First line anlotinib plus liposomal doxorubicin for locally advanced or metastatic soft tissue sarcoma: A prospective, single-arm trial
2022-07-23XinSunRanxinZhangJieXuLuXieWeiGuo
Xin Sun, Ranxin Zhang, Jie Xu, Lu Xie, Wei Guo
Musculoskeletal Tumor Center, Peking University People's Hospital, Beijing 100044, China
ABSTRACT Objective: To examine the efficacy and safety of anlotinib as firstline therapy to treat locally advanced or metastatic soft-tissue sarcoma. Methods: This is a single-arm trial. Treatment-naïve patients (≥14 years) with locally advanced or metastatic soft tissue sarcoma were eligible. Each treatment cycle lasted for 3 weeks, and included liposomal doxorubicin (40-50 mg/m2) on day 1 and anlotinib (12 mg) on days 8-21. Starting from the 9th cycle, treatment consisted of only anlotinib. Treatment continued until disease progression or intolerable toxicities. The primary efficacy end point was progression-free survival (PFS).Results: Eight patients were enrolled between July 25, 2019 and January 8, 2020. The median number of treatment cycles was 5.5. Within 5.9 months median follow-up, PFS events occurred in 4 (4/8, 50%) patients. The median PFS was 11.3 months and the 6-month PFS rate was 56%. No patients attained complete response and 2 patients (fibrosarcoma, 1 patient and undifferentiated pleomorphic sarcoma, 1 patient) achieved partial response. Three patients (fibrosarcoma, 2 patients and synovial sarcoma, 1 patient) had stable disease. The objective response rate was 25% (2/8) for the study population, and the disease control rate was 75% (6/8). No new safety concerns emerged. Conclusions: Anlotinib plus liposomal doxorubicin demonstrated antitumor activities in previously untreated locally advanced or metastatic soft tissue sarcomas. Due to the small sample size, further investigations with a larger population should be undertaken to confirm the study findings.
KEYWORDS: Soft-tissue sarcoma; Multikinase inhibitor; Anlotinib; Antiangiogenesis; Liposomal doxorubicin; Progressionfree survival
1. Introduction
Soft tissue sarcoma is a heterogeneous group of malignant tumors of mesenchymal origin, and includes four major histological subtypes: liposarcoma, leiomyosarcoma, synovial sarcoma and others[1]. Liposarcoma represents approximately one quarter of soft tissue sarcomas in the extremities and nearly half of retroperitoneal soft tissue sarcomas[2,3]. Fibrosarcoma is a rare form of soft tissue sarcoma in adults, accounting for 3.6% and 5%-10% of soft tissue sarcomas in the USA and China, respectively[4,5]. Slightly fewer than 40 000 new cases of soft tissue sarcoma were reported in China in 2014[6,7], with a crude incidence rate of 2.9/100 000. In the USA, approximately 13 000 new cases and 5 350 deaths were reported in 2020[8].
Surgical treatment, with or without radiation, remains the preferred modality for local soft tissue sarcomas. Doxorubicin, either alone or in combination with ifosfamide, has been the standard of care for metastatic soft tissue sarcomas for many years[9,10], with a median survival of 8-13 months and a 2-year survival rate of only 30%[11-16], at the cost of significant toxicities[17,18].
Significance
Soft tissue sarcomas, a heterogeneous group of malignant tumors of mesenchymal origin, are highly vascularized and could be amenable to antiangiogenic therapy. In this prospective single-arm trial, we examined the potential efficacy and safety of antiangiogenic drug anlotinib plus liposomal doxorubicin for locally advanced or metastatic soft tissue sarcoma in the first line setting. Anlotinib plus liposomal doxorubicin showed an objective response in two out of eight patients and achieved disease control in six out of eight patients. The findings support further clinical development of the antiangiogenic regimen for soft tissue sarcomas.
