Factors associated with trabecular bone score in postmenopausal women with type 2 diabetes and normal bone mineral density
2022-07-15OlgaFazullinaAntonKorbutVadimKlimontov
lNTRODUCTlON
Type 2 diabetes (T2D) and bone fractures have been recognized as a widespread comorbidity leading to excess mortality and an enormous healthcare burden[1,2]. Recent data from the Continuous National Health and Nutrition Examination Survey (NHANES) indicate an increasing prevalence of osteoporosis and osteopenia in the US among T2D patients[3]. People with T2D have higher risk of vertebral and some non-vertebral fractures than non-diabetic individuals[4,5], regardless of normal or even increased bone mineral density (BMD)[6,7]. This “diabetic paradox” has been attributed to the modified effect of hyperglycemia, obesity and related factors on BMD[8]. As BMD assessment may lead to underestimation of a fracture risk in T2D, additional parameters of bone health should be taken into consideration.
I waited until it was dark, snuck up to the old lady’s house, and put the envelope of retribution through the letter slot in her door. My soul felt redeemed4 and I couldn’t wait for the freedom of, once again, looking straight into the old lady’s eyes.
In recent years, the Trabecular Bone Score (TBS) on lumbar spine dual X-ray absorptiometry (DXA) images is increasingly applied for the assessment of bone microarchitecture. It had been demonstrated that low TBS is associated with both prevalent and incident fractures; therefore, TBS was incorporated in the Fracture Risk Assessment tool (FRAX) algorithm[9]. The impaired bone microarchitecture is considered as a major contributor to fracture risk in T2D[10]. Accordingly, the utility of TBS for osteoporotic fracture risk assessment was shown in postmenopausal women with T2D[11,12]. Individuals with diabetes as compared to those without have significantly lower TBS[13,14]; the difference is greater in women[13]. It could be speculated that the reduction of TBS is an earlier event in the deterioration of bone health in T2D than BMD decrease. However, at present, data on TBS in postmenopausal women with T2D and normal BMD is limited, and predictors of the TBS decrease in these women need to be refined.
A growing body of evidence indicates the pivotal role of hyperglycemia-related biochemical abnormalities, as well as obesity and dysregulated adipokine production, in the pathogenesis of increased bone fragility in T2D[15,16]. Nevertheless, the role of diabetes-related factors and fat accumulation at early stages of bone metabolic disease in T2D needs further research.
Therefore, in this study we aimed to identify clinical and body composition parameters that affect TBS in postmenopausal women with T2D and normal BMD.
MATERlALS AND METHODS
Design
A non-interventional cross-sectional comparative study was conducted.
To be included in the study, women had to meet the following criteria:(1) Caucasian origin; (2) Age 50-75 years; (3) Time since menopause ≥ 1 year; (4) Known T2D duration ≥ 1 year; and (5) Normal BMD assessed by DXA.
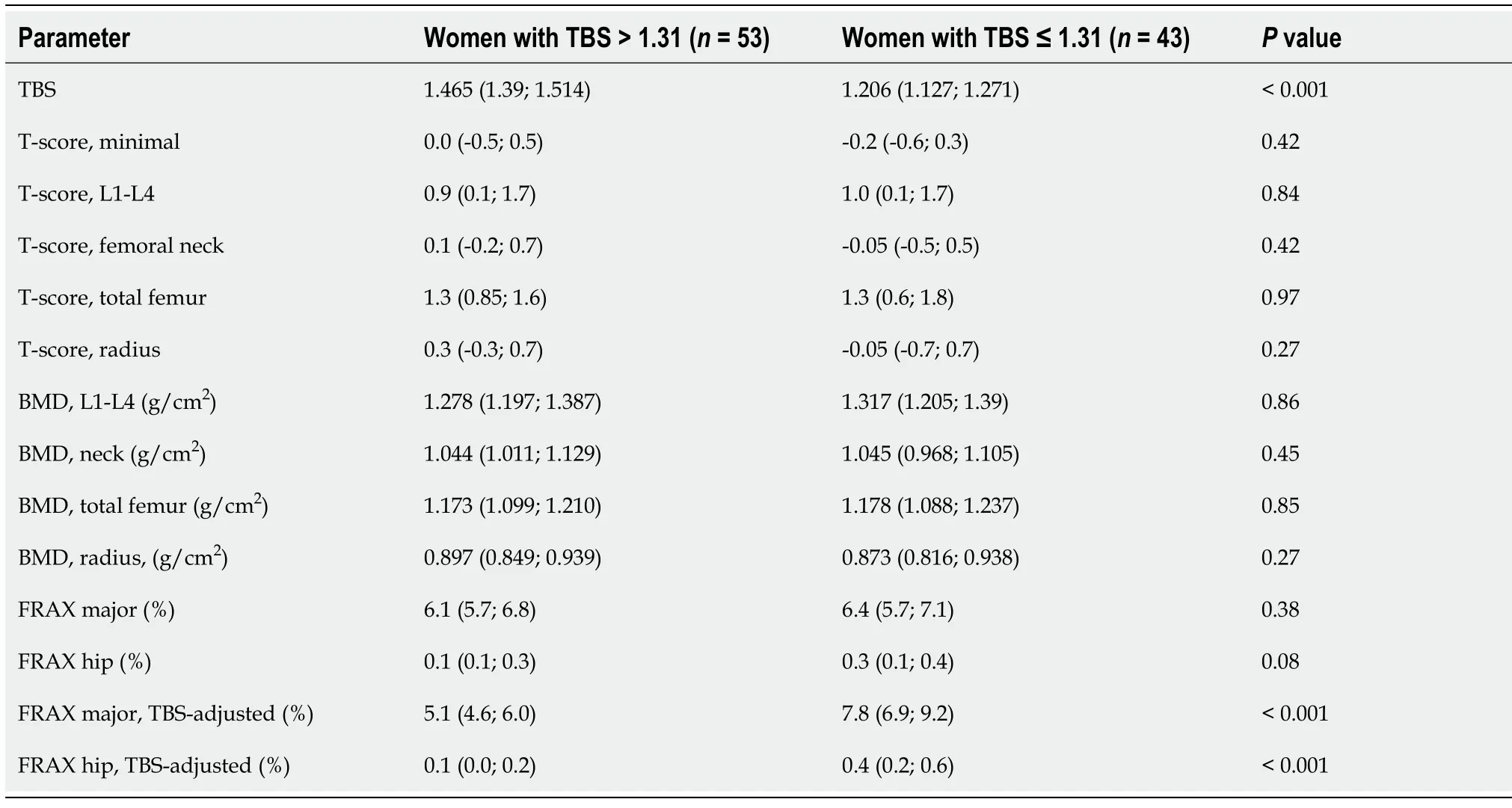
