含苯丙氨酸和1H-茚-1-酮类氯虫苯甲酰胺类似物的合成及杀虫活性
2022-08-10方佳琪郝树林杜晓华
方佳琪, 郝树林, 杜晓华
(浙江工业大学 催化加氢中心,浙江省绿色农药清洁生产技术研究重点实验室,浙江省绿色农药协同创新中心,杭州 310014)
氯虫苯甲酰胺是一种双酰胺类杀虫剂,具有高效、低毒等特点,已得到广泛应用[1-5],但害虫对其已产生严重的抗性[6-10],而对其进行结构改造是延缓抗性发展的有效途径之一[11-13]。天然氨基酸是高效、安全的生物活性分子,笔者通过在氯虫苯甲酰胺中引入不同种类的氨基酸发现,引入带有苯环的氨基酸相比其他不带苯环的氨基酸具有更高的生物活性[14]。此外,茚酮类化合物在自然界中占据重要地位,已被广泛应用于天然产物、医药、生物、材料科学等领域[15-17]。在许多天然产物和药物分子的核心结构中都存在茚酮结构片段,如5-氯-2,3-二氢-1-茚酮是合成新农药茚虫威的重要中间体[18-20],部分茚二酮衍生物具有很好的除草活性[21-22]。基于此,本研究拟将具有不同取代基的苯丙氨酸衍生物引入氯虫苯甲酰胺的分子结构中,并经进一步成环反应,合成了含1H-茚-1-酮的氯虫苯甲酰胺类似物,初步评价了其杀虫活性,并利用分子对接方法评估了目标化合物的作用机制。目标化合物的设计策略见图式1,合成路线见图式2。
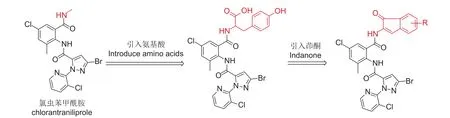
图式1 目标化合物的设计策略Scheme 1 Design strategy of target compounds
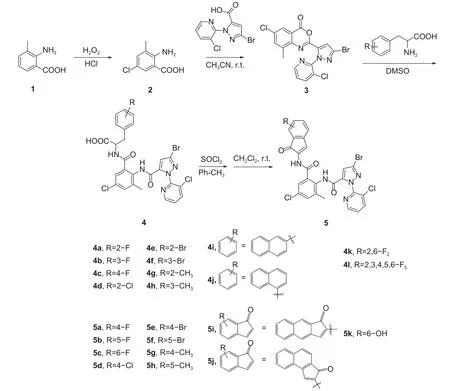
图式2 目标化合物4 和5 的合成路线Scheme 2 Synthetic routes of target compounds 4 and 5
1 实验部分
1.1 仪器与试剂
SM-3 磁力搅拌器,上海志威电器有限公司;DF-101S 集热式恒温加热磁力搅拌器,杭州惠创仪器设备有限公司;RE-52AA 旋转蒸发器,上海亚荣生化仪器厂;SHB-III 循环水式多用真空泵,杭州惠创仪器设备有限公司;AG135 电子分析天平,梅特勒-托利多集团 (Mettler Toledo);1260 Infinity 高效液相色谱仪、6890/5973 HP-5MS 气质联用仪和6545 Q-TOF 高分辨液质联用仪,安捷伦科技有限公司;Trace GC Ultra 气相色谱仪和LCQ Fleet 液质联用仪,赛默飞世尔科技有限公司;B-545 熔点仪,瑞士步琦有限公司;AVANCE III 核磁共振波谱仪 (400、500、600 MHz),布鲁克光谱仪器公司。全部试剂均为市售分析纯化学试剂。
1.2 化合物的合成
1.2.1 中间体5-氯-3-甲基-2-氨基苯甲酸 (2) 的合成 中间体2 参照文献[16]方法合成,结构表征数据与文献一致。收率77%,熔点228~230 ℃(文献[16]收率为80%,熔点229~230 ℃)。
1.2.2 中间体6-氯-2-(3-溴-1-(3-氯吡啶-2-吡啶基)-1H-吡唑-5-基)-8-甲基-4H-3,1-苯并噁嗪-4-酮的合成 (3) 的合成 中间体3 参照文献[16] 方法合成,其结构表征数据与文献一致。收率82%,熔点199~202 ℃(文献[16]收率为90.2%,熔点199~200 ℃)。
1.2.3 目标化合物4a~4l 的合成 向100 mL 三口瓶中加入化合物3 (1 mmol),分别加入不同取代基的苯丙氨酸 (1 mmol),10 mL 二甲基亚砜,升温至60 ℃反应6 h,加入水 (30 mL),搅拌均匀后用浓盐酸调至pH=1.0,析出固体,抽滤得目标化合物4a~4l。
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(2-氟苯基)丙酸 (4a):棕色固体,产率98%,m.p. 126~128 ℃;1H NMR (400 MHz,DMSOd6),δ:12.86 (s,1H),10.14 (s,1H),8.67 (d,J=8.0 Hz,1H),8.44 (d,J= 4.6 Hz,1H),8.10 (d,J= 8.0 Hz,1H),7.55 (dd,J= 8.0,4.7 Hz,1H),7.45 (d,J= 1.8 Hz,1H),7.24 (d,J= 1.9 Hz,1H),7.23 (s,1H),7.19(d,J= 8.1 Hz,1H),7.15 (d,J= 6.1 Hz,1H),7.14~7.03(m,1H),6.99-6.95 (m,1H),4.57~4.51 (m,1H),3.20~3.15 (m,1H),2.93~2.87 (m,1H),2.09 (s,3H);13C NMR (101 MHz,DMSO-d6),δ: 172.80,165.92,161.18(d,1JC-F= 243.8 Hz),155.92,148.83,147.55,139.75,139.72,139.60,135.21,132.19,132.13,132.98(d,3JC-F=4.5 Hz),131.35,129.07(d,3JC-F=8.2 Hz),128.26,127.31,127.03,126.17,124.81 (d,2JC-F=15.3 Hz),124.56 (d,4JC-F= 3.4 Hz),115.41(d,2JC-F= 21.7 Hz),111.24,52.88,30.58,18.21; HRMS:C26H19BrCl2FN5O4[M-H]-,计算值631.990 9,测试值631.988 2.