Soft tissue sarcomas are highly vascularized. Markers for angiogenesis, e.g., microvessel density, circulating vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF), have been shown to correlate with patient prognosis[19-21]. Molecular targeted therapy in the second line setting has led to notable gains in survival outcomes, bringing new hopes for soft tissue sarcoma patients. In a phase Ⅲ trial, pazopanib, a multitargeted tyrosine kinase inhibitor (TKI), significantly extended the progression-free survival (PFS) in metastatic non-adipocytic soft-tissue sarcoma patients who had failed standard chemotherapy (4.6 months, 95% CI 3.7-4.8 vs. placebo 1.6 months, 95% CI 0.9-1.8)[22]. In a phaseⅡtrial, second line treatment with regorafenib, a multikinase inhibitor, prolonged PFS in leiomyosarcoma or synovial sarcoma patients but not in liposarcoma patients[23], adding support to distinct sensitivity of different subtypes of soft-tissue sarcoma to specific treatment regimens[3]. However, olaratumab, a recombinant human PDGF receptor α (PDGFRα) antibody, only led to a marginal survival benefit in patients with anthracycline-naïve locally advanced or metastatic soft tissue sarcoma when added to doxorubicin (PFS 6.6 months, 95% CI 4.1-8.3 vs. doxorubicin alone 4.1 months, 95% CI 2.8-5.4) in a phaseⅡtrial[24]. A phase Ⅲ, double-blinded randomized trial also failed to demonstrate notable benefits in overall survival with olaratumab plus doxorubicin vs. doxorubicin alone in patients with anthracycline-naïve advanced soft tissue sarcoma (20.4 vs. 19.7 months)[25]. The failure of molecular targeted therapy to achieving survival gains in advanced soft tissue sarcoma patients in the first line setting highlights the need for novel therapeutic agents that confer survival advantages beyond those achieved with the current standard of care-anthracyclines.
VEGF promotes immunosuppressive tumor microenvironment by increasing the expansion of suppressive immune cells and suppressing effector T cell development[26-28]. Antiangiogenic therapy could lead to vessel normalization and enhance the transmigration of immune cells, tipping the immune inhibitory tumor microenvironment towards a more immune active state[29,30]. However, antiangiogenic TKIs (e.g., sorafenib and lenvatinib) in combination with chemotherapy have yielded rather disappointing outcomes[31-33], suggesting that suppression of additional proangiogenic factors may be necessary. Anlotinib is a multikinase inhibitor that targets VEGF receptor (VEGFR), FGF receptor, PDGFR, and c-Kit and exerts a broad spectrum of inhibitory effects on tumor angiogenesis and growth[34-38]. It has potent antiangiogenic activities with a low IC50for VEGFR-2 (0.2 nmol/L vs. lenvatinib 4 nmol/L and sorafenib 90 nmol/L) and VEGFR-3 (0.7 nmol/L vs. lenvatinib 5.2 nmol/L and sorafenib 20 nmol/L) [34-38]. In a phaseⅠtrial, anlotinib demonstrated activities against an array of solid tumors that include soft tissue sarcoma with manageable toxicities[39]. In a subsequent phaseⅡtrial in advanced soft tissue sarcoma patients who progressed after anthracycline-based chemotherapy, anlotinib achieved a 12-week PFS rate of 68%, and an objective response rate (ORR) of 13%[40]. Anlotinib has been approved as 2nd-line treatment for advanced soft tissue sarcoma in China[41].
We conducted this prospective single-arm trial to examine the potential efficacy and safety of anlotinib plus liposomal doxorubicin as first line treatment for locally advanced or metastatic soft tissue sarcoma.
