Lymph node metastasis from non-melanoma skin cancer
2022-07-20RobbieWoodsJackWoodsConallFitzgeraldEhabAlameerJosephLopezBhuvaneshSinghJatinShahDepartmentofHeadNeckSurgeryMemorialSloanKetteringCancerCenterNewYorkNY0065USA
Robbie S. R. Woods, Jack F. C. Woods, Conall W. R. Fitzgerald, Ehab Alameer, Joseph Lopez,Bhuvanesh Singh, Jatin P. ShahDepartment of Head & Neck Surgery, Memorial Sloan Kettering Cancer Center, New York, NY 0065, USA.
2Plastic & Reconstructive Surgery, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA.
Abstract The management of non-melanoma skin cancers metastatic to the neck is challenging due to variability in biological behavior and patterns of regional lymphatic spread. Metastatic non-melanoma skin cancers to the parotid and neck often behave aggressively, with a high incidence of local recurrence after treatment and reduced five-year survival outcomes. Patterns of lymphatic spread are different from those seen in mucosal squamous cell carcinoma, with higher prevalence of disease in the parotid and superficial lymphatics. These factors require that treatment is individualized to achieve optimal outcomes. Traditionally, the management of non-melanoma skin cancers metastatic to lymph nodes has involved surgical excision followed by adjuvant radiation therapy. However,novel systemic therapies are showing promising results and their role in the management of these cancers is evolving.
Keywords: Neck, non-melanoma cutaneous malignancy, skin neoplasms, lymph nodes, lymphatic metastasis
INTRODUCTION
Patterns of spread
In contrast to mucosal origin malignancies which predominantly metastasize to lymph nodes in levels I-V in the neck (shown in Figure 1), cutaneous malignancies of the head and neck can also metastasize to the pre- and post-auricular, parotid, suboccipital, and superficial lymphatic systems, depending on tumor location.

Figure 1. Assigned lymph node levels of the lateral neck. Level IA: Submental nodes. Level IB: Submandibular nodes. Level IIA: Upper jugular nodes anterior to the spinal accessory nerve. Level IIB: Upper jugular nodes posterior to the spinal accessory nerve. Level III: Mid jugular nodes. Level IV: Lower jugular nodes. Level VA: Posterior triangle nodes superior to the level of the inferior border of cricoid cartilage. Level VB: Posterior triangle nodes inferior to the level of the inferior border of cricoid cartilage. Level VI: Central compartment nodes.
Patterns of lymphatic drainage are predictable based on the location of the primary tumor, shown in Figure 2. This information can be used to guide the management of regional diseases from specific primary sites[1]. As a general rule, a line joining the helix of one ear to the helix of the opposite ear in a coronal plane separates the watershed areas of the scalp. Tumors located anterior to this line generally metastasize to preauricular, periparotid, intraparotid, and anterior cervical lymph nodes (levels I-IV), whereas tumors of the scalp posterior to this line usually metastasize to the postauricular and suboccipital lymph nodes, as well as those in the posterior triangle of the neck and deep jugular chain[1]. For example, cutaneous malignancies on the cheek, eyelids, pinna, forehead, and temple have been reported as draining initially to preauricular,periparotid, and parotid nodes[2]. Most lymph nodes within the parotid are found superficial to the facial nerve, and these are typically involved in cutaneous malignancy; however, a small number of nodes can be present deep in the nerve[3,4].
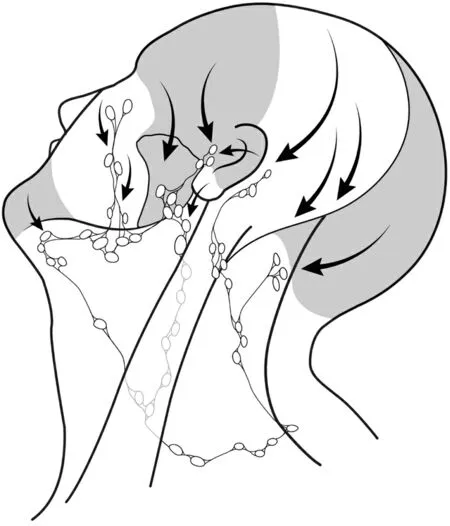
Figure 2. Patterns of metastatic spread to lateral neck lymph nodes from non-melanoma skin cancer of the head and neck.