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(3-氟苯基)丙酸 (4b):黄色固体,产率99%,m.p. 98~99 ℃;1H NMR (500 MHz,DMSOd6),δ:10.19 (s,1H),8.61 (d,J= 7.9 Hz,1H),8.46(dd,J= 4.8,1.5 Hz,1H),8.12 (dd,J= 8.1,1.6 Hz,1H),7.58 (dd,J= 8.1,4.7 Hz,1H),7.50 (d,J= 2.4 Hz,1H),7.28 (s,1H),7.26 (d,J= 2.7 Hz,1H),7.25~7.22 (m,1H),7.21~7.04 (m,1H),7.02 (d,J= 7.7 Hz,1H),7.00~6.96 (m,1H),4.58~4.53 (m,1H),3.14~3.11 (m,2H),2.97~2.93 (m,1H),2.14 (s,3H);13C NMR (126 MHz,DMSO-d6),δ:172.74,165.89,162.75 (d,1JC-F= 244.2 Hz),161.45,155.96,148.81,147.49,139.77,139.67,139.50,137.71,135.23,132.13 (d,3JC-F=11.0 Hz),131.31,130.35 (d,3JC-F= 7.8 Hz),128.24,127.26,126.99,126.13,125.62,116.32 (d,2JC-F=20.9 Hz),113.59 (d,2JC-F= 21.3 Hz),111.20,54.05,36.59,18.19; HRMS:C26H19BrCl2FN5O4[M-H]-,计算值631.990 9,测试值631.990 8.
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(4-氟苯基)丙酸 (4c):棕色固体,产率89%,m.p. 125~127 ℃;1H NMR (400 MHz,DMSOd6),δ:12.86 (s,1H),10.21 (s,1H),8.63 (d,J=7.8 Hz,1H),8.47-8.46 (m,1H),8.13 (d,J= 8.0 Hz,1H),7.58 (dd,J= 8.0,4.7 Hz,1H),7.50 (d,J= 1.6 Hz,1H),7.29 (s,1H),7.27 (d,J= 1.9 Hz,1H),7.22 (dd,J=8.2,5.6 Hz,2H),7.05~7.00 (m,2H),4.55~4.49 (m,1H),3.11~3.06 (m,1H),2.96~2.91 (m,1H),2.14 (s,3H);13C NMR (101 MHz,DMSO-d6),δ:172.93,165.98,161.47 (d,1JC-F= 242.4 Hz),156.03,148.83,147.53,139.76,139.72,139.54,135.35,134.20 (d,4JC-F=3.0 Hz),132.12,131.37,131.30,128.27,127.30,127.04,126.24,115.29(d,2JC-F= 21.1 Hz),111.19,54.30,35.99,18.19; HRMS:C26H19BrCl2FN5O4[M-H]-,计算值631.990 9,测试值631.990 9.
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(2-氯苯基)丙酸 (4d):黄色固体,产率91%,m.p. 99~100 ℃;1H NMR (500 MHz,DMSOd6),δ:10.17 (s,1H),8.71 (d,J= 8.2 Hz,1H),8.49(dd,J= 4.7,1.6 Hz,1H),8.14 (dd,J= 8.1,1.6 Hz,1H),7.60 (dd,J= 8.1,4.8 Hz,1H),7.49 (d,J= 2.4 Hz,1H),7.37 (dd,J= 7.9,1.3 Hz,1H),7.29 (d,J= 2.5 Hz,1H),7.27 (dd,J= 7.6,1.8 Hz,1H),7.24 (s,1H),7.20~7.17 (m,1H),7.14~7.11 (m,1H),4.67~4.62 ( m,1H),3.02~2.97 (m,2H),2.13 (s,3H);13C NMR (126 MHz,DMSO-d6),δ:165.92,158.45,155.92,147.53,144.09,140.80,139.79,139.71,139.62,139.45,135.60,135.20,133.69,132.09,131.88,131.33,129.53,128.82,128.21,127.34,127.00,126.05,111.25,52.32,34.90,18.21; HRMS:C26H19BrCl3N5O4[M-H]-,计算值647.961 3,测试值647.959 3.
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(2-溴苯基)丙酸 (4e):黄色固体,产率83%,m.p. 100~102 ℃;1H NMR (400 MHz,DMSOd6),δ:12.89 (s,1H),10.16 (s,1H),8.76 (d,J= 8.1 Hz,1H),8.49 (dd,J= 4.6,1.1 Hz,1H),8.14 (dd,J=8.0,1.0 Hz,1H),7.60 (dd,J= 8.1,4.7 Hz,1H),7.54(d,J= 7.7 Hz,1H),7.50 (d,J= 1.7 Hz,1H),7.29 (d,J=1.9 Hz,1H),7.26 (d,J= 7.0 Hz,1H),7.23 (s,1H),7.18~7.14 (m,1H),7.11~7.08 (m,1H),4.67~4.65 (m,1H),3.33~3.28 (m,1H),3.04~2.97 (m,1H),2.12 (s,3H);13C NMR (101 MHz,DMSO-d6),δ:172.41,165.51,155.46,148.36,147.14,139.34,139.31,139.25,136.73,134.73,132.42,131.80,131.71,131.50,130.90,128.67,127.77,127.48,126.88,126.60,125.65,124.04,110.84,51.77,36.83,17.79; HRMS:C26H19Br2Cl2N5O4[M-H]-,计算值691.910 8,测试值691.908 1.
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(3-溴苯基)丙酸 (4f):黄色固体,产率87%,m.p. 100~103 ℃;1H NMR (400 MHz,DMSOd6),δ:12.89 (s,1H),10.18 (s,1H),8.66 (d,J= 8.0 Hz,1H),8.47 (dd,J= 4.6,1.1 Hz,1H),8.12(dd,J= 8.0,1.0 Hz,1H),7.58 (dd,J= 8.1,4.7 Hz,1H),7.50 (d,J= 1.8 Hz,1H),7.45 (s,1H),7.36 (d,J= 7.6 Hz,1H),7.29 (s,1H),7.26 (d,J= 1.9 Hz,1H),7.21~7.14 (m,2H),4.58~4.52 (m,1H),3.14~3.09(m,1H),2.95~2.89 (m,1H),2.14 (s,3H);13C NMR(101 MHz,DMSO-d6),δ:172.75,165.91,155.92,148.83,147.53,140.96,139.76,139.70,139.53,135.19,132.41,132.19,132.14,131.33,130.71,129.73,128.64,128.26,127.32,127.03,126.18,121.85,111.20,53.99,36.45,18.21; HRMS:C26H19Br2Cl2N5O4[M-H]-,计算值691.910 8,测试值691.910 7.