2. Subjects and methods
2.1. The study population
This prospective single-arm trial enrolled patients with pathologically proven locally advanced or metastatic soft tissue sarcoma who had received no prior therapy with anthracyclines or other antitumor treatment. The trial was conducted at Renmin Hospital of Peking University. For enrollment, patients must be at least 14 years of age, had at least one measurable lesion according to RECIST 1.1, had a predicted life expectancy at >3 months, adequate organ function, and an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1. The main exclusion criteria were prior treatment with anlotinib or other small molecule antiangiogenic TKIs, or monoclonal antibodies (e.g., sunitinib, sorafenib, bevacizumab, and regorafenib), systemic antitumor treatment including cytotoxic therapy, signal transduction inhibitors, immunotherapy in the preceding 4 weeks or mitomycin C in the preceding 6 weeks, and extended-field radiotherapy in the preceding 4 weeks or limited field radiotherapy for the sole purpose of tumor assessment in the preceding 2 weeks. We also excluded patients with malignancy within the preceding 3 years, or with known central nervous system metastasis. Additional eligibility criteria are detailed in Supplementary Methods.
2.2. Ethical issues and registration
The trial was conducted in accordance with the provisions of the Declaration of Helsinki and the International Conference on Harmonisation guidelines for Good Clinical Practice and approved by the institutional review board (Approval No. 2018PHD008-01). All patients provided written informed consents before enrollment. The trial is registered with ClinicalTrials.gov (NCT03880695). The study protocol adhered to the SPIRIT statement and the reporting of the study adhered to the CONSORT statement[42,43].
2.3. The study intervention
Each treatment cycle lasted for 3 weeks and included liposomal doxorubicin (40-50 mg/m2) on day 1 and anlotinib (12 mg/day, Chiatai Tianqing Pharmaceutical, China) on days 8-21. Dose reduction (to 75% and 50% of the initial dosage for liposomal doxorubicin and to 10 or 8 mg/d for anlotinib) was allowed at the discretion of the attending physician. Starting from the 9th cycle, treatment consisted of only anlotinib. Treatment cycles continued until disease progression, intolerable toxicities, or withdrawal of consent.
Best supportive care was provided. Patients were allowed to receive diuretics and angiotensin converting enzyme inhibitors for hypertension, granulocyte colony stimulating factor for leukopenia, erythropoietin for anemia, thrombopoietin and interleukin-11 for thrombocytopenia. Non-anthracyclines-based perioperative adjuvant/neoadjuvant chemotherapy was allowed (with a wash-out period of 6 months).
2.4. Patient assessment
Patients were assessed every 6 weeks for the first 24 weeks by RECIST v1.1 and every 12 weeks in the first 2 years after the last treatment cycle and every 24 weeks thereafter until disease progression or data cutoff date, whichever occurred earlier. Radiological evaluation included enhanced computed tomography (CT) scan or magnetic resonance imaging (MRI), plain chest CT scan (whole body bone imaging or PET/CT scan every 6 months if necessary) every 6 weeks for the first 24 weeks by RECIST v1.1 and every 12 weeks thereafter. Quality of life (QoL) was evaluated using WHOQOL-BREF.
2.5. Study end points
The primary efficacy end point was PFS, as calculated from date of medication initiation to the date of disease progression or death of any cause. Secondary end points included objective response rate, as defined by the proportion of patients who achieved confirmed complete response (CR) or partial response (PR) assessed by investigators per RECIST 1.1 guidelines, and disease control rate (DCR), as defined by the proportion of patients who achieved complete response, partial response, or stable disease (SD) as their best overall response for more than 4 weeks.
2.6. Safety evaluation
Adverse events (AEs) were graded and recorded according to NCI CTCAE version 4.0 and coded using MedDRA 22.0. Safety events included AEs and severe AEs (SAEs).
2.7. Statistical analysis
This was not a randomized controlled trial. Nonetheless, we estimated sample size requirement based on superiority over the first line MAID regimen. Specific assumptions included a PFS of 6 months with the MAID regimen, and a PFS of 9 months with the study drug, a power (1-β) of 0.80 and α=0.05, and a dropout rate of 20%. The calculation yielded 48 patients, with 28 primary end point events.
All analyses, including the primary efficacy end point of PFS and AEs, were conducted in a modified intention-to-treat population that included all patients who had received at least one dose of the study medications. PFS was estimated using the reverse Kaplan-Meier estimator method. All statistical analyses were conducted using SPSS 25.0.