RISK OF REGIONAL METASTASIS
Although non-melanoma skin cancer (NMSC) has a lower propensity for advanced disease than cutaneous melanoma, the absolute mortality burden has recently surpassed that of melanoma[5]. Cutaneous squamous cell carcinoma (SCC) and Merkel cell carcinoma (MCC) have the highest risk of metastasis to regional nodes[6,7]. Other NMSCs can also present with regional diseases in the parotid and neck, such as cutaneous adnexal tumors, cutaneous sarcomas and, rarely, basal cell carcinoma (BCC). The presence of lymph node disease is a poor prognostic factor in NMSC. For SCC, it has been suggested that the presence of nodal metastasis is a stronger predictor of prognosis than features present in the primary tumor[8].
Squamous cell carcinoma
Approximately 3.7%-5.8% of cases of cutaneous SCC present with regional disease[9-12]. However, higher rates of 33%-47% are reported in association with several clinical and histological features[13,14]. These include tumor location (ear, lip, or temple), size > 2 cm, depth of invasion > 6 mm or beyond subcutaneous fat,histologic type, degree of differentiation, perineural invasion, and immunosuppression[15-18].Immunocompromise is a significant independent risk factor for the aggressive behavior of metastatic cutaneous SCC to the neck[19,20]. Presence of disease in regional nodes from cutaneous SCC has been associated with five-year survival rates ranging from 35%-70%, depending on tumor stage, surgery, and adjuvant treatment[14,16,21-25].
Merkel cell carcinoma
MCC is a neuroendocrine carcinoma with a high propensity for regional spread of over 50% and, even in cases without clinical evidence of lymph node metastasis, the rate of nodal micrometastasis on sentinel lymph node biopsy can be 30%-38%[26]. MCC of unknown primary occurs in 5%-25% of cases and carries improved survival and different tumor characteristics compared to tumors with presence of a primary[27-30].Increasing nodal disease burden has been suggested as a prognosticator in MCC[31].
Basal cell carcinoma
While BCC is the most common primary cutaneous malignancy found in the head and neck[32], it rarely metastasizes to lymph nodes. Rates of nodal disease from primary BCC are reported between 0.0028% to 0.55%[33]. Regional spread from BCC is associated with male gender, fair skin, and history of radiation, as well as primary tumor factors such as increased size, deeper invasion, perineural invasion, basosquamous histology, and primary site in the head and neck[33-36].
Adnexal tumors
Cutaneous adnexal tumors, including those involving hair follicles, sweat ducts, ceruminous glands,sebaceous glands, apocrine glands, and eccrine glands, may occur sporadically or as part of genetic syndromes. Rates of regional spread are uncertain due to the rare and heterogeneous nature of these tumors; however, the presence of lymph node metastases portends significantly worse overall survival[37]. It has been suggested that these tumors are more likely to demonstrate nodal involvement with aggressive local histology, and that lymphatic disease in early-stage tumors is rare[38,39], but high rates of up to 89% have also been reported[40].
Cutaneous sarcoma
Regional lymph node metastasis is reported at up to 23% in angiosarcoma[41]and 10%-20% in pleomorphic dermal sarcoma[42]. However, other non-melanoma cutaneous malignancies of the head and neck, such as atypical fibroxanthoma, leiomyosarcoma, or dermatofibrosarcoma, rarely spread regionally, with rates less than 10% in most of these tumors[43].
CLINICAL ASSESSMENT
Clinical evaluation of the entire skin of the head and neck, including the scalp, must be performed to assess for multifocal lesions or in-transit metastatic disease. A thorough examination of the parotid and neck should be performed in all patients with cutaneous malignancy of the head and neck. In cases that present with clinical nodal disease, anatomical structures are particularly important to consider, including underlying cartilage, underlying bone, parotid gland, external auditory canal, temporomandibular joint,lateral temporal bone, motor nerves, such as the facial nerve branches, and cutaneous sensory nerves, such as the greater auricular and auriculotemporal nerves. For facial skin lesions, involvement of the nasal cartilages and tarsal plates for lesions of the eyelid, as well as the involvement of the terminal divisions of the trigeminal nerve, should be carefully assessed.
