu
Abstract
Key Words: Acute kidney injury; Cirrhosis; Nomogram; Prognosis
lNTRODUCTlON
Acute kidney injury (AKI) is a common and severe complication occurring in patients with cirrhosis,manifesting as an abrupt increase in serum creatinine (SCr) levels and an acute significant reduction in urine output (UO) in a short period[1,2]. Many diagnostic criteria and classifications of AKI have been developed to identify AKI and improve prognosis over the last two decades, such as the Risk, Injury,Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) classification in 2004[3], the Acute Kidney Injury Network (AKIN) classification in 2007[4], and the Kidney Disease: Improving Global Outcomes (KDIGO) classification in 2012[5]. However, the concept of AKI in patients with cirrhosis was debated for many years until the International Club of Ascites (ICA) proposed a new classification of AKI in 2015[1]. These classifications are based on SCr levels and/or UO. One of the main differences between the ICA classification and other classifications is the abandonment of the UO criteria[1]. Although these criteria have been established, a single criterion that comprehensively evaluates and diagnoses AKI is still unavailable.
The incidence and mortality of AKI in patients with cirrhosis varies substantially among studies[2,6,7]. In some studies, the incidence of AKI ranges from 20%-50% in patients with cirrhosis[2,8]. Moreover,studies have reported that the mortality rate of AKI was also unacceptably high in patients with cirrhosis, reaching up to 80%[6,9]. Therefore, identifying cirrhotic patients with AKI who are at high risk of mortality is very important and may be helpful for providing timely medical interventions to improve the prognosis of these patients. Currently, many studies have investigated risk factors for mortality in patients with cirrhosis[10] and other complications, such as gastroesophageal variceal bleeding[11,12], ascites[13], bacterial infections[14] and metabolic acidosis[15]. Moreover, a number of studies have focused on identifying the risk factors for the development of AKI in patients with cirrhosis[16-19] and other conditions[20-22]. Furthermore, numerous studies have also evaluated the effect of AKI on the mortality of patients with cirrhosis[16,23-25]. A systematic review reported that the risk of death from AKI was increased more than 6-fold in patients with cirrhosis[24]. However, few studies have focused on analyzing the risk factors for mortality in patients with cirrhosis who are diagnosed with AKI. Additionally, based on clinician demand, a quantitative predictive model for determining the prognosis of cirrhotic patients with AKI also must be developed to predict the risk of mortality and distinguish patients with a high risk of mortality.
Therefore, the purpose of the present study was to investigate the risk factors for mortality of cirrhotic patients with AKI and establish a nomogram for predicting the overall survival of these patients to identify high-risk patients and guide timely treatments to improve the prognosis of these patients.
MATERlALS AND METHODS
Patients
We conducted a retrospective study at the Department of Gastroenterology and Hepatology and the Department of Infectious Diseases, The First Affiliated Hospital of Nanchang University, a tertiary care referral hospital in China, between January 2015 and December 2016. The inclusion criteria were as follows: (1) Patients who were diagnosed with cirrhosis; and (2) Patients who met the criteria for at least one of the RIFLE, AKIN, KDIGO and ICA classifications. These patients were hospitalized. The exclusion criteria were as follows: (1) Patients aged less than 18 and more than 80 years; (2) Patients with a previously history renal dysfunction or chronic kidney disease; (3) Patients who underwent liver or renal transplantation; (4) Patients who received renal replacement therapy (including hemodialysis and peritoneal dialysis) before admission; (5) Patients with malignancy or severe cardiopulmonary disease; (6) Patients who were discharged or died < 48 h after admission; (7) Pregnant patients; and (8)Patients without complete data.
According to the inclusion and exclusion criteria, 382 consecutive patients with cirrhosis who were diagnosed with AKI were screened, and 250 eligible patients were recruited and randomly divided into the training cohort (n= 173) and the validation cohort (n= 77) using the built-in random packet function of SPSS 23.0 software according at a ratio of 1:2. The flow chart for inclusion and exclusion is shown in Figure 1.
The study was approved by the Ethics Committee of The First Affiliated Hospital of Nanchang University (No. AF-SG-04-2.0).
Data collection
Demographics and clinical variables, namely, demographic data (age, sex and body weight), mean arterial pressure (MAP), underlying liver disease, cirrhosis-related complications, diabetes, biochemical analysis (routine blood tests, serum biochemical tests, coagulation function tests,etc.), days of the hospital stay, and comorbidities were recorded and collected from every cirrhotic patient with AKI. UO was recorded for all patients during hospitalization. The Child-Turcotte-Pugh (CTP), model for endstage liver disease (MELD) and MELD-Na scores at admission were calculated accordingly.
Definitions
Cirrhosis was diagnosed based on the medical history, physical signs and symptoms, endoscopic signs of portal hypertension, radiological evidence of liver nodularity, and liver biopsy[26].
According to the consensus of the Asian Pacific Association for the Study of the Liver[27], acute-onchronic liver failure (ACLF) was defined as acute hepatic insult manifesting as jaundice and coagulopathy complicated within 4 wk by ascites and/or encephalopathy in patients with previously diagnosed or undiagnosed chronic liver disease associated with high mortality.
AKI was defined and classified using the RIFLE, AKIN, KDIGO or ICA classifications(Supplementary Table 1). The peak AKI stage based on the SCr level or UO criterion during hospitalization was used. For the RIFLE, KDIGO and ICA classifications, the last SCr level recorded within the previous 3 mo before admission was used as the baseline SCr level, but the SCr level at admission was used as the baseline SCr level for patients without a previous SCr measurement[1]. The SCr level at admission was also considered the baseline SCr level for the AKIN classification. The glomerular filtration rate (GFR) was used for the RIFLE classification, and the eGFR was calculated using the revised Schwartz method[28]. UO was used in the RIFLE, AKIN and KDIGO classifications.
Calculations of the CTP, MELD and MELD-Na scores
The CTP score was calculated as described in previous reports and classified as grade A (5-6 points),grade B (7-9 points), and grade C (10-15 points)[29]. The computed formula for the MELD score was 3.8× ln (bilirubin, mg/dL) + 11.2 × ln (international normalized ratio) + 9.6 × ln (creatinine, mg/dL) + 6.4(constant for liver disease etiology)[30,31]. The computed formula for the MELD-Na score was MELD +1.59 × [135 - (Na, mmol/L)], and sodium ion concentrations ranged from 125 to 140 mmol/L[32].
Management of patients
A comprehensive medical intervention was administered to every patient, including supportive therapy, prevention and treatment of complications, and reduction or withdrawal of all unnecessary nephrotoxic medications. Patients also received albumin, vasoconstrictors (norepinephrine and terlipressin), intravenous antibiotics, diuretics, proton pump inhibitors or continuous renal replacement therapy if required. Twenty-six patients (10.4%) received continuous replacement therapy.
Follow-up and outcomes
After the diagnosis of AKI, regular follow-up was performed for all cirrhotic patients with AKI by telephone and electronic medical records. Patients were followed for a maximum of 180 d or until death,liver or renal transplantation, or the end of the study period. Follow-up data on the prognosis were collected and evaluated. The primary endpoint of the study was the mortality rate.
Statistical analysis
All continuous variables were tested for a normal distribution using the Kolmogorov-Smirnov test.Variables that met the normal distribution are presented as the mean ± SD and were analyzed using Student’sttest, while nonnormally distributed variables are summarized as medians and interquartile ranges and were analyzed using the Mann-WhitneyUtest. Categorical variables are presented as numbers and percentages and were analyzed using theχ2test or Fisher’s exact test. Cumulative survival was evaluated using the Kaplan-Meier method and compared between groups using the log-rank test.The univariate analysis of risk factors of death in cirrhotic patients with AKI was performed using Cox regression analysis, and multivariate Cox analysis with the forward Wald method was subsequently performed for all variables withPvalues less than 0.2.
A nomogram was developed based on the weighted sum of each independent variable, with weights equal to the hazard ratios from the multivariate Cox model to predict the prognosis of cirrhotic patients with AKI. The area under the receiver operating characteristic curve (AUROC), calibration curve and decision curve analysis (DCA) were used to evaluate its predictive performance. All tests were twotailed, andPvalues less than 0.05 were considered indicative of statistical significance. Data were analyzed using SPSS software, version 23.0 (SPSS Inc., Chicago, IL, Untied States) and R software,version 3.5.3.