3. Results
3.1. Patient characteristics
Between July 25, 2019 and January 8, 2020, 8 patients (median age: 52 years; 4 men and 4 women) were enrolled. Due to the low prevalence of soft tissue sarcomas, the planned sample size (48 patients) was not reached, and the trial was terminated. The histologic types included fibrosarcoma (n=4), undifferentiated pleomorphic sarcoma (n=2), liposarcoma (n=1) and synovial sarcoma (n=1) (Table 1). Five patients had stage Ⅳ soft tissue sarcoma, 2 patients had stage Ⅲ soft tissue sarcoma and 1 patient had stage ⅠB soft tissue sarcoma. Five patients had distant metastasis (4 to the lungs and 1 to the liver).

Table 1. Patient demographic and baseline characteristics.
The median number of treatment cycles was 7 (range 1 to 16 cycles). Four patients discontinued treatment due to progressive disease and 4 patients withdrew from the study (1 patient withdrew consent and 3 patients received other antitumor therapy). No patients were receiving treatment as of the study cutoff date (December 2020).
3.2. Efficacy measures
The median follow-up duration was 5.9 months (95% CI 0.3-11.5 months). PFS events occurred in 4 patients (4/8, 50%). The median PFS was 11.3 months (range not estimable). The 3-month PFS rate was 75% and 6-month PFS rate was 56% (Figure 1A and Table 2).
In the intention-to-treat population, no patients attained complete response while 2 patients achieved partial response, with an objective response rate of 25% (2/8). Per histologic subtypes, 1 of 4 patients with fibrosarcoma and 1 of 2 patients with undifferentiated pleomorphic sarcoma had objective response (partial response in both cases). In addition, 4 patients had stable disease, with a disease control rate of 75% (6/8). Per histologic subtypes, 2 of 4 patients with fibrosarcoma had stable disease and 3 of the 4 fibrosarcoma patients achieved disease control. Furthermore, 1 patient with synovial sarcoma and 1 patient with liposarcoma had stable disease. Two patients developed progressive disease (Table 3). Five patients (2 with partial response and 3 with stable disease) experienced reduction in target lesion size from the baseline and 3 patient (1 with stable disease and 2 with progressive disease) had an increase in target lesion size from the baseline (Figure 1B). Furthermore, patients with >30% reduction in target lesion size relative to the baseline tended to have a longer duration of treatment compared to those with <30% reduction in target lesion size (Figure 1C).
3.3. Safety
Treatment-related adverse events (TRAEs) of any grade occurred in 6 patients. TRAEs led to anlotinib dose reduction in 4 patients and discontinuation in 1 patient due to pneumothorax. Hand-foot syndrome was the most common TRAE, occurring in 3 patients, followed by hypertension, epistaxis, and oral ulcerative mucositis, occurring each in 2 patients (Table 4). Grade 3 TRAEs were reported in 3 patients, including hand-foot syndrome in 2 patients and pneumothorax in 1 patient. The patient with pneumothorax recovered after treatment. No treatment-related death was reported.
3.4. QoL
Two patients completed the QoL survey using WHOQOL-BREF. Both experienced progressive disease, however, their QoL scores were not negatively affected with tumor growth. For patient No. 3, who was diagnosed as fibrosarcoma with a PFS of only 1.4 months, she felt better after treatment, with her QoL score increased from 60 at the screening phase to 80 at the end of the study. Particularly, the patient had negative feelings quite often during screening stage while seldom had negative feelings at the time of exit from the study. Her choice also changed from “poor” to “neither poor nor good” for the question on “How well are you able to move around?”. As for the other patient (No. 5), who had undifferentiated pleomorphic sarcoma and a PFS of only 0.7 month, he had a high QoL score (90) at the screening phase to the end of the study. Besides, he also felt a need of less medical care in daily life and had better sleeping. These two cases indicated that even though the treatment did not result in tumor response, the patients still could benefit from treatment with improved QoL.