SCC has a significant propensity for perineural spread or direct nerve invasion by nodal disease, and so particular care must be taken to assess and manage sensory and motor nerves in these cases. Thorough clinical examination should also include the extent of involvement of the skin, particularly in morpheaform BCC and dermal lymphatic permeation by aggressive cancers.
Metachronous presentation of regional lymph node metastasis from NMSC is common and can be difficult to distinguish from primary mucosal site metastasis. In these cases, histological assessment, such as for high-risk human papillomavirus by in situ hybridization, can be helpful, and a thorough evaluation for a mucosal primary is critical.
For clinically suspected nodal disease, as well as for primary tumors with a high risk of nodal involvement,imaging should be performed to further evaluate the presence and extent of nodal metastases. This typically will include contrast-enhanced computed tomography (CT) scanning of the head, neck, and chest. CT scans are also valuable to assess bone invasion by primary tumors in extensive lesions. Magnetic resonance imaging (MRI) is valuable for the assessment of suspected perineural invasion or parapharyngeal involvement, while Positron emission tomography/CT imaging can be used in some cases for the assessment of distant metastases, in addition to ruling out a mucosal primary site in those with metachronous presentations. Ultrasound-guided fine-needle aspiration or core biopsy can be used to establish diagnosis.
STAGING
NMSC of the head and neck is staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) classification system[44]. For tumors involving the temporal bone, the most commonly used staging system is the modified University of Pittsburgh system[45].
There has been some debate around the use of the AJCC staging system in cutaneous SCC[46,47]. The nodal system is the same as for mucosal SCC, but it is likely that stratification does not adequately reflect the differences between nodal spread of mucosal and cutaneous SCC[48,49]. For example, very high rates of extranodal extension in cutaneous SCC means that nodes greater than 6 cm in size without extranodal extension are extremely rare and so the N3a stage may be better reclassified based on other features such as the number of nodal metastases[44,49]. It has been suggested that an increasing number of metastatic lymph nodes, separately from the presence of extranodal extension, is an independent predictor of disease-specific survival in cutaneous SCC of the head and neck and could be incorporated into AJCC staging[50-52].However, alternative staging systems for regional spread of cutaneous SCC have been proposed, which may give better stratification[53]. These staging systems are often used for research purposes leading to inconsistency in the significance of parotid involvement in staging. Examples of commonly used staging systems include the Brigham and Women’s system[54], Clark’s N1S3 system[55], O’Brien’s parotid and neck node system[56], and the Immunosuppression, Treatment, Extranodal spread, and Margin status prognostic score[57].
For MCC, multiple staging systems have been described[58]; however, a distinct AJCC TNM staging system,separate from other NMSC, is now most commonly used. Any regional nodal involvement is classified as stage N1, while regional nodal involvement with in-transit metastasis is classified as stage N3. Stage N2 disease is classified by the presence of in-transit metastasis without nodal disease present. Overall, stage III disease is denoted by any primary tumor size or unknown primary, with regional lymph node involvement.
For cutaneous adnexal tumors of the head and neck, regional lymph node staging is important due to its impact on prognosis[37]. Staging, along with other NMSC, is according to the 8th edition AJCC system.
SURGICAL MANAGEMENT
Multiple guidelines exist to assist decision making in the management of NMSC. However, this can be a challenge as evidence from high-quality clinical trials is rare[59]. When possible, surgery remains the mainstay of management of this disease, including in cases of regional spread.
Squamous cell carcinoma
Reported rates of metastatic spread from the superficial parotid nodes to the cervical lymph nodes in SCC range from 13%-35%[56,60-62]. Moreover, since metachronous metastasis is common, some research has suggested elective treatment of the neck and parotid in advanced stage or high-risk cutaneous SCC[21,63-68].However, no prospective study has demonstrated a clinically substantial survival benefit to elective neck dissection compared to therapeutic neck dissection after nodal metastasis has developed, and recent data reports worse overall survival in those undergoing elective neck dissection[69,70]. Elective treatment of the neck in regionally metastatic head and neck NMSC remains controversial and prospective trials are needed to clarify optimal management.