RESULTS
Baseline characteristics of the study patients
The characteristics of the patients in the training and validation cohorts are summarized in Table 1.Among the 250 patients, the mean age was 53.6 ± 13.3 years, and most of the patients were male(190/250, 76.0%). Notably, 25.6% (64/250) of the patients presented AKI at the time of admission, and 74.4% (186/250) developed AKI during hospital stay. Only 7 (2.8%) of these patients had compensated cirrhosis, and 243 (97.2%) patients had decompensated cirrhosis. The main etiology of these patients in both datasets was hepatitis B, accounting for 69.6% (174/250) of the entire cohort. Most of the patients(239/250, 95.6%) had ascites, and 60.8% (152/250) of the patients had ACLF. The main causes of AKI were hypovolemia (136/250, 54.4%), followed by infections (54/250, 21.6%), nephrotoxicity (21/250,8.4%) and other factors (39/250, 15.6%). In the comparison of the training cohort and validation cohort,although the MAP, albumin level and serum sodium level were significantly different (allP< 0.05), a significant difference in mortality was not observed between the training (75.1%) and validation (79.2%)datasets (P= 0.298). Based on RIFLE, AKIN, KDIGO and ICA classifications, 234, 221, 241 and 211 of all 250 patients were diagnosed with AKI, respectively. No patients who underwent liver transplantation.
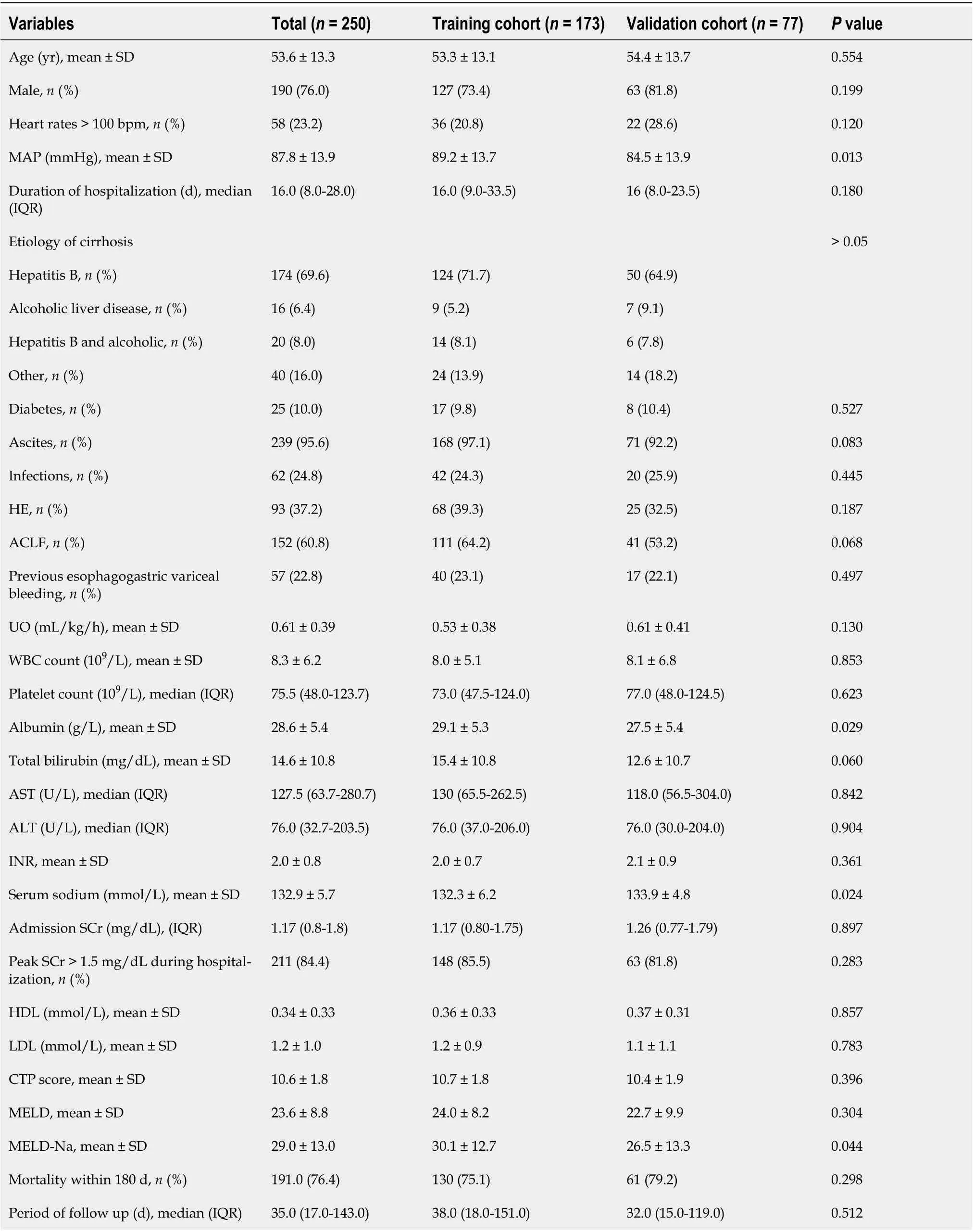
Table 1 Demographic and baseline clinical characteristics of cirrhotic patients with acute kidney injury
Mortality of cirrhotic patients with AKI
The median period of follow-up for all 250 patients after the diagnosis of AKI was 35.0 (17.0-143.0) d.The overall 30-d mortality, 90-d mortality and 180-d mortality rates were 46.4% (116/250), 68.8%(172/250) and 76.4% (191/250), respectively. Among these patients who died, the most common cause of mortality was hepatic failure (74/191, 38.7%), followed by infections (41/191, 21.5%), renal failure(34/191, 17.8%), variceal bleeding (24/191, 12.6%), and other causes (19/191, 9.9%). The median followup periods in the training and validation cohorts were 38.0 (18.0-151.0) and 32.0 (15.0-119.0) d,respectively. The mortality rates of the training cohort were 45.1%, 68.2% and 75.1% at 30 d, 90 d and 180 d, respectively. The mortality rates at 30 d, 90 d and 180 d were 49.4%, 70.1% and 79.2% in the validation cohort, respectively. No significant difference in mortality was observed during follow-up in either dataset (P=0.511; Figure 2).
Potential risk factors for mortality in the training cohort
During the follow-up period, 130 cirrhotic patients with AKI in the training cohort died. We performed a Cox regression analysis to identify risk factors of death from cirrhotic patients with AKI (Table 2). The univariate Cox regression analysis showed that a heart rate > 100 bpm, UO, presence of diabetes, total bilirubin level, international normalized ratio (INR), serum sodium level, high-density lipoprotein level,admission SCr level, peak SCr level > 1.5 mg/dL, overt hepatic encephalopathy (HE) or ACLF, and CTP, MELD and MELD-Na scores were potential risk factors of mortality. Multicollinearity between CTP, MELD and MELD-Na scores and other predictors was avoided by excluding these predictive models from the multivariate analysis. Subsequently, the multivariate Cox regression analysis revealed that the presence of diabetes [Hazard ratio (HR) = 1.795; 95% confidence interval (CI): 1.048-3.076;P=0.001], HE (HR = 1.986; 95%CI: 1.381-2.856;P< 0.001), INR (HR = 1.390; 95%CI: 1.081-1.787;P= 0.010),serum sodium level (HR = 0.964; 95%CI: 0.937-0.992;P= 0.011) and peak SCr level > 1.5 mg/dL (HR =2.026; 95%CI: 1.109-3.702;P= 0.022) were potential risk factors for mortality in cirrhotic patients with AKI.
Establishment of a prediction nomogram in the training cohort
A nomogram incorporating these potential risk factors recorded after the diagnosis of AKI during hospitalization was constructed to predict the probability of death within 180 d for cirrhotic patients with AKI (Figure 3). Each predictor had a number with weights equal to the HR of the multivariate Cox regression model, and the estimated probability of death for cirrhotic patients with AKI was calculated by adding the scores of each predictor.