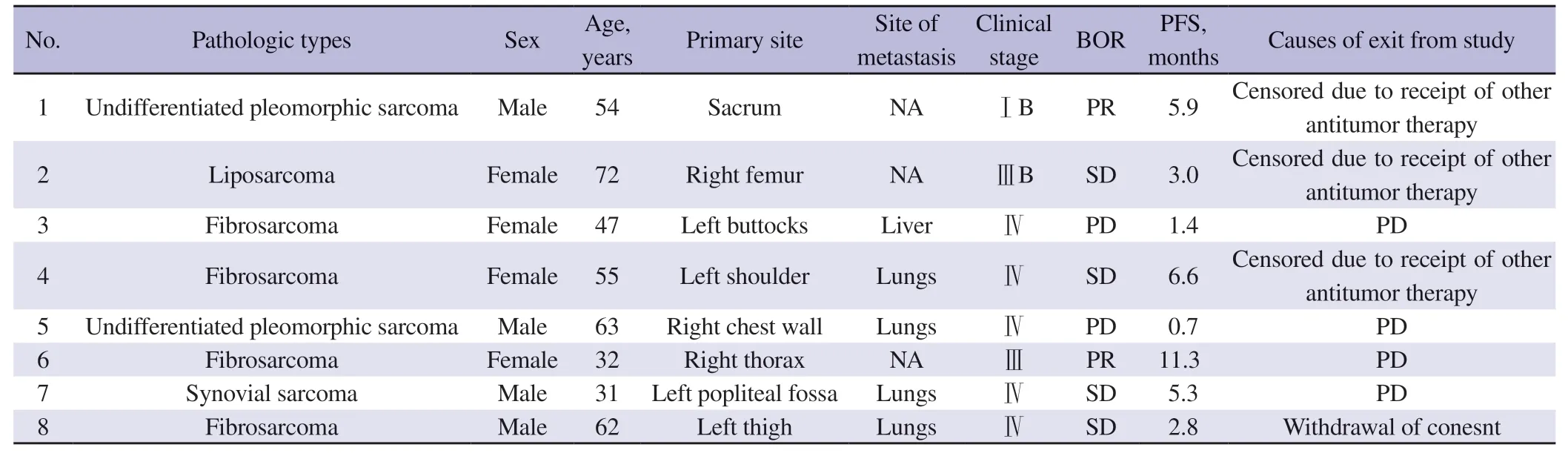
Table 2. Individual demographic and disease characteristics and treatment outcomes of the study population.

Table 3. Best objective responses by tumor types.

Table 4. Treatment-related adverse events (TRAEs) and grade 3 and above TRAEs.
4. Discussion
In the current trial, first line treatment with anlotinib plus liposomal doxorubicin in patients with locally advanced or metastatic soft tissue sarcoma achieved an objective response rate of 25%, a median PFS of 11.3 months and a 6-month PFS rate of 56%. The median PFS (11.3 months) in this trial was more favorable than that achieved with olaratumab plus doxorubicin (6.6 months) and ifosfamide plus sorafenib (4.8 months)[18,24]. Though the PFS appears to be impressive, given the limited size of the study cohort and half of the cohort censored, the data should be interpreted with caution when it is compared with the data of other drugs. The objective response rate (25%) is also higher than that previously reported with doxorubicin alone (11.9%-23.3%), bevacizumab plus doxorubicin (12%) or olaratumab plus doxorubicin (18.2%)[15,18,24], suggesting that the combination regimen was more effective in shrinking the tumors; 3 patients (partial response in 1 case and stable disease in 2 cases) experienced reduction in target lesion size and went on to receive other anti-tumor therapy. Meanwhile, the disease control rate (75%) is comparable to that with olaratumab plus doxorubicin (74.2%) [18,24]. The findings indicate that the therapeutic regimen of anlotinib plus liposomal doxorubicin could offer a chance to patients with locally advanced or metastatic soft tissue sarcoma to accept other treatment modalities.