For clinically node-negative high-risk SCC, depending on their age, co-morbidities, and risk factors,sentinel lymph node biopsy or observation with or without imaging may be considered alternatives to elective neck dissection and parotidectomy[21,70,71]. Although sentinel lymph node biopsy is a viable tool in head and neck melanoma and is also used in MCC, it is rarely used in other head and neck cutaneous malignancies outside of clinical trials[72-74]. Issues with sentinel lymph node mapping in the head and neck include proximity of the injection site to the draining basins, unpredictable lymphatic drainage, multiple sentinel nodes per patient, higher risk of false-negative results, and mapping to the parotid gland in 19%-44% of cases with a small chance of risk to the facial nerve in dissection[75].
Up to 75% of nodal metastases from cutaneous SCC of the head and neck are present in the parotid gland[76]. Typical management of this presentation, when resectable, is surgery followed by adjuvant radiation[77], because radiation alone for clinically positive disease is associated with worse survival outcomes[78]. For cases with involved cervical nodes, in which parotid nodes are not clinically present but the primary tumor site is associated with a high risk for parotid spread, either parotidectomy can be carried out alongside the neck dissection, or adjuvant radiation can be used to treat the parotid. It is also important to resect the external jugular lymphatics in these cases and level V for tumors based posteriorly[2,64,72,79].
For patients with parotid nodal metastases who are otherwise clinically node-negative, additional elective selective neck dissection would include levels I-III for anterior facial primaries, levels II-III for anterior scalp and external ear primaries, and levels II-V including postauricular and suboccipital nodes for posterior scalp and neck primaries[79].
Although it has been suggested that total parotidectomy be considered when positive parotid nodes are identified[69,80,81], there is potential for higher complications from this, and so it may only be beneficial in cases with definite deep lobe involvement[56,82,83]. Radical or extended radical parotidectomy may be necessary depending on the involvement of adjacent structures[81]. When metastatic cutaneous SCC is adherent to the temporal bone, some form of lateral temporal bone surgery should be carried out[84], which may be aggressive and require adjuvant radiation[85].
Trigeminal, facial and cervical plexus nerves can be at risk of cutaneous malignancy, usually by the primary tumor itself, but also potentially by regional nodal disease, particularly if extranodal extension is present[69,86]. This is usually due to direct invasion by nodal disease, but can also be due to perineural spread,and the parotid gland is more likely involved than the neck. The likelihood of nerve sacrifice is highest in patients with nodal disease within the body of the parotid gland and when the primary tumor itself demonstrates perineural invasion[87]. For patients with clinical perineural invasion, available evidence suggests that survival is improved by surgical resection if feasible, followed by postoperative radiation[88-91].Facial nerve function is an important outcome for patients with cancer of the parotid and temporal bone region[92], and ideally, it should only be sacrificed when directly compromised by disease[69,82]. If oncologic resection is not possible without sacrificing the nerve, it may require partial or complete resection even if it is functioning preoperatively. For example, radical resection to ensure clear margins may be necessary for tumors at the stylomastoid foramen even when the nerve is clinically intact[84], but efforts should be made to preserve the nerve where possible. Intraoperative frozen section to ensure satisfactory clearance of perineural disease may potentially remove any individual effect on survival[85]. Parotid nodal disease may directly involve the auriculotemporal nerve or spread to it via anastomoses with the facial nerve, with subsequent spread to the infratemporal fossa and foramen ovale[93-95], in which case partial mandibular resection may be needed to access the infratemporal fossa.
Merkel cell carcinoma
Status of spread to the lymph nodes is the most important predictor of survival in MCC. Survival has been reported at 52% for MCC with metastasis to regional nodes, compared to 71% in cases without metastasis[96]. As micrometastasis is common, and independent of size or depth, sentinel lymph node biopsy is recommended for all patients with MCC and is included in the AJCC 8th edition staging system[97]. When disease is identified in sentinel nodes, lymph node dissection is suggested, although there are no prospective studies demonstrating its benefit, and so radiation may be an alternative[96,98].
Basal cell carcinoma
Regional lymph node metastasis from BCC is exceedingly rare but portends a poorer prognosis than localized disease[35]. Due to its rarity, no guidelines are established. However, treatment has typically been with therapeutic neck dissection and possible adjuvant radiation.
Adnexal tumors
For cutaneous adnexal tumors, lymph node metastases identified after sentinel lymph node biopsy in earlystage tumors are rare[38], so it may best be applied selectively, possibly only in patients with local recurrence[39]. One study suggested that apocrine carcinoma demonstrates high rates of lymphatic spread,and so elective node dissection should be considered in this histology, although no cases in this study were in the head and neck, in which regional spread may be less common[40,99].