Validation of the prediction nomogram
In the training cohort, the AUROC of the nomogram in predicting mortality at 30, 90 and 180 d were 0.757 (95%CI: 0.685-0.830), 0.792 (95%CI: 0.726-0.859) and 0.801 (95%CI: 0.726-0.870), respectively, which exhibited good discrimination for the prognosis of cirrhotic patients with AKI (Figure 4A). The calibration plots of 30-, 90- and 180-d survival showed satisfactory agreement between the predicted prognosis and actual prognosis probability in the training cohort (Figure 4B-D). In the validation cohort,the nomogram also displayed good discrimination with AUROCs of 0.763 (95%CI: 0.656-0.869), 0.817(95%CI: 0.716-0.917) and 0.862 (95%CI: 0.765-0.958) in predicting mortality at 30, 90 and 180 d,respectively (Figure 5A). The calibration plots also showed good agreement between the predicted and actual prognosis probabilities in the validation cohort (Figure 5B-D). Based on the results, the nomogram had good discrimination and calibration in predicting the prognosis of cirrhotic patients with AKI.
Performance of the nomogram in predicting mortality
We compared the performance of the nomogram with CTP, MELD and MELD-Na scores in predicting the overall survival of cirrhotic patients with AKI, and the results are shown in Table 3. There were no significant differences between the nomogram and the CTP, MELD and MELD-Na scores in predicting 30-d mortality for either the training or validation cohort (allP> 0.05; Figure 6A and B). However, the AUROC of the nomogram in predicting mortality at 90 d was significantly higher than that of the CTP,MELD and MELD-Na scores in both the training and validation cohort (allP< 0.05; Figure 6C and D).Moreover, the AUROC of the nomogram in predicting mortality at 180 d was also larger than that of the CTP, MELD and MELD-Na scores in both the training and validation cohort (allP< 0.05; Figure 6E and F). With regard to clinical usefulness, DCA of the nomogram was depicted and compared with the CTP,MELD and MELD-Na scores. Compared with the CTP, MELD and MELD-Na scores, medical intervention guided by the nomogram increased the net benefit for 90- and 180-d overall survival but nor 30-d overall survival in the training dataset (Figure 7A-C). In the validation dataset, the similar result was obtained: The nomogram showed the best net benefit for 90- and 180-d overall survival but not 30-d overall survival (Figure 7D-F). Taken together, these results showed that the nomogram better predicted the overall survival of cirrhotic patients with AKI than the CTP, MELD and MELD-Na scores.
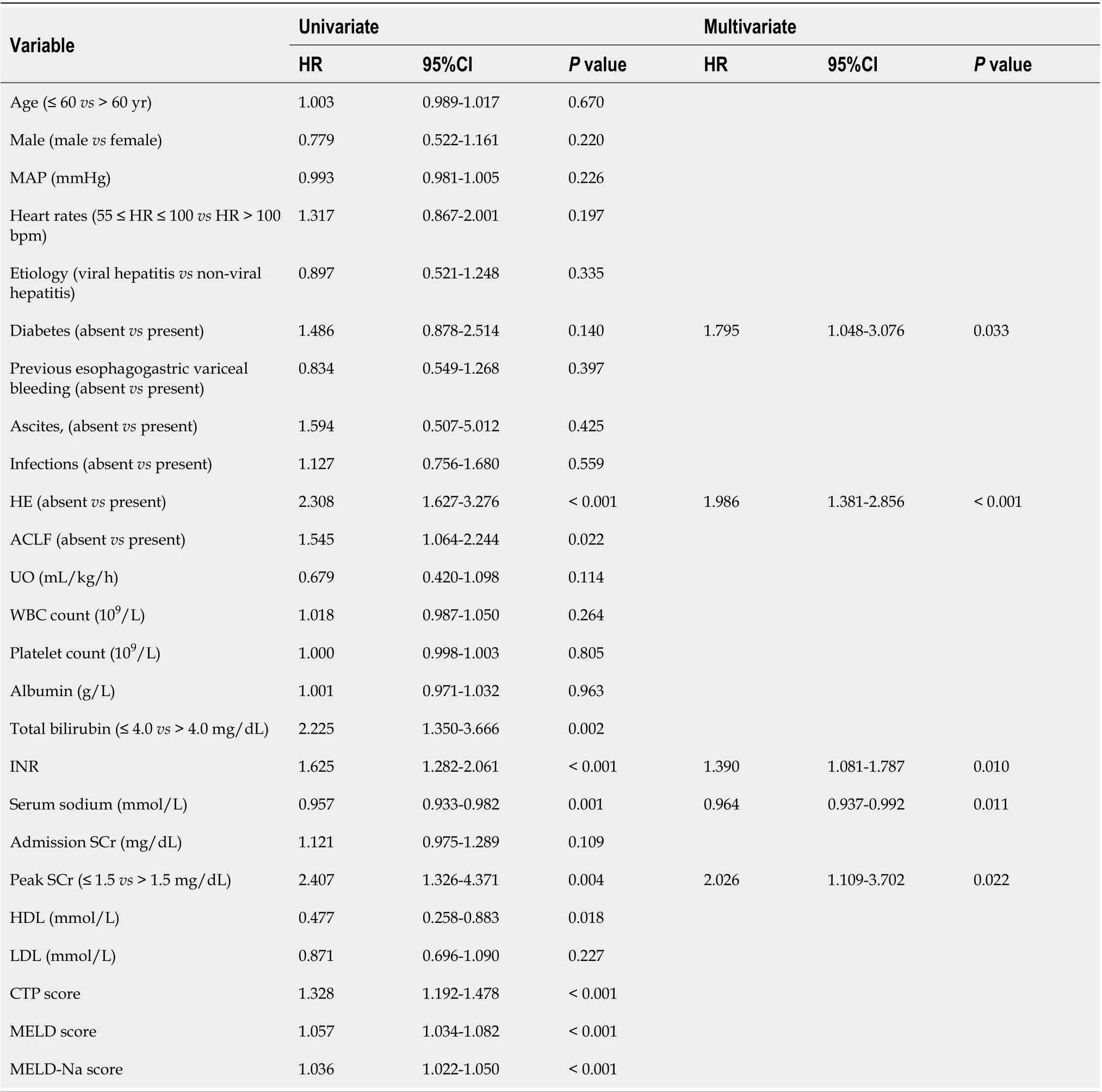
Table 2 Cox regression analysis of predictors for death of cirrhotic patients with acute kidney injury in the training cohort
According to the nomogram, the score of each patient was calculated and utilized for a stratified analysis. Based on the Youden index, the optimal cutoff value of the nomogram score in predicting mortality within 180 d in the training cohort was 98, with a sensitivity of 56.1% and a specificity of 90.7%. Based on the threshold of 98, patients in the training cohort were divided into two subgroups,with high risk (> 98 points,n= 77) and low risk (≤ 98 points,n= 96) of mortality. The high-risk group had a significantly higher mortality rate within 180 d than the low-risk group (94.8%vs59.4%,P< 0.001;Figure 8A). In the validation cohort, the same cutoff value was used, and the sensitivity and specificity of the nomogram were 55.6% and 92.9%, respectively. Patients from the validation cohort were also stratified into a high-risk group (n= 36) and a low-risk group (n= 41), and the mortality rate was significantly different between both risk groups (97.2%vs68.3%,P< 0.001; Figure 8B).