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(邻甲苯基) 丙酸 (4g):黄色固体,产率92%,m.p. 90~91 ℃;1H NMR (400 MHz,DMSO-d6),δ:12.82 (s,1H),10.18 (s,1H),8.72 (d,J= 8.0 Hz,1H),8.48 (dd,J= 4.6,1.1 Hz,1H),8.20~8.09 (m,1H),7.59 (dd,J= 8.1,4.7 Hz,1H),7.49 (d,J= 1.7 Hz,1H),7.28 (d,J= 1.9 Hz,1H),7.26 (s,1H),7.11 (d,J= 7.6 Hz,2H),7.08~7.01 (m,1H),6.99~6.97 (m,1H),4.57~4.51 (m,1H),3.16~3.11 (m,1H),2.92~2.86 (m,1H),2.27 (s,3H),2.13 (s,3H);13C NMR(101 MHz,DMSO-d6),δ:172.85,165.54,155.52,148.38,147.12,139.34,139.29,139.15,136.06,135.81,134.85,131.75,131.65,130.90,130.01,129.42,127.80,126.87,126.59,126.52,125.77,125.63,110.81,52.44,34.13,18.95,17.78; HRMS:C27H22BrCl2N5O4[M-H]-,计算值628.015 9,测试值628.015 9.
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(间甲苯基) 丙酸 (4h):黄色固体,产率95%,m.p. 96~97 ℃;1H NMR (400 MHz,DMSO-d6),δ:12.82 (s,1H),10.19 (s,1H),8.61 (d,J= 7.9 Hz,1H),8.48-8.46 (m,1H),8.13 (d,J= 8.0 Hz,1H),7.59 (dd,J= 8.1,4.7 Hz,1H),7.49 (d,J=1.8 Hz,1H),7.26 (s,1H),7.25 (d,J= 2.1 Hz,1H),7.11~7.08 (m,1H),7.03 (s,1H),6.99~6.97 (m,2H),4.54~4.48 (m,1H),3.09~3.04 (m,1H),2.91~2.86 (m,1H),2.23 (s,3H),2.13 (s,3H);13C NMR (101 MHz,DMSO-d6),δ:172.61,165.44,155.53,148.35,147.10,139.35,139.29,139.13,137.52,137.11,134.84,131.76,131.66,130.87,129.84,128.10,127.79,127.08,126.91,126.72,126.07,125.83,110.78,53.92,36.46,21.02,17.77; HRMS:C27H22BrCl2N5O4[M-H]-,计算值628.015 9,测试值628.016 0.
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(萘-2-基)丙酸 (4i):黄色固体,产率97%,m.p. 216~217 ℃;1H NMR (500 MHz,DMSOd6),δ:10.50 (s,1H),8.45 (dd,J= 4.7,1.6 Hz,1H),8.08 (dd,J= 8.1,1.5 Hz,2H),7.82~7.77 (m,1H),7.69(dd,J= 8.7,3.7 Hz,2H),7.65 (s,1H),7.56 (dd,J=8.1,4.7 Hz,1H),7.43 (d,J= 2.4 Hz,1H),7.42 (d,J=3.8 Hz,1H),7.40 (d,J= 1.1 Hz,1H),7.40 (s,1H),7.38 (d,J= 1.7 Hz,1H),7.30 (d,J= 2.4 Hz,1H),4.06~4.02 (m,1H),3.39~3.35 (m,2H),3.18~3.14 (m,1H),2.13 (s,3H);13C NMR (126 MHz,DMSO-d6),δ:165.32,156.12,148.88,147.50,139.69,139.34,137.20,135.88,133.37,132.15,131.82,131.29,128.58,128.25,127.83,127.79,127.73,127.60,127.32,126.97,126.14,125.94,125.56,111.39,60.21,56.12,37.94,21.22,18.30,14.56; HRMS:C30H22BrCl2N5O4[M-H]-,计算值664.015 9,测试值664.014 8.
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(萘-1-基)丙酸 (4j):黄色固体,产率99%,m.p. 214~215 ℃;1H NMR (500 MHz,DMSO
d6),δ:10.52 (s,1H),8.47 (d,J= 4.7 Hz,1H),8.29~8.27 (m,2H),8.14~8.12 (m,1H),7.94~7.77 (m,2H),7.66 (d,J= 8.2 Hz,1H),7.60~7.57 (m,1H),7.50~7.48 (m,2H),7.41 (d,J= 2.3 Hz,1H),7.35 (d,J= 7.0 Hz,1H),7.30 (d,J= 2.8 Hz,2H),7.26~7.23(m,1H),4.06~4.02 (m,1H),3.80~3.77 (m,1H),3.27~3.22 (m,1H),2.10 (s,3H);13C NMR (126 MHz,DMSO-d6),δ:165.51,155.95,148.96,147.50,139.68,139.20,135.84,133.79,132.32,131.69,131.17,129.70,129.26,128.95,128.29,127.39,127.33,126.97,126.87,126.16,125.95,125.73,124.37,111.38,60.21,55.94,35.76,21.22,18.36,14.56;HRMS:C30H22BrCl2N5O4[M-H]-,计算值664.015 9,测试值664.015 8.