Previous studies with sorafenib and lenvatinib failed to show that targeting the VEGF pathway per se is effective for soft tissue sarcomas[24,31-33], suggesting that inhibition of other targets or targets beyond the VEGF pathway is necessary for achieving meaningful clinical response. The promising objective response rate and disease control rate with anlotinib plus liposomal doxorubicin could be due to its broad spectrum of inhibitory targets, and specifically higher affinity for VEGFR, FGFR, PDGFR and c-Kit[34-38,44]. In the current trial, 2 patients with progressive disease who had completed QoL assessment showed improved QoL, with better sleep, fewer negative feelings and less dependency for medical treatment for daily life functioning, indicating that anlotinib plus liposomal doxorubicin could lead to improved QoL in advanced soft tissue sarcoma patients, even in cases of progressive disease.
Despite the low response rate of fibrosarcoma to radiotherapy and chemotherapy, patients with fibrosarcoma often require a combination of local radiotherapy and chemotherapy. Notably, in the current study, 1 out of 4 fibrosarcoma patients and 1of 2 undifferentiated pleomorphic sarcoma patients showed an objective response. This stood in contrast to the low objective response rate with anlotinib alone in patients with fibrosarcoma (11%) and undifferentiated pleomorphic sarcoma (7%) who progressed after anthracycline-based chemotherapy[40]. The relatively poor prognosis in the patient with liposarcoma in this trial (stable disease and PFS 3 months) is consistent with previous studies of regorafenib[23] and pazopanib in patients with liposarcoma[22]. The variability of soft tissue sarcoma histology is well documented and could affect tumor response rate to chemotherapy and TKI therapy, highlighting the need for subtype-specific therapy in this highly heterogeneous group of diseases. The recently approved drugs, including trabectedin, eribulin, and pazopanib, have all been limited to select histologic subtypes[22,45,46].
Overall, anlotinib plus liposomal doxorubicin had manageable toxicities. Grade 3 and above TRAEs occurred in 3 patients. Handfoot syndrome, hypertension, oral ulcerative mucositis, and epistaxis were the most frequent TRAEs. Grade 3 treatment-related hand-foot syndrome occurred in 2 patients and pneumothorax in 1 patient. The toxicity profile is consistent with earlier studies of anlotinib for soft tissue sarcomas and other tumor types[39,40]. In contrast to frequent hematologic toxicities with regorafenib[23], no treatment-related hematologic toxicities or cardiotoxicities were reported in this trial.
Two of the 8 patients in this trial went on to undergo surgery after treatment with anlotinib plus doxorubicin, raising the possibility that this treatment regimen could be helpful in downgrading unresectable soft tissue sarcoma to allow curative surgery.
A key limitation of this trial is the very small sample size due to difficulty in patient recruitment. Efficacy assessment, including PFS, objective response rate and disease control rate, is thus not sufficiently robust. Another limitation is the lack of a control arm.
In conclusion, anlotinib in combination with liposomal doxorubicin produced promising antitumor activities as first line treatment for locally advanced or metastatic soft tissue sarcomas. However, the results are based on very small sample size, and must be verified by larger trials in the future.
Conflicts of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Acknowledgements
We thank Chia Tai Tianqing Pharmaceutical Group for providing anlotinib.
Funding
The authors received no extramural funding for the study.
Authors’ contributions
X.S. and W.G. conceptualized the study; X.S., R.Z., and J.X. curated the data; X.S. and J.X. carried out the formal analysis; X.S. carried out the investigation and developed the methodology and X.S. and W.G. were responsible for project administration; X.S. and R.Z. provided the resources; X.S. was responsible for software; supervision was done by X.S, W.G; X.S. validated the data and X.S. and L.X. were responsible for data visualization; roles/writingoriginal draft were by X.S; writing-review and editing were carried out by X.S. and W.G.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Impact of climate change on tropical health in the Central African Republic
- Biopharmaceuticals for prevention of COVID-19: A scoping review
- Patterns in the relationship between acute COVID-19/long COVID-19 and quality of life: A cross-sectional study of patients attending a tertiary care hospital in Turkey
- Disseminated histoplasmosis in a 17-year-old Nigerian male patient: A case report