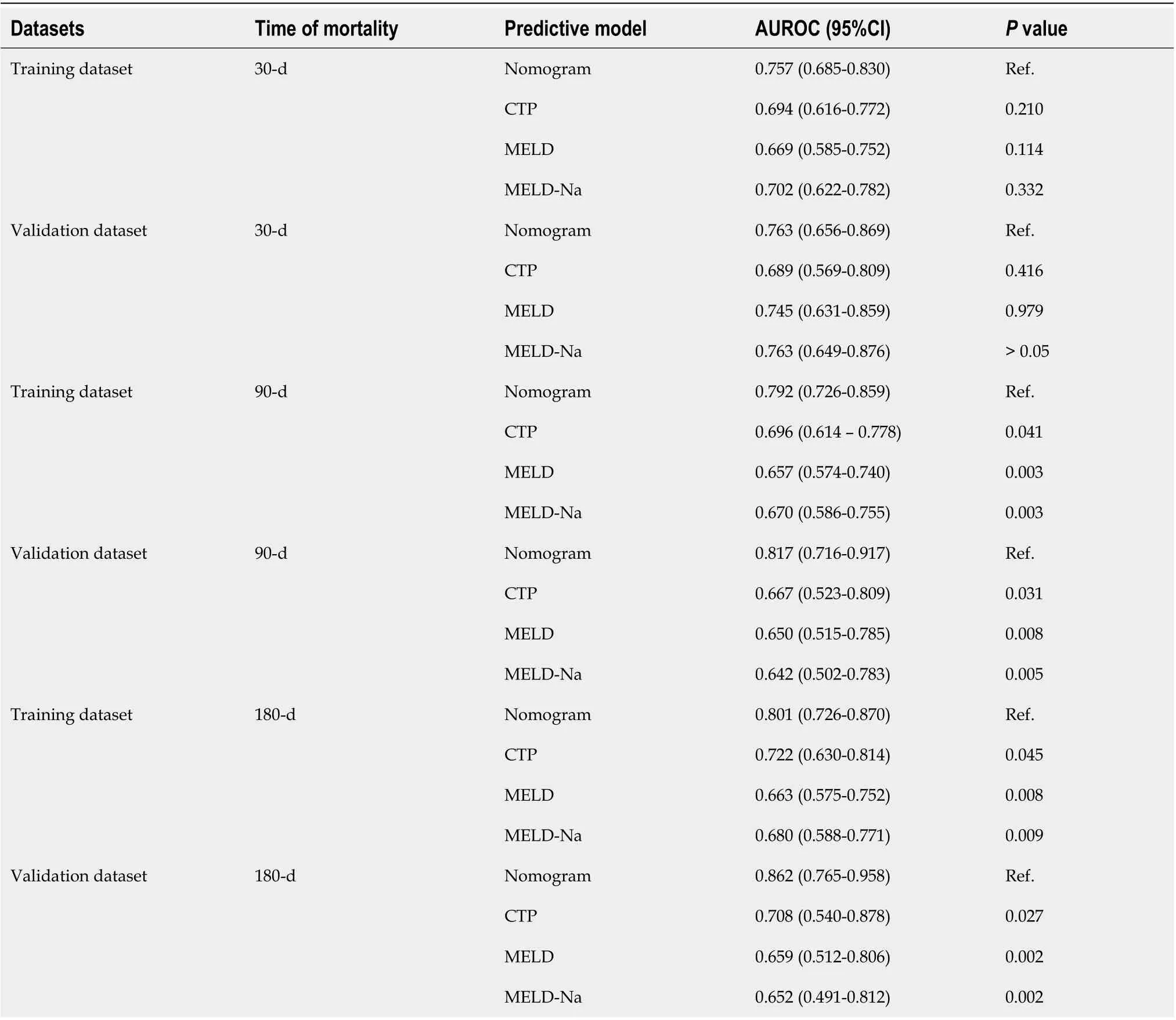
Table 3 Comparison of models in predicting the death of cirrhotic patients with acute kidney injury
DlSCUSSlON
In this study, we conducted a Cox regression analysis to identify the risk factors for mortality within 180 d in cirrhotic patients with AKI, and these predictors included the presence of diabetes, HE, INR, serum sodium level and peak SCr levels. A nomogram incorporating these predictors was developed and showed good predictive discrimination and calibration for the mortality of these patients. Moreover,compared with the CTP, MELD and MELD-Na scores, the nomogram showed better performance for predicting the overall survival of cirrhotic patients with AKI. To our knowledge, this is the first to establish a quantitative nomogram based on these risk factors for the overall survival of cirrhotic patients with AKI.
AKI is a very common and life-threatening complication in patients with cirrhosis and has an unacceptably high mortality rate[33]. Therefore, many AKI criteria and classifications have been developed over the past two decades to comprehensively evaluate and diagnose AKI as early as possible and improve the prognosis. Currently, four main classifications are used: The RIFLE[3], AKIN[4], KIDGO[5] and ICA[1] criteria. SCr level and UO, which are established markers of kidney function,were the basis for the development of these AKI classifications. The use of the SCr level alone to diagnose with AKI may not be appropriate. First, SCr levels are influenced by various factors, such as age, race, sex, body weight, tubular creatinine secretion and the effect of bilirubin on creatinine assays[34,35]. Second, in patients with advanced cirrhosis in particular, the diagnostic value of SCr levels was diminished because of the reduced hepatic production of creatinine from creatine and muscle wasting[7,36]. Finally, a greater number of AKI cases could be detected earlier using UO in combination with the SCr level[37-39]. Notably, UO is also affected by many factors, such as the body fluid volume of the patients and the use of diuretics, which may decrease the diagnostic and predictive value of the UO criteria for AKI[37]. As a result, any single criterion for AKI may not be able to comprehensively identify and evaluate these patients. Therefore, in the present study, patients who met the criteria for at least one of the RIFLE, AKIN, KDIGO and ICA classifications were recruited as comprehensively as possible, to analyze the clinical characteristics of cirrhotic patients with AKI and identify risk factors for mortality.
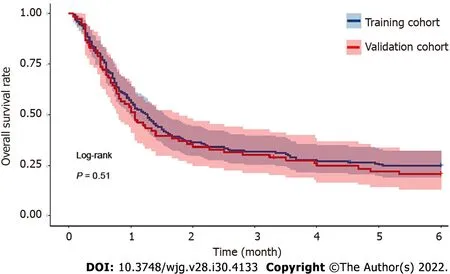
Figure 2 Kaplan-Meier analysis of the overall survival of cirrhotic patients with acute kidney injury within 180 d in both the training and validation cohorts.
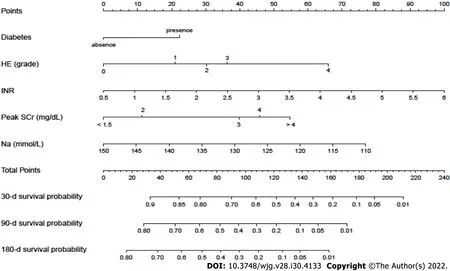
Figure 3 A nomogram for predicting the 30-d, 90-d and 180-d overall survival of cirrhotic patients with acute kidney injury. HE: Hepatic encephalopathy; SCr: Serum creatinine; INR: International normalized ratio.
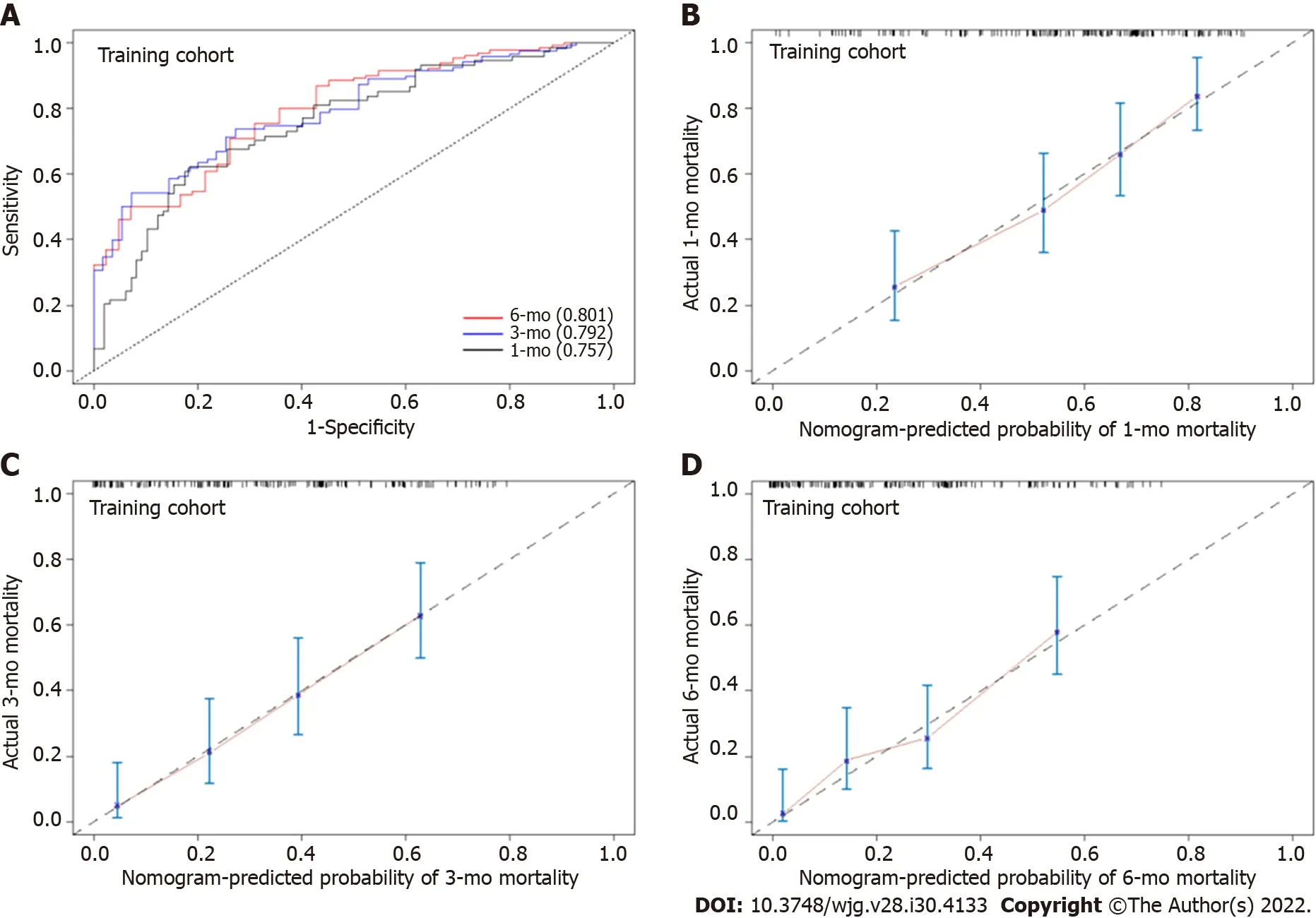
Figure 4 The area under the receiver operating characteristic curve and calibration curve of the nomogram for predicting the overall survival of cirrhotic patients with acute kidney injury in the training cohort. A: The area under the receiver operating characteristic curve of the nomogram for mortality at 30-, 90- and 180-d in the training cohort; B: Calibration curve of the nomogram in predicting 30-d overall survival in the training cohort; C:Calibration curve of the nomogram in predicting 90-d overall survival in the training cohort; D: Calibration curve of the nomogram in predicting 180-d overall survival in the training cohort.