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(2,6-二氟苯基)丙酸 (4k):棕色固体,产率73%,m.p. 120~122 ℃;1H NMR (400 MHz,DMSO-d6),δ:12.90 (s,1H),10.17 (s,1H),8.79 (d,J= 8.0 Hz,1H),8.48 (d,J= 4.5 Hz,1H),8.14 (d,J=8.0 Hz,1H),7.60 (dd,J= 8.0,4.7 Hz,1H),7.50 (s,1H),7.32 (s,1H),7.29 (s,1H),7.27~7.22 (m,1H),7.02~6.98 (m,2H),4.60~4.54 (m,1H),3.21~3.16 (m,1H),3.03~2.98 (m,1H),2.13 (s,3H);13C NMR (101 MHz,DMSO-d6),δ:172.38,165.78,162.91,161.65(d,1JC-F= 237.5 Hz),160.38,155.86,148.84,147.53,139.70,139.59,135.11,132.18 (d,4JC-F= 3.4 Hz),131.36,129.51,128.29,127.30,127.04,126.19,113.37(d,2JC-F= 20.1 Hz),111.65(d,2JC-F= 25.1 Hz),111.21,52.17,24.82,18.19; HRMS:C26H18BrCl2F2N5O4[M-H]-,计算值649.981 5,测试值649.981 5.
2-(2-(3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺基)-5-氯-3-甲基苯甲酰胺基)-3-(全氟苯基)丙酸 (4l):棕色固体,产率71%,m.p. 119~121 ℃;1H NMR (400 MHz,DMSO-d6),δ:13.12 (s,1H),10.20 (s,1H),8.81 (d,J= 8.1 Hz,1H),8.46 (dd,J= 4.8,1.5 Hz,1H),8.14 (dd,J= 8.1,1.5 Hz,1H),7.60 (dd,J= 8.1,4.7 Hz,1H),7.52 (d,J= 2.4 Hz,1H),7.32 (d,J= 2.4 Hz,1H),7.27 (s,1H),4.63~4.58(m,1H),3.27~3.22 (m,1H),3.11~3.05 (m,1H),2.15(s,3H);13C NMR (101 MHz,DMSO-d6),δ:171.89,165.90,156.00,148.75,147.48,139.77,139.73,139.68,135.08,132.25,132.19,131.42,128.17,127.28,127.00,126.08,123.44,117.11,112.86,112.05,111.08,51.61,24.93,18.15; HRMS:C26H15BrCl2F5N5O4[M-H]-,计算值703.953 2,测试值703.953 2.
1.2.4 目标化合物5a~5k 的合成 向100 mL 三口瓶中加入化合物4 (1 mmol)、15 mL 甲苯和0.6 g氯化亚砜 (5 mmol),升温回流5 h,待反应结束后,旋转蒸发得到的酰氯直接用于下一步;将0.2 g 无水氯化铝 (1.5 mmol) 和15 mL 二氯甲烷加入100 mL 三口瓶中,在0 ℃下搅拌30 min,再加入所得酰氯,放置室温反应6 h,加入20 mL 水淬灭反应,用二氯甲烷萃取 (30 mL × 3),再用饱和氯化钠溶液洗涤 (30 mL × 3),无水硫酸镁干燥,抽滤,滤液脱除溶剂,再经过硅胶柱层析 (石油醚、乙酸乙酯的体积比为1 : 1-1 : 100) 分离纯化后得到目标化合物5a~5k。
3-溴-N-(4-氯-2-((4-氟-1-氧代-1H-茚-2-基)氨基甲酰基)-6-甲基苯基)-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺(5a):棕色固体,产率43%,m.p. 255~257 ℃;1H NMR(600 MHz,DMSO-d6),δ:8.24~8.22 (m,2H),8.13 (s,1H),7.90 (d,J= 2.4 Hz,1H),7.74 (d,J= 1.8 Hz,1H),7.55 (dd,J= 8.1,4.7 Hz,1H),7.48~7.44 (m,2H),7.28~7.25 (m,1H),7.22~7.18 (m,1H),7.16~7.12(m,1H),6.80 (s,1H),1.89 (s,3H);13C NMR (151 MHz,DMSO-d6),δ:182.15,174.76,164.69,161.47,159.86 (d,3JC-F=13.3 Hz),148.76,147.45,143.51,142.97,140.41,139.57 (d,2JC-F=23.5 Hz),139.16,137.00 (d,1JC-F= 244.4 Hz),132.94,129.74,128.41,127.29,126.78,125.65 (d,4JC-F= 3.0 Hz),123.66,121.31,120.07 (d,3JC-F=12.7 Hz),116.61 (d,2JC-F=21.7 Hz),112.30,99.99,16.22; HRMS:C26H15BrCl2FN5O3[M-H]-,计算值611.964 7,测试值611.962.
3-溴-N-(4-氯-2-((5-氟-1-氧代-1H-茚-2-基)氨基甲酰基)-6-甲基苯基)-1-(3-氯吡啶-2-基) -1H-吡唑-5-甲酰胺(5b):棕色固体,产率53%,m.p. 262~263 ℃;1H NMR(500 MHz,DMSO-d6),δ:8.31~8.20 (m,2H),7.92 (s,1H),7.86 (d,J= 2.5 Hz,1H),7.68 (d,J= 2.5 Hz,1H),7.58 (dd,J= 8.0,4.8 Hz,1H),7.54~7.17 (m,2H),7.16~7.10 (m,2H),7.09~6.95 (m,2H),1.88 (s,3H);13C NMR (126 MHz,DMSO-d6),δ:166.79,162.62(d,1JC-F= 243.3 Hz),159.82,149.15,147.16,144.16,143.78,140.44,138.85,138.55,135.80 (d,3JC-F=8.0 Hz),135.56,134.10,133.01,132.35,131.12 (d,3JC-F=8.4 Hz),128.61,127.05,126.70,126.04,123.41,121.68,116.46(d,2JC-F=21.2 Hz),115.54 (d,2JC-F=22.2 Hz),112.84,16.16; HRMS:C26H15BrCl2FN5O3[M-H]-,计算值611.964 7,测试值611.964 6.