RECONSTRUCTION
Large defects in the parotid and neck region after clearance of nodal metastases may require a pedicled or free flap[100], particularly in cases where adjuvant radiation is likely or good cosmesis is sought. However,adjuvant radiation can result in significant free flap volume loss and so overcorrection is sometimes required to ensure adequate function after radiation[101]. In cases where the primary tumor involves the skull base, closure is usually done in layers to prevent cerebrospinal fluid leak and close dead space[102].
For larger defects that include parotidectomy and neck dissection, an anterolateral thigh flap is typically employed[103]. Alternative local flaps to reconstruct the parotid region, for example, in cases where free tissue transfer may be contraindicated, include the temporalis muscle flap, supraclavicular island flap, scalp flap,preauricular flap, or temporoparietal fascia flap.
In cases with extensive perineural invasion, static facial reanimation can be used and this is sometimes incorporated into the flap of choice[102]. Dynamic reconstruction can be carried out using autologous nerve grafts such as the greater auricular or sural nerves, the latter of which can be used for multiple anastomoses.Nerve allografts can also be used for dynamic reconstruction, as well as masseteric or hypoglossal nerve transfers, with oral commissure symmetry better in hypoglossal nerve use and time to the first movement quicker in masseteric nerve use[104].
RADIATION
Radiation in regional lymph node metastases from NMSC is typically reserved for the adjuvant setting.However, for cases undergoing parotidectomy for parotid nodal disease, elective neck radiation can be an alternative to elective neck dissection with adjuvant radiation[105]. Radiation or chemoradiation as primary treatment can be considered in patients deemed unfit for surgical management[106].
For node-positive cases, adjuvant treatment is typically given to all patients with nodal disease greater than 3 cm with no extracapsular extension, those with extranodal extension, or those with incompletely excised nodal disease. Some evidence suggests that it may also be considered with one positive lymph node ≤ 3 cm with no extracapsular extension[107,108]. The recommended dose of adjuvant radiation is 60 Gy in 2.0 Gy once-daily fraction, 5 days per week, or a biologically equivalent dose[109]. Techniques such as Intensitymodulated radiation treatment (IMRT), or volumetric modulated arc therapy are preferable. Non-IMRT techniques, including 3-dimensional conformal radiation and electron or proton beam therapy, are acceptable if adequate tumor coverage is achieved while constraints on organs at risk are met[109].Brachytherapy use is limited to previously irradiated patients with incompletely resectable nodal disease.
The role of concurrent chemotherapy with radiation is controversial and is less certain than in cases of mucosal SCC. Some research has suggested a lower chance of recurrence[110], but there has been no evidence to suggest improvement in overall survival by the addition of chemotherapy[23], and it is possible that outcomes could be worse with the use of concurrent chemotherapy[111]. Concurrent chemotherapy is typically used in cases of mucosal SCC demonstrating extranodal extension, and it has been associated with worse outcomes in cutaneous SCC, but this feature in cutaneous SCC is associated with higher nodal burden and may be different from the tumor biology associated with mucosal disease[50]. Thus, further research is needed to determine if there is any role for concurrent chemotherapy with radiation in the adjuvant setting for NMSC. Recent studies evaluating the use of radiotherapy with immunotherapy, such as cemiplimab or pembrolizumab, in locally advanced unresectable cutaneous SCC have shown promising results and further research is needed to clarify the role of concurrent radiation with immunotherapy[112-114].
Separately, MCC is a highly radiosensitive tumor. Treatment of the neck includes either elective nodal radiation[115,116], or sentinel node biopsy followed by adjuvant radiation or lymph node dissection if positive,or by observation alone if negative[116,117]. Elective nodal radiation may be avoided in T1 cases with negative sentinel nodes[118,119]. Conventionally fractionated radiation with a dose of 50 Gy in 25 fractions is used in the elective setting[120], however, single fraction treatment with 8 Gy has also been proposed[121]. For lymph node metastases, adjuvant radiation is advocated to improve locoregional control, but has not been shown to improve overall survival[122]. Higher radiation doses up to 60 Gy have been suggested for definitive treatment of unresectable diseases, especially for treating bulky diseases[123].