The mortality rate of cirrhotic patients with AKI displays substantial heterogeneity among studies.Xionget al[9] reported that the mortality rates were 76.1% and 86.7% in-hospital and at 6 mo,respectively, for patients with cirrhosis who were diagnosed with AKI upon admission to the intensive care unit (ICU), which was consistent with the results of previous studies[7,40]. The results were significantly higher than the values reported in our present study (52.4% in-hospital mortality and 76.4% 180-d mortality). Another study conducted by Kumaret al[41] showed that the mortality rate at 30 d was 46.7% for cirrhotic patients with AKI, which was consistent with our result of 46.4%. A metaanalysis conducted by Tariqet al[42] revealed that the overall in-hospital mortality, 30-d mortality, and 90-d mortality rates of patients with liver cirrhosis were 34.6%, 42.4%, and 47.1%, respectively, which was lower than the results from our study (52.4% in-hospital mortality, 46.4% 30-d mortality, and 68.8%90-d mortality). The heterogeneity of the mortality rate may be mainly affected by the difference in the severity of the patient’s condition. Moreover, some serious complications of patients with cirrhosis may also contribute to the high mortality rate. For instance, in the present investigation, 60.8% (152/250) of patients had cirrhosis combined with ACLF, resulting in an increase in the AKI mortality rate, which was supported by previous studies by Zanget al[43] and Shiet al[44]. Furthermore, the literature has indicated that the mortality of AKI in patients with cirrhosis who were admitted to the ICU was significantly higher than that of patients admitted to regular wards[7,42]. In addition, the use of different AKI criteria to identify patients among studies also resulted in different the mortality rate[6,7].Notably, the types of AKI may also affect the prognosis of these patients. AKI is usually divided into three subtypes: Prerenal AKI, acute tubular necrosis (ATN) and hepatorenal syndrome (HRS)[9]. AKI is closely associated with the prognosis of patients with cirrhosis. A retrospective study showed that compared with non-AKI patients, patients with prerenal AKI had a 2.37-fold higher risk of in-hospital death, patients with ATN had a 6.878-fold higher risk, and patients with HRS had a 12.98-fold higher risk[9]. The result seems to indicate that cirrhotic patients with HRS have a higher risk of death than those with ATN and prerenal AKI. Moreover, patients with HRS-AKI have a worse prognosis than those with non-HRS-AKI[2]. Taken together, the varying mortality of AKI in patients with cirrhosis may be influenced by the severity of the liver disease, the diversity of AKI classifications, survey populations, complications of cirrhosis, and the subtypes of AKI, making the evaluation of AKI difficult and in many cases impossible.
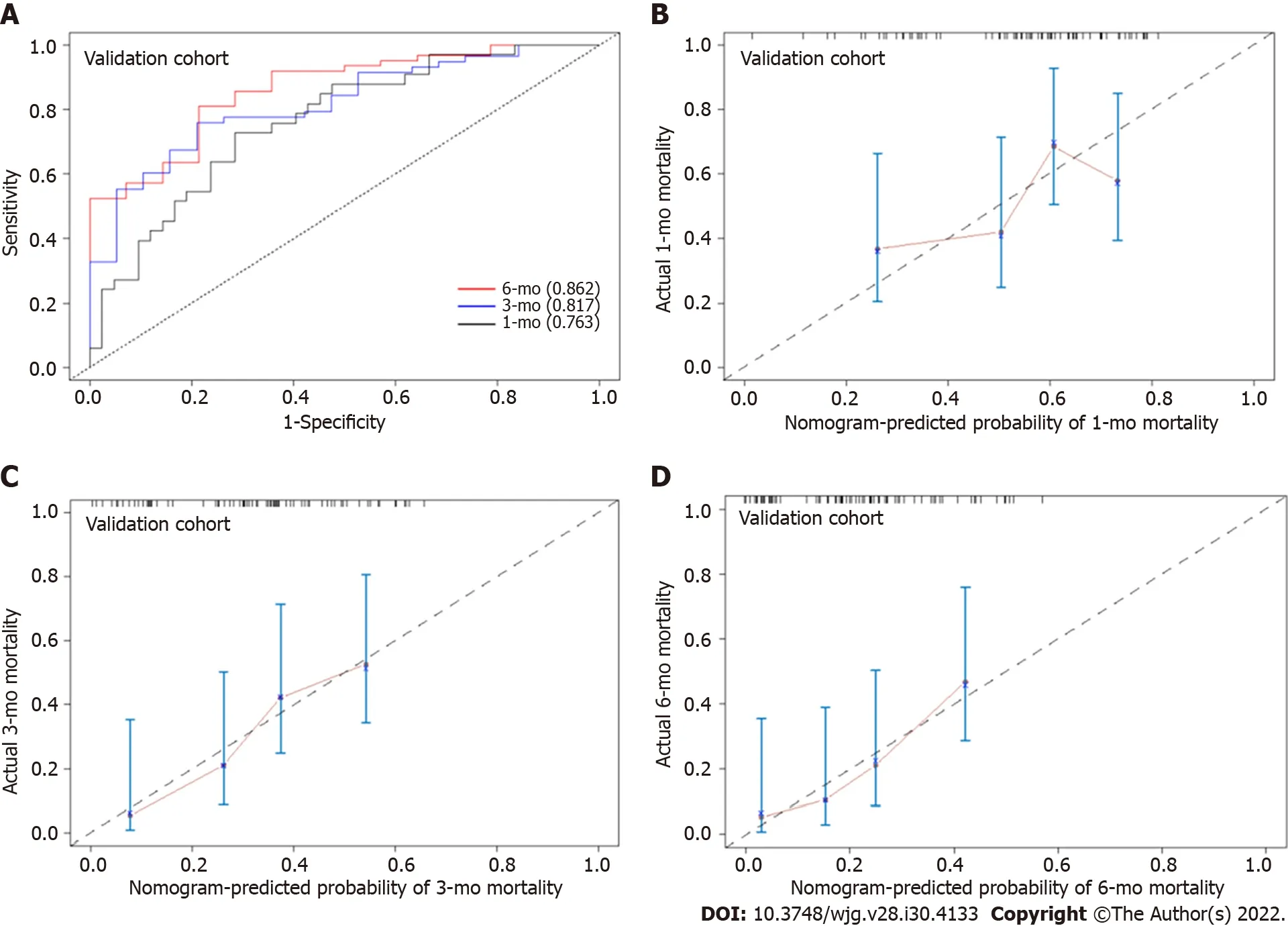
Figure 5 The area under the receiver operating characteristic curve and calibration curve of the nomogram for predicting the overall survival of cirrhotic patients with acute kidney injury in the validation cohort. A: The area under the receiver operating characteristic curve of the nomogram for mortality at 30-, 90- and 180-d in the validation cohort; B: Calibration curve of the nomogram in predicting 30-d overall survival in the validation cohort;C: Calibration curve of the nomogram in predicting 90-d overall survival in the validation cohort; D: Calibration curve of the nomogram in predicting 180-d overall survival in the validation cohort.