3-溴-N-(4-氯-2-((6-氟-1-氧代-1H-茚-2-基)氨基甲酰基)-6-甲基苯基)-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺(5c):黄色固体,产率45%,m.p. 230~232 ℃;1H NMR(600 MHz,DMSO-d6),δ:10.56 (s,1H),8.48 (dd,J=4.7,1.5 Hz,1H),8.24~8.19 (m,2H),8.15 (dd,J=8.1,1.5 Hz,1H),7.85 (d,J= 2.4 Hz,1H),7.76 (d,J=2.4 Hz,1H),7.60 (dd,J= 8.1,4.7 Hz,1H),7.47 (d,J=1.4 Hz,2H),7.22 (t,J= 8.9 Hz,2H),2.26 (s,3H);13C NMR (151 MHz,DMSO-d6),δ:166.66,164.18(d,1JC-F=252.5 Hz),161.30,156.09,148.96,147.61,140.75,139.69,139.36,135.54(d,3JC-F= 8.8 Hz),134.95,133.80,132.55,132.54,132.25,131.79,130.01 (d,4JC-F= 3.0 Hz),128.41,127.67,127.46,127.30,127.14,124.74,116.42(d,2JC-F=21.9 Hz),111.34,18.18; HRMS:C26H15BrCl2FN5O3[M-H]-,计算值611.964 7,测试值611.964 7.
3-溴-N-(4-氯-2-((4-氯-1-氧代-1H-茚-2-基)氨基甲酰基)-6-甲基苯基)-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺(5d):棕黄色固体,产率58%,m.p. 271~272 ℃;1H NMR(500 MHz,DMSO-d6),δ:8.13 (dd,J= 8.1,1.5 Hz,1H),8.08 (dd,J= 4.7,1.6 Hz,1H),8.00 (s,1H),7.91(dd,J= 2.6,0.7 Hz,1H),7.65 (dd,J= 2.5,1.0 Hz,1H),7.48 (dd,J= 8.0,4.7 Hz,1H),7.39 (dd,J= 8.0,1.2 Hz,1H),7.31~7.28 (m,2H),7.20~7.17 (m,1H),7.13 (dd,J= 7.8,1.7 Hz,1H),7.01 (s,1H),1.99 (s,3H);13C NMR (126 MHz,DMSO-d6),δ:170.76,166.61,160.76,149.03,147.22,143.84,143.70,140.05,138.77,138.07,135.80,135.48,133.72,132.30,132.22,130.77,130.06,129.11,128.11,127.76,126.89,126.35,123.39,121.49,112.95,16.04;HRMS:C26H15BrCl3N5O3[M-H]-;,计算值627.935 1,测试值627.932 7.
3-溴-N-(4-氯-2-((4-溴-1-氧代-1H-茚-2-基)氨基甲酰基)-6-甲基苯基)-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺(5e):棕黄色固体,产率52%,m.p. 246~248 ℃;1H NMR(500 MHz,DMSO-d6),δ:8.11 (dd,J= 8.1,1.5 Hz,1H),8.06 (dd,J= 4.7,1.6 Hz,1H),7.94 (s,1H),7.93(d,J= 2.6 Hz,1H),7.65 (dd,J= 2.6,1.0 Hz,1H),7.59~7.54 (m,1H),7.47 (dd,J= 8.1,4.7 Hz,1H),7.26~7.19 (m,2H),7.13~7.08 (m,1H),6.96 (s,1H),5.75 (s,1H),1.76 (s,3H);13C NMR (126 MHz,DMSOd6),δ:160.85,148.96,147.21,143.66,140.03,138.81,137.88,135.58,133.75,133.27,132.39,131.13,129.24,128.32,128.07,127.09,126.32,124.25,123.45,121.40,112.95,60.21,55.35,21.22,16.05,14.55; HRMS:C26H15Br2Cl2N5O3[M-H]-,计算值671.884 6,测试值671.882 7.
3-溴-N-(4-氯-2-((5-溴-1-氧代-1H-茚-2-基)氨基甲酰基)-6-甲基苯基)-1-(3-氯吡啶-2-基)-1H-吡唑-5-甲酰胺(5f):棕黄色固体,产率50%,m.p. 229~231 ℃;1H NMR(600 MHz,DMSO-d6),δ:8.29 (dd,J= 4.7,1.5 Hz,1H),8.24 (dd,J= 8.1,1.5 Hz,1H),8.15 (s,1H),7.93(d,J= 2.5 Hz,1H),7.79~7.74 (m,1H),7.59~7.62 (m,1H),7.57 (dd,J= 8.1,4.6 Hz,1H),7.51 (d,J= 2.1 Hz,1H),7.33~7.26 (m,2H),6.74 (s,1H),5.76 (s,1H),1.92 (s,3H);13C NMR (151 MHz,DMSO-d6),δ:164.91,159.96,148.72,147.54,143.53,142.83,140.46,139.64,139.27,137.67,136.37,134.15,134.01,133.12,133.05,131.73,128.62,128.54,127.40,127.03,126.87,123.69,122.73,121.24,112.19,16.27; HRMS:C26H15Br2Cl2N5O3[M-H]-,计算值671.884 6,测试值671.884 7.
3-溴-N-(4-氯-2-甲基-6-((4-甲基-1-氧代-1H-茚-2-基)氨基甲酰基) 苯基)-1-(3-氯吡啶-2-基 )-1H-吡唑-5-甲酰胺(5g):黄色固体,产率44%,m.p. 212~214 ℃;1H NMR(500 MHz,DMSO-d6),δ:10.57 (s,1H),8.47 (dd,J=4.7,1.6 Hz,1H),8.43 (dd,J= 7.9,1.3 Hz,1H),8.15(dd,J= 8.0,1.6 Hz,1H),7.85 (d,J= 2.5 Hz,1H),7.76 (d,J= 2.5 Hz,1H),7.59 (dd,J= 8.1,4.7 Hz,1H),7.50 (s,1H),7.46 (s,1H),7.40~7.37 (m,1H),7.32 (d,J= 7.6 Hz,1H),7.14-7.11 (m,1H),2.50 (s,3H),2.26 (s,3H);13C NMR (126 MHz,DMSO-d6),δ:166.78,161.59,156.05,148.96,147.55,140.65,140.39,139.66,139.37,134.95,133.83,132.93,132.32,132.19,131.96,131.60,131.15,129.51,128.41,127.65,127.44,127.09,126.73,124.76,111.36,19.93,18.20; HRMS:C27H18BrCl2N5O3[M-H]-,计算值607.989 7,测试值607.989 5.