While most adnexal tumors are resistant to radiation, the role of radiation is not clear due to heterogeneity of tumors. Adjuvant radiotherapy may be considered in patients with microcystic adnexal carcinoma, as well as certain others such as syringocystadenocarcinoma papilliferum and pilomatrix carcinoma[124,125].
SYSTEMIC THERAPY
Immunotherapy has been revolutionary in the management of melanoma, but the role of systemic therapy in NMSC is not clear. Hedgehog pathway inhibitors, and more recently immune checkpoint inhibitors, have been approved for use in metastatic BCC or advanced BCC not amenable to surgery or radiation[126]. For MCC, avelumab, an anti-programmed death-ligand 1 (PD-L1) inhibitor, has been recommended as firstline treatment for metastatic MCC due to results showing median overall survival of 20.3 months with its use in a phase two study[127]. A recent study, in which two-thirds of MCC patients had locoregional disease at presentation, demonstrated that treatment with neoadjuvant nivolumab resulted in a pathological complete response in 47.2%[128].
For advanced unresectable or metastatic SCC, traditional chemotherapy and/or epidermal growth factor receptor inhibitors have been used with modest success, with median overall survival of 15.3-16.2 months[129]. Phase two trial data on the use of cemiplimab in metastatic and locally advanced cutaneous SCC have shown complete or partial response rates of 47%[130], and so cemiplimab is now the mainstay in most clinical regimens[5]. In the phase two study by Migdenet al., an intravenous dose of cemiplimab (3 mg per kilogram of body weight) was administered every 2 weeks and patients were assessed for a response every 8 weeks[130]. In this study, complete or partial response was observed in 49% of patients with distant metastasis and 43% of patients with regional metastasis[130]. In another phase two study where patients received pembrolizumab, 200 mg intravenously every three weeks, complete and partial response rates were 17% and 33%, respectively, for locally advanced unresectable disease, while complete and partial response rates were 11% and 25%, respectively, for recurrent or metastatic disease[131]. In a separate phase two study of pembrolizumab, higher response rates were seen in patients with PD-L1-positive disease (55%)compared to PD-L1-negative disease (17%)[132]. Importantly, responses seem to be durable with manageable safety in these studies.
Taxanes are the primary treatment in advanced unresectable angiosarcoma, while research on vascular endothelial growth factor inhibitors, checkpoint inhibitors, and eribulin mesylate has not shown significant promise[133]. For adnexal malignancies, chemotherapy may be beneficial in patients with metastatic disease,while anti-estrogenic treatment and human epidermal growth factor receptor 2 inhibitors have been used in some cases with variable outcomes[124].
CONCLUSION
Cutaneous malignancy of the head and neck can spread to the superficial and/or deep lymphatic network,including pre- and post-auricular nodes, parotid nodes, suboccipital nodes, and deep jugular chain nodes,depending on the location and features of the primary tumor. Patient factors such as immunocompromise need to be considered. Clinical evaluation of the parotid and neck should be performed in all patients with head and neck NMSC. Pre-operative imaging is also recommended.
Treatment for lymphatic spread of cutaneous malignancy of the head and neck typically involves surgical resection with adjuvant radiation. Appropriate management of anatomical structures in the head and neck is important to ensure the best surgical outcomes. New systemic therapies, such as cemiplimab, are demonstrating promising results in clinical trials. Management in a multidisciplinary setting is recommended.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the study and performed data interpretation and manuscript drafting: Woods RSR, Woods JFC, Fitzgerald CWR, Alameer E, Lopez J, Singh B, Shah JP
Supervised the writing process: Singh B, Shah JP
Approved the final version of the report: Woods RSR, Woods JFC, Fitzgerald CWR, Alameer E, Lopez J,Singh B, Shah JP
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflict of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
杂志排行
Journal of Cancer Metastasis and Treatment的其它文章
- Anti-angiogenic drugs in cancer therapeutics: a review of the latest preclinical and clinical studies of anti-angiogenic agents with anticancer potential
- Molecular pathology of thymoma and thymic carcinoma
- Tumors due to chronic exposure to benzene and biomarkers of exposure
- Sensitivity of MCF-7 mammosphere CSCs to neutron radiation
- AUTHOR INSTRUCTIONS