Considering the high mortality rate of AKI in patients with cirrhosis, the identification of risk factors for mortality in these patients is meaningful. However, few studies conducted to date have focused on investigating the risk factors for death in cirrhotic patients with AKI. A study conducted by Kumaret al[41] showed that the presence of jaundice, HE, SCr level > 1.5 mg/dL at admission, CTP score, and MELD score were independent predictors of mortality for cirrhotic patients with AKI. Another study by Panet al[40] found that MAP, total bilirubin levels, acute respiratory failure and sepsis were closely associated with the prognosis of cirrhotic patients with AKI who were admitted to the ICU. In the present study, the serum sodium level, INR, peak SCr level > 1.5 mg/dL, the presence of diabetes and HE were considered potential risk factors for death in cirrhotic patients with AKI. Notably, the CTP,MELD and MELD-Na scores were excluded from the multivariate Cox regression analysis because of the increased probability of multicollinearity. An increase in INR is one of the manifestations of synthesis dysfunction in the liver. Similarly, HE is also an important feature of liver failure. Once HE occurs in patients with chronic liver disease, the prognosis is very poor, with a 1-year survival rate of less than 50% and a 3-year survival rate of less than 25%[46]. Studies have shown that INR is associated with the death of patients with cirrhosis[46,47]. Moreover, a serum sodium imbalance is very common in cirrhotic patients with AKI and affect the prognosis of these patients[48-50]. Furthermore, the SCr level represents the status of renal function. Patients with a peak SCr > 1.5 mg/dL had a higher shortterm mortality rate than those with a peak SCr ≤ 1.5 mg/dL[51,52]. A study showed that a SCr > 1.5 mg/dL was an independent predictor of mortality in cirrhotic patients with AKI[41]. In addition,diabetes was also considered an independent risk factor for mortality in cirrhotic patients with AKI in this study. Although the mechanisms underlying the relationship between diabetes and mortality in cirrhotic patients with AKI remain unclear, several studies have documented that the presence of diabetes and poorly controlled blood glucose levels are associated with the prognosis of patients with cirrhosis[53-55]. Diabetes is an independent risk factor for the development of AKI or acute kidney disease[56,57]. Consequently, we postulate that the presence of diabetes is associated with the prognosis of cirrhotic patients with AKI. Overall, the recovery of liver and kidney function, correction of electrolyte imbalances, and monitoring and control of blood glucose levels in patients with diabetes may improve the poor prognosis of cirrhotic patients with AKI.
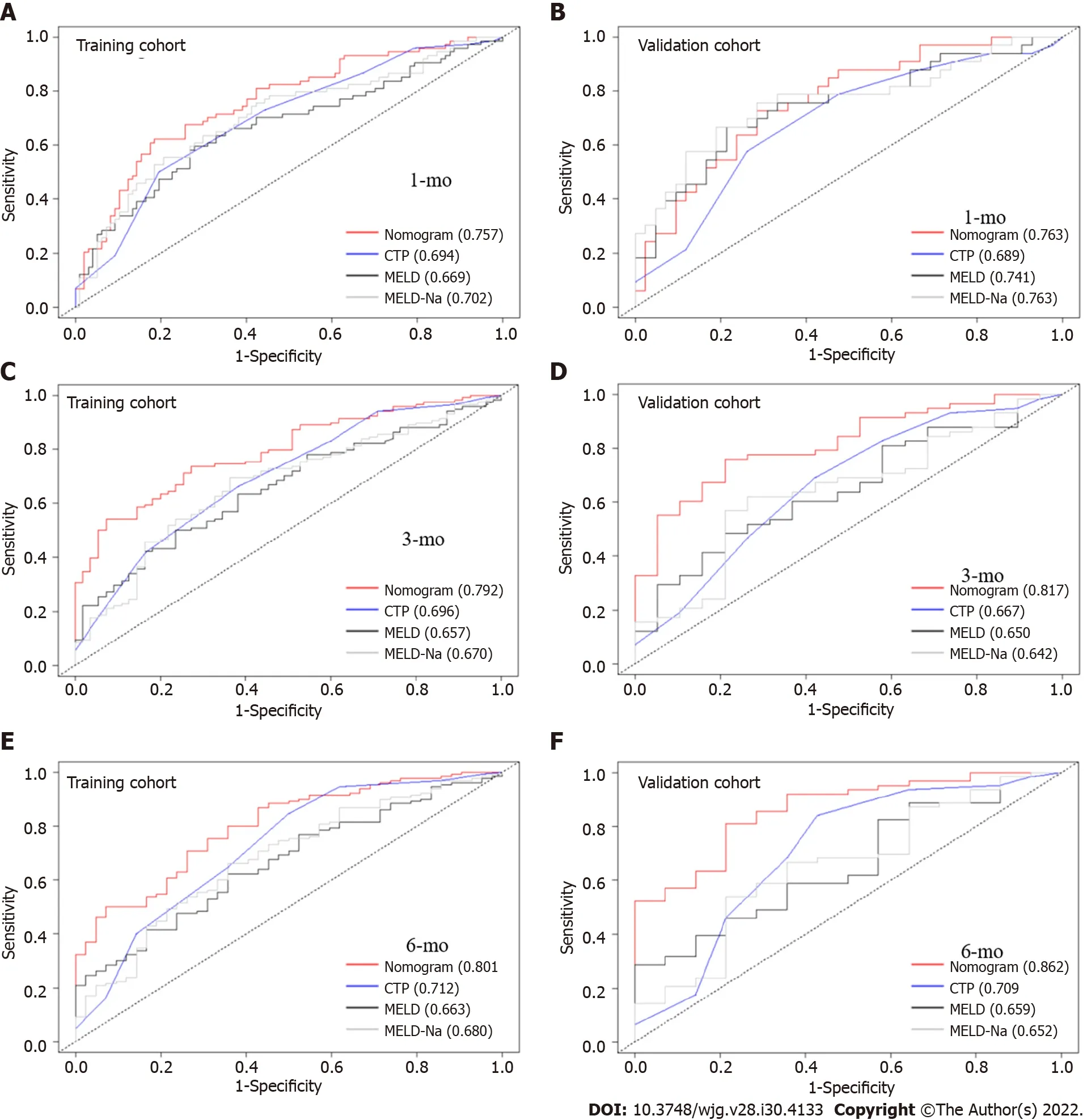
Figure 6 Comparison of the performance of the nomogram and the other scores in predicting mortality of cirrhotic patients with acute kidney injury. A: The area under the receiver operating characteristic curve (AUROC) of the nomogram and the Child-Turcotte-Pugh (CTP), model for end-stage liver disease (MELD) and MELD-Na scores in predicting 30-d mortality in the training cohort; B: The AUROCs of the nomogram and the CTP, MELD and MELD-Na scores in predicting 30-d mortality in the validation cohort; C: The AUROCs of the nomogram and the CTP, MELD and MELD-Na scores in predicting 90-d mortality in the training cohort; D: The AUROCs of the nomogram and the CTP, MELD and MELD-Na scores in predicting 90-d mortality in the validation cohort; E: The AUROCs of the nomogram and the CTP, MELD and MELD-Na scores in predicting 180-d mortality in the training cohort; F: The AUROCs of the nomogram and the CTP,MELD and MELD-Na scores in predicting 180-d mortality in the validation cohort. CTP: Child-Turcotte-Pugh; MELD: Model for end-stage liver disease.