3-溴-N-(4-氯-2-甲基-6-((5-甲基-1-氧代-1H-茚-2-基)氨基甲酰基) 苯基)-1-(3-氯吡啶-2-基 )-1H-吡唑-5-甲酰胺(5h):黄色固体,产率49%,m.p. 218~219 ℃;1H NMR(500 MHz,DMSO-d6),δ:10.59 (s,1H),8.46 (d,J=4.6 Hz,1H),8.14 (d,J= 8.0 Hz,1H),8.05 (d,J= 7.6 Hz,1H),7.89 (s,1H),7.86~7.79 (m,1H),7.79~7.68 (m,1H),7.58 (dd,J= 8.2,4.7 Hz,1H),7.51~7.41 (m,1H),7.38 (d,J= 3.5 Hz,1H),7.33 (d,J= 7.2 Hz,1H),7.28 (d,J= 7.3 Hz,1H),2.34 (s,3H),2.27 (s,3H);13C NMR (126 MHz,DMSO-d6),δ:166.83,161.29,156.11,148.88,147.52,140.57,139.68,139.48,138.59,134.86,133.68,133.26,133.23,132.89,132.72,132.17,130.01,129.19,128.33,127.66,127.38,127.07,125.07,111.28,99.99,21.31,18.21; HRMS:C27H18BrCl2N5O3[M-H]-,计算值607.989 7,测试值607.989 0.
3-溴-N-(4-氯-2-甲基-6-((1-氧代-1H-环戊[b] 萘-2-基)氨基甲酰基)苯基)-1-(3-氯吡啶-2-基 )-1H-吡唑-5-甲酰胺 (5i):棕色固体,产率45%,m.p. 205~207 ℃;1H NMR(400 MHz,DMSO-d6),δ:8.27 (s,1H),8.04 (s,1H),7.86 (s,1H),7.79 (d,J= 7.5 Hz,1H),7.73 (d,J=6.5 Hz,2H),7.62 (s,2H),7.52~7.48 (m,2H),7.43 (s,2H),7.28 (s,1H),7.22~7.18 (m,1H),6.65 (s,1H),1.99 (s,3H);13C NMR (126 MHz,DMSO-d6),δ:165.66,160.05,148.93,147.25,143.66,143.48,140.38,139.12,138.06,136.07,133.99,133.09,132.83,129.94,129.17,129.11,128.65,128.29,127.98,127.30,127.23,126.76,125.57,123.68,121.51,112.34,60.21,21.23,16.27,14.55; HRMS:C30H18BrCl2N5O3[M-H]-,计算值643.989 7,测试值643.987 7.
3-溴-N-(4-氯-2-甲基-6-((3-氧代-3H-环戊[a] 萘-2-基)氨基甲酰基)苯基)-1-(3-氯吡啶-2-基 )-1H-吡唑-5-甲酰胺 (5j):棕色固体,产率51%,m.p. 215~216 ℃;1H NMR(400 MHz,DMSO-d6),δ:8.50 (s,1H),8.35 (s,1H),8.09 (dd,J= 4.7,1.4 Hz,1H),7.99 (dd,J= 8.1,1.5 Hz,1H),7.87 (d,J= 2.4 Hz,1H),7.86~7.75 (m,2H),7.72~7.65 (m,1H),7.61 (d,J= 1.7 Hz,1H),7.50~7.42 (m,2H),7.41~7.30 (m,2H),7.35~7.29 (m,1H),7.25 (d,J= 7.1 Hz,1H),7.11 (s,1H),1.67 (s,3H);13C NMR (126 MHz,DMSO-d6),δ:165.66,160.05,148.93,147.25,143.66,143.48,140.38,139.12,138.06,136.07,133.99,133.09,132.83,129.94,129.17,129.11,128.65,128.29,127.98,127.30,127.23,126.76,125.57,123.68,121.51,112.34,60.21,21.23,16.27,14.55; HRMS:C30H18BrCl2N5O3[M-H]-,计算值643.989 7,测试值643.989 5.
3-溴-N-(4-氯-2-((6-羟基-1-氧代-1H-茚-2-基)氨基甲酰基)-6-甲基苯基)-1-(3-氯吡啶-2-基) -1H-吡唑-5-甲酰胺(5k):黄色固体,产率44%,m.p. 209~211 ℃;1H NMR(400 MHz,DMSO-d6),δ:10.56 (s,1H),10.54 (s,1H),8.48 (dd,J= 4.7,1.5 Hz,1H),8.15 (dd,J= 8.1,1.5 Hz,1H),8.05 (d,J= 8.8 Hz,2H),7.84 (d,J= 2.4 Hz,1H),7.72 (d,J= 2.2 Hz,1H),7.59 (dd,J= 8.1,4.7 Hz,1H),7.50 (s,1H),7.34 (s,1H),6.81 (d,J= 8.8 Hz,2H),2.25 (s,3H);13C NMR (126 MHz,DMSOd6),δ:167.10,161.96,159.47,156.05,148.96,147.55,140.61,139.65,139.40,135.82,134.45,134.10,133.48,132.20,129.31,128.41,127.47,127.41,127.09,125.10,124.66,116.62,111.30,18.20; HRMS:C26H16BrCl2N5O4[M-H]-,计算值609.969 0,测试值609.969 1.
1.3 杀虫活性测定
将供试化合物用含体积分数0.1%吐温80 的DMF 溶液溶解,配制成质量分数为1.0% 的母液,然后用蒸馏水稀释至100、20、4 和0.8 mg/L,备用。
采用浸叶法[23]测定,供试靶标为黏虫Mythinma separata。将适量玉米叶在配制好的药液中浸润后取出,自然阴干,放入垫有滤纸的培养皿中。接黏虫3 龄中期幼虫,10 头/皿,重复4 次。置于24~27 ℃观察室内培养,2 d 后调查结果。以毛笔触动虫体,无反应则视为死虫。运用DPS 数据处理系统计算死亡率,并校正死亡率。
1.4 分子对接
采用AutoDock 4.2 软件的Lamarckian 遗传算法分别对氯虫苯甲酰胺和5k 与小菜蛾鱼尼汀受体(RyR)N端结构域 (NTD) 的晶体结构 (PDB 代码:5Y9V)[24]进行了分子对接。
2 结果与分析
2.1 目标化合物的合成
本研究先以2-甲基-3-氨基苯甲酸为起始原料,经过卤化生成5-氯-3-甲基-2-氨基苯甲酸;再与3-溴-1-(3-氯吡啶-2-吡啶基)-1H-吡唑-5-甲酸进行缩合反应,生成6-氯-2-(3-溴-1-(3-氯吡啶-2-吡啶基)-1H-吡唑-5-基)-8-甲基-4H-3,1-苯并噁嗪-4-酮(3);再引入苯丙氨酸片段合成目标化合物4;最后通过环化反应合成目标化合物5。在合成目标化合物4 时,对反应条件进行了优化。结果表明:在进行缩合反应时,发现使用二甲基亚砜作为溶剂反应效果最好,当温度为60 ℃时产率最高,其中4f 产率最高(95%)。在环化反应中,如图式3所示,用氯化亚砜作为酰化试剂,化合物4 生成酰氯后经过烯醇化,亚硫酰氯从空间位阻较小的一侧进行Hell-Volhard-Zelinsky 型取代反应,然后HCl 和一氧化硫与二氧化硫和单质硫处于平衡状态,通过协同消除得到脱氢酰氯,最后由分子内傅克酰基化反应生成目标化合物5[25-26]。

图式3 成环反应过程Scheme 3 Ring-forming reaction process
2.2 杀虫活性及其构效关系分析
杀虫活性测定结果(表1)表明,大部分目标化合物在100 mg/L 下对黏虫的致死率为100%。对活性较高的化合物进行低浓度筛选,当质量浓度降至20 mg/L 时,化合物4a、5i、5k 的致死率仍为100%;当浓度降至4 和0.8 mg/L 时,化合物5k 对黏虫的致死率分别为90%和70%。部分化合物的LC50值见表2。化合物4a、5i、5k 的LC50值分别为3.80、2.28 和0.55 mg/L.
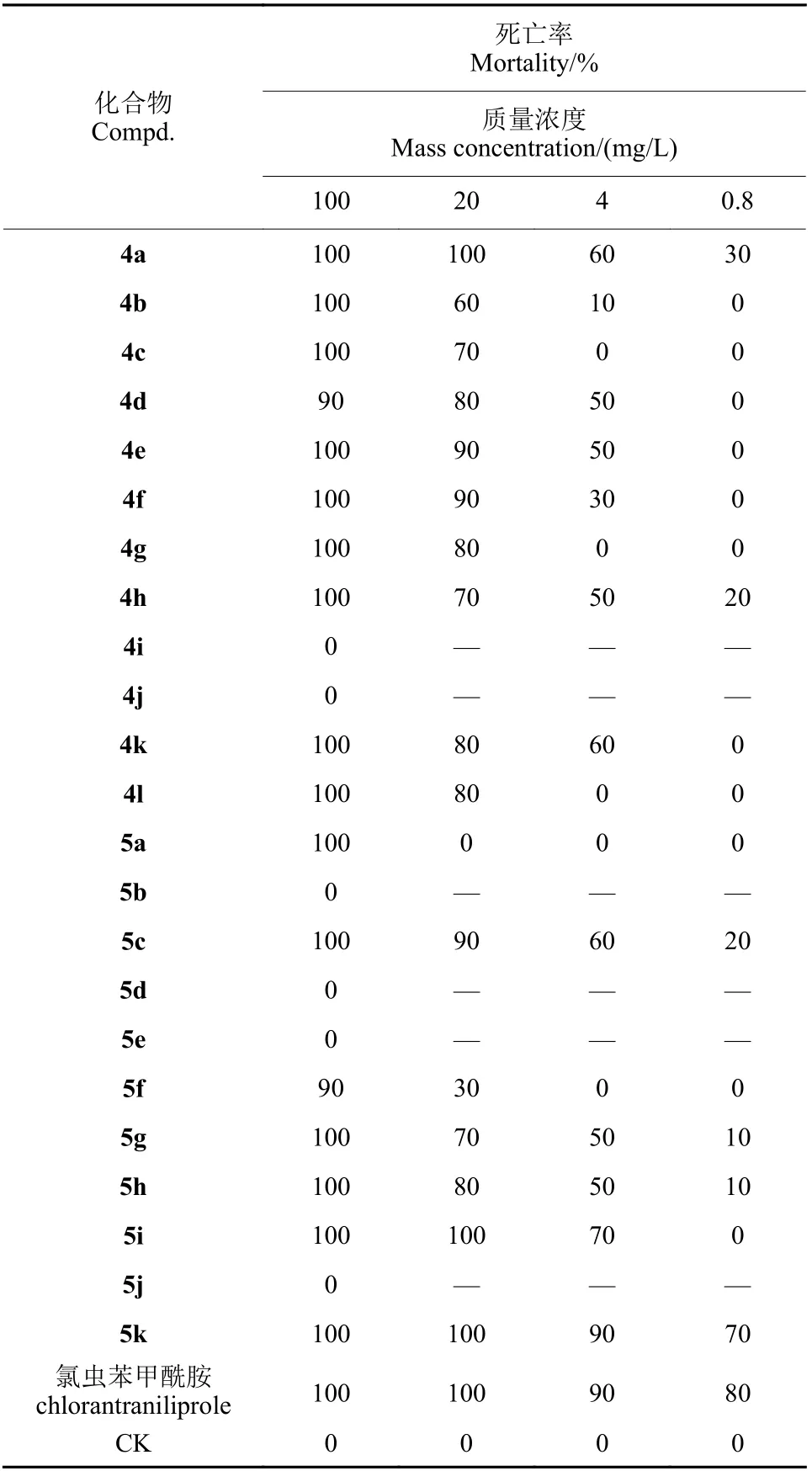
表1 目标化合物对黏虫的杀虫活性Table 1 Insecticidal activities of the target compounds against oriental armyworm
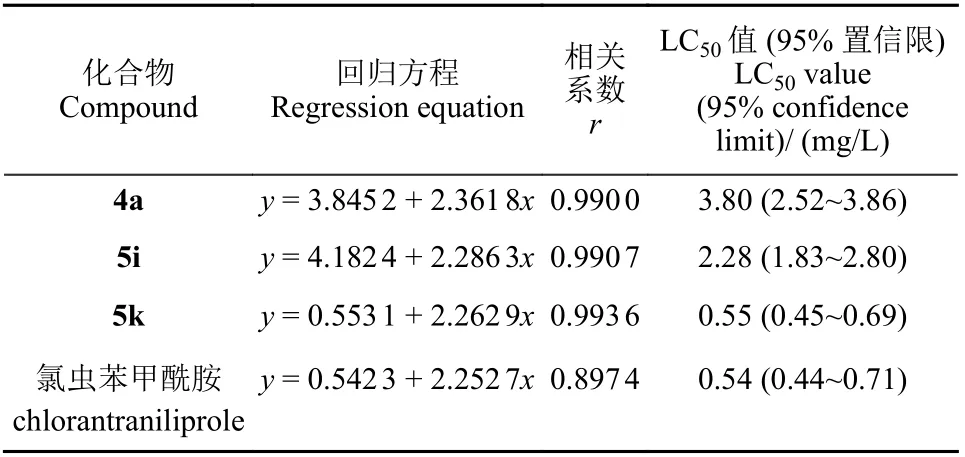
表2 部分目标化合物的LC50 值Table 2 The LC50 value of some target compounds(95% confidence limit)