Currently, many studies have investigated the risk factors for the development of AKI in patients with numerous acute and chronic diseases, or have established a model for the development of AKI[20,58-60]. However, studies focused on predicting the prognosis of cirrhotic patients with AKI are scarce.According to Kumaret al[41], the AUROCs of CTP and MELD scores were 0.82 and 0.84, respectively,for predicting 30-d mortality in cirrhotic patients with AKI who met the ICA classification. Fanget al[45]reported that CTP and MELD scores had AUROCs of 0.61 and 0.757, respectively, for predicting inhospital mortality of cirrhotic patients with AKI. Moreover, another prospective study conducted by Panet al[40] indicated that CTP and MELD scores had AUROCs of 0.622 and 0.776, respectively, for predicting the in-hospital mortality of cirrhotic patients with AKI who met the RIFLE classification. In the present study, the AUROCs of CTP and MELD scores were 0.694 and 0.669, respectively, for predicting 30-d mortality of cirrhotic patients with AKI in the training cohort, respectively. The discriminative ability of CTP and MELD scores differed among studies, which may be explained by the analysis of different patients. Patients with cirrhosis who were diagnosed with AKI in the study by Kumaret al[41] only met the ICA criteria, and those patients in the studies by Fanget al[45] and Panet al[40] only met the RIFLE criteria, while the study population in our cohort met the criteria for at least one of the main four classifications. In addition, Fanget al[45] developed an MBRS (MAP + bilirubin + respiratory failure + sepsis) score (AUROC = 0.898) (including MAP, serum bilirubin level and presence of acute respiratory failure and sepsis) in 2008, a predictive model for cirrhotic patients with AKI, that had better discriminative ability than the CTP and MELD scores. Another prospective study by their team published in 2012 further validated the discrimination and calibration of the MBRS score[40]. The AUROCs and goodness-of-fit of the MBRS were 0.863 and 1.160, respectively, which were superior to the CTP and MELD scores in terms of discriminative ability[40]. Notably, the development of the MBRS score was based on patients admitted to the ICU who may suffer from multiple organ failures and comorbidities, but the authors did not clearly determine whether the MBRS score was applicable to patients admitted to regular wards, which requires further validation in the future. Moreover, some clinical data incorporated in the MBRS score, such as the fraction of inspired oxygen, are difficult to obtain in the regular ward and have a complex calculation, which may result in the score not being widely used in clinical practice.
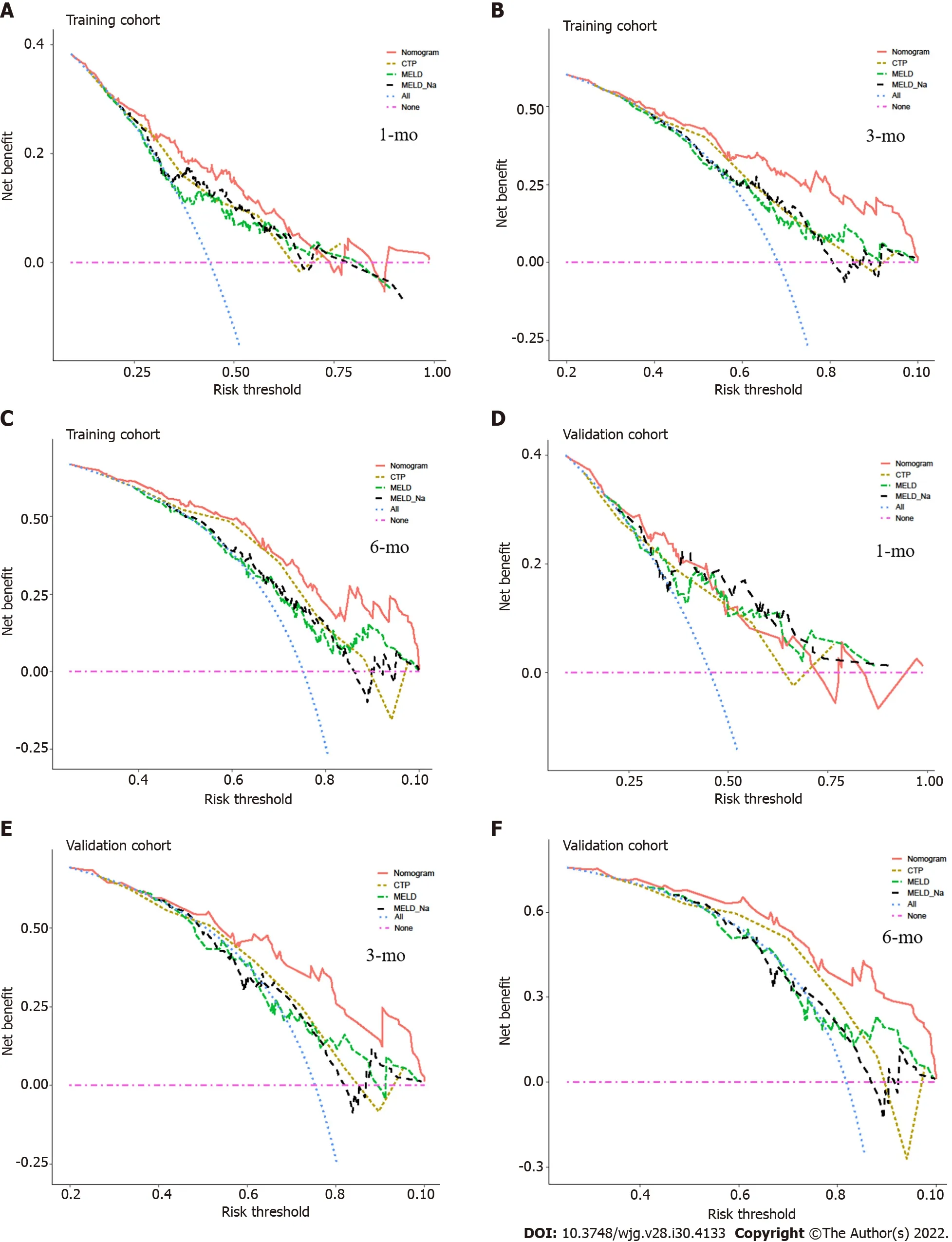
Figure 7 Comparison of the decision curve analysis curve of medical interventions in patients based on the nomogram and the other scores. A: The decision curve analysis (DCA) curve of medical interventions in patients from the training cohort based on the nomogram, Child-Turcotte-Pugh(CTP), model for end-stage liver disease (MELD) and MELD-Na scores at 30 d; B: The DCA curve of medical interventions in patients from the training cohort based on the nomogram, CTP, MELD and MELD-Na scores at 90 d; C: The DCA curve of medical interventions in patients from the training cohort based on the nomogram,CTP, MELD and MELD-Na scores at 180 d; D: The DCA curve of medical interventions in patients from the validation cohort based on the nomogram, CTP, MELD and MELD-Na scores at 30 d; E: The DCA curve of medical interventions in patients from the validation cohort based on the nomogram, CTP, MELD and MELD-Na scores at 90 d; F: The DCA curve of medical intervention in patients from the validation cohort based on the nomogram, CTP, MELD and MELD-Na scores at 180 d.CTP: Child-Turcotte-Pugh; MELD: Model for end-stage liver disease.
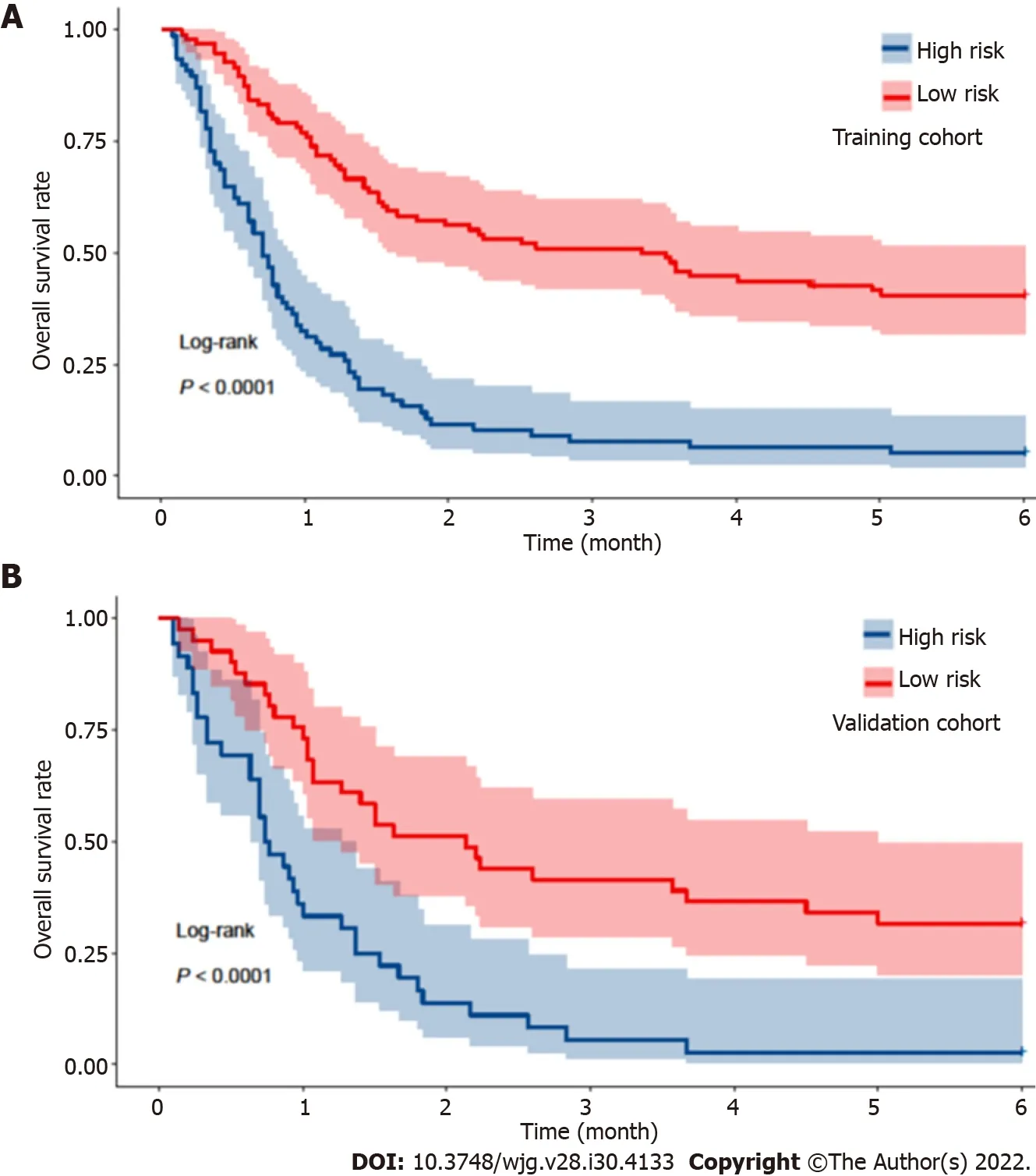
Figure 8 Kaplan-Meier analysis of the cirrhotic patients with acute kidney injury stratified into high-risk and low-risk mortality groups according to the nomogram score, with cutoff of 98. A: Overall survival rates were compared between patients with a high and low risk of mortality in the training cohort; B: Overall survival rates were compared between patients with a high and low risk of mortality in the validation cohort.
In the present study, we developed a quantitative and visual nomogram for predicting the overall survival of cirrhotic patients with AKI based on these potential risk factors of mortality. The nomogram could be used to calculate the scores corresponding to each potential risk factor, and the predicted probability corresponding to the sum of the scores represented the risk of death for patients with cirrhosis and AKI. For example, patients with cirrhosis presenting AKI and diabetes had SCr level of 2 mg/dL, INR 2, grade 1 HE and blood sodium level of 130 mmol/L. The total score of the patient was approximately 118 points (SCr level of 2 mg/dL, approximately 11 points; INR 2, approximately 26 points; grade 1 HE, approximately 21 points; diabetes, approximately 22.5 points; and blood sodium level of 130 mmol/L, approximately 37.5 points.), and the probability of the overall survival at 30, 90 and 180 d was approximately 53%, 23% and 15%, respectively. Compared with the CTP, MELD and MELD-Na scores, the nomogram had better discriminative power for mortality at 90 and 180 d in both the training and validation datasets (allP< 0.05). Moreover, with regard to clinical decisions, the DCA curve indicated that the nomogram had more net benefits than the CTP, MELD and MELD-Na scores for predicting mortality at 90 and 180 d in both the training and validation datasets. However, the AUROC and DCA curves were not significantly different between the nomogram and the CTP, MELD and MELD-Na scores for predicting mortality at 30 d in both the training and validation datasets.Overall, the nomogram was also superior to the CTP, MELD and MELD-Na scores in predicting the prognosis of cirrhotic patients with AKI, especially 90- and 180-d mortality. Furthermore, a score for each patient was calculated based on the nomogram and utilized for stratifing patients into two risk groups for mortality. The high-risk group had a significantly higher mortality rate within 180 d than the low-risk group (allP< 0.001) in both the training and validation cohorts. The mortality rates of high-risk patients were as high as 94.8% in the training cohort and 97.2% in the validation cohort. As a result, the nomogram might select patients with a high-risk mortality to allow treatments to be provided as soon as possible, which may improve the prognosis of cirrhotic patients with AKI. In addition, compared with the MBRS score, the five variables included in the nomogram were all convenient and easily accessible in clinical practice. Since the use of nomograms for evaluation in clinical practice can be time-consuming and complicated, our next step will be to develop software that can be embedded in electronic medical systems to guide clinicians in the timely treatment of these patients and reduce patient mortality without increasing the working time and burden of clinicians.
Several limitations of the present study should also be considered. First, this study analyzed a small sample and employed a retrospective design in which selection biases were unavoidable. Second, this investigation was performed at one academic tertiary-care medical center; the results may not be extrapolated to other centers. In addition, a prospective, large-scale and multicenter study will be needed to validate the reliability of the nomogram.
CONCLUSlON
In conclusion, the presence of HE and diabetes, serum sodium level, INR and peak SCr level > 1.5 mg/dL were considered potential risk factors for mortality. A nomogram based on these risk factors was established, and compared with the CTP, MELD and MELD-Na scores, the nomogram exhibited better predictive performance for the overall survival of cirrhotic patients with AKI. According to the score obtained from the nomogram, patients with a high risk of mortality could be selected for suitable individualized treatments, which may improve the prognosis of cirrhotic patients with AKI.
ARTlCLE HlGHLlGHTS

FOOTNOTES
Author contributions:Wan YP, Wang AJ and Zhang W equally contributed to this work; Wang AJ and Zhu X contributed to the concept; Wan YP, Wang AJ and Zhang W designed this study; Wan YP performed the manuscript writing; Zhang W performed data analysis; Zhang H and Peng GH contributed to samples collection and data collection and validation; Zhu X were clinical experts and performed the manuscript revision; all authors read and approved the final version of the manuscript.
Supported bythe National Natural Science Foundation of China, No. 81960120 and No. 82160115.
lnstitutional review board statement:This study was reviewed and approved by the Ethics Committee of The First Affiliated Hospital of Nanchang University (No. AF-SG-04-2.0).
lnformed consent statement:Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent. For full disclosure, the details of the study are published on the home page of The First Affiliated Hospital of Nanchang University.
Conflict-of-interest statement:We have no financial relationships to disclose.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Yi-Peng Wan 0000-0002-8156-4670; An-Jiang Wang 0000-0001-6670-6645; Wang Zhang 0000-0002-8438-6718; Hang Zhang 0000-0003-3205-5455; Gen-Hua Peng 0000-0002-1369-0333; Xuan Zhu 0000-0002-1240-0947.
S-Editor:Yan JP
L-Editor:A
P-Editor:Yu HG
杂志排行
World Journal of Gastroenterology的其它文章
- Role of one-step nucleic acid amplification in colorectal cancer lymph node metastases detection
- Current perspectives on the role of liver transplantation for Langerhans cell histiocytosis: A narrative review
- Gut microbiota, inflammatory bowel disease and colorectal cancer
- Thrombocytopenia in chronic liver disease: Physiopathology and new therapeutic strategies before invasive procedures
- P2X7 receptor blockade decreases inflammation, apoptosis, and enteric neuron loss during Clostridioides difficile toxin A-induced ileitis in mice
- Serological profiling of Crohn’s disease and ulcerative colitis patients reveals anti-microbial antibody signatures