初步构效关系分析可知,对于目标化合物4,当苯环上取代基为氟、氯、溴、甲基、苯基及羟基时,对应化合物的杀虫活性高于其他化合物,且邻位取代氟的活性略优于间-氟、对-氟、2,6-二氟和2,3,4,5,6-五氟。对于目标化合物5,当取代基为羟基时,对应化合物的杀虫活性高于其他化合物,具有进一步研究的价值。
2.3 分子对接
氯虫苯甲酰胺的作用靶标是RyR[27],为了评估目标化合物的作用机制,采用AutoDock 4.2 软件的Lamarckian 遗传算法分别对氯虫苯甲酰胺和化合物5k 与小菜蛾RyRN端结构域 (NTD) 的晶体结构 (PDB 代码:5Y9V) 进行了分子对接,所得理论结合模式分别如图1 和图2。可以看出:氯虫苯甲酰胺和化合物5k 均可通过氢键、π-π 堆积作用等方式与靶标结合,结合能分别为 -36 132 J/mol(-8.63 kcal/mol)和 -38 811 J/mol (-9.27 kcal/mol),说明5k 与氯虫苯甲酰胺一样也是作用于RyR;然而,二者作用的氨基酸残基有区别:氯虫苯甲酰胺是由苯环上的Cl 原子以及两个酰胺上的H 原子分别与Thr-145、Val-193 和Val-170 形成氢键作用,而化合物5k 则是由吡唑上的N 原子和茚酮上的羟基与His-195 和Gly-101 形成氢键作用,这说明将氯虫苯甲酰胺的N-甲基替换成5k 的茚酮会导致空间结构发生明显变化,从而使它们与靶标结合的氨基酸残基不同。
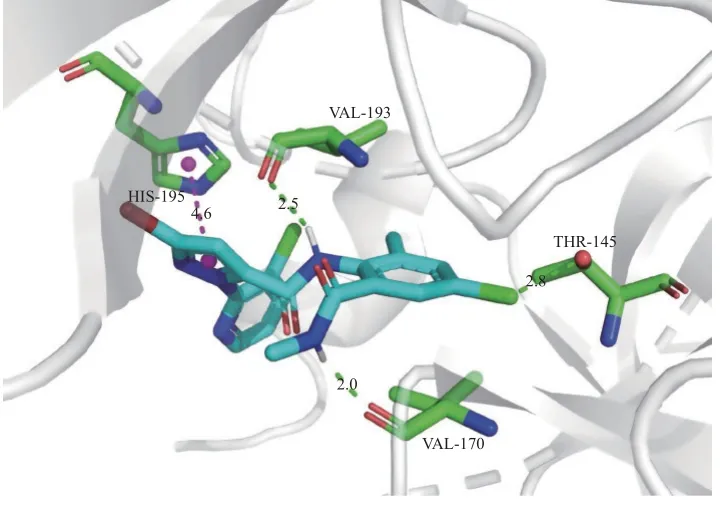
图1 氯虫苯甲酰胺与RyR N 端结构域之间的理论结合模式Fig. 1 Theoretical binding mode between chlorantraniliprole and N-terminal domain of RyR
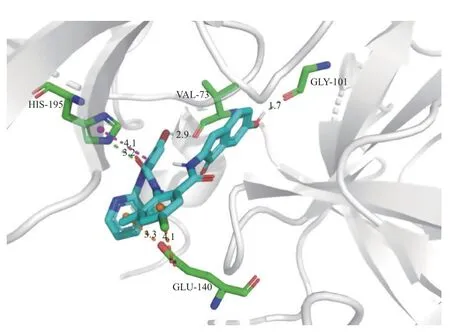
图2 5k 与RyR N 端结构域之间的理论结合模式Fig. 2 Theoretical binding mode between 5k and N-terminal domain of RyR
3 结论
以2-甲基-3-氨基苯甲酸为起始原料,经过卤化、缩合、引入苯丙氨酸片段及傅克反应合成了结构新颖的含苯丙氨酸和1H-茚-1-酮片段的双酰胺类化合物。初步生物活性测试结果表明,大部分化合物在100 mg/L 下对黏虫均具有较高的杀虫活性,其中化合物4a、5i、5k 在20 mg/L 下对黏虫有100%的致死率,化合物5k 在4 和0.8 mg/L下对黏虫分别有90%和70%的致死率,其LC50值为0.55 mg/L。引入茚酮结构以后大部分化合物的活性有所提高,因此利用苯丙氨酸以及1H-茚-1-酮作为新双酰胺类杀虫剂的药效基团具有深入研究的价值。分子对接的结果表明,化合物5k 和氯虫苯甲酰胺一样,也是作用于鱼尼汀受体(RyR),但与靶标结合的氨基酸残基不同,这种差异可能对抗性治理有益。后续将进一步优化结构,并对有氯虫苯甲酰胺已产生抗性的害虫进行生物活性测试。