Six women with TBS > 1.31 and 14 women with TBS ≤ 1.31 had at least one fracture in their medical history (
= 5.64,
= 0.02). Two women with TBS > 1.31 had a low-energy fracture (humerus, tibia) in anamnesis. In the group of patients with TBS ≤ 1.31, nine women reported low-energy fractures of spine (
= 2), radius (
= 4), femur neck (
= 1) and humerus (
= 2). A difference in the prevalence of lowenergy fractures was statistically significant (
=6.05,
= 0.01). At the same time, there were no differences in BMD and T-score between two groups (Table 2). The 10-year risk of low-grade hip fractures was higher in those with TBS ≤ 1.31 (all
< 0.0001). The inclusion of TBS data in the FRAX algorithm exacerbated the differences between the groups.
As you have hitherto always behaved well in my service I will not send you to prison; but leave your place instantly and never let me see your face again
Ethical issues
The study protocol was approved by the Ethical Committee of the clinic of Research Institute of Clinical and Experimental Lymphology - Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences (protocol N. 104 from 20 December 2014). All study participants provided informed written consent prior to study enrollment.
Methods
The BMD and T-score at the lumbar spine (L1-L4), femur, femoral neck and forearm were assessed by DXA (Lunar Prodigy Advance bone densitometer, GE healthcare, Madison, WI, United States; database NHANES III; the Least significant change is 0.028 g/cm
for L1-L4, 0.033 g/cm
for femur, and 0.055 g/cm
for radius 33%). The TBS was estimated with the use of TBS iNsight software (version 3.0.2.0, GE healthcare). The Body Composition software (GE healthcare) was applied for assessment of body composition parameters, including bone mineral component, fat mass and lean mass, and fat distribution. Fat distribution patterns were differentiated based on the ratio of fat mass in the abdominal and hip areas (android and gynoid fat mass respectively)[18].
Potentially eligible subjects were screened at the clinic of Research Institute of Clinical and Experimental Lymphology - Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences (Novosibirsk, Russa), a tertiary referral center. All women underwent a detailed clinical examination, which included the assessment of glycemic control, in-depth screening/monitoring of diabetic complications and associated diseases. Women who met the inclusion criteria (1-4) and did not have the exclusion criteria underwent DXA to determine body composition, BMD and TBS. Those with abnormal BMD (T-score ≤ -1 SD) were excluded. The rest of the participants were divided into 2 groups:1) women with normal TBS (>1.31); 2) women with TBS reduction (≤1.31). The cut-off TBS value was chosen according to the results of meta-analysis [17]. The risk factors for TBS reduction were estimated by univariate and multivariate regression analysis and analysis of receiver operating characteristic (ROC)-curves.
The FRAX tool (web version 4.3, country-specific algorithm, https://www.sheffield.ac.uk/FRAX/to ol.aspx?country=13) was used to determine the ten-year risk of low-energy fractures. Both TBSunadjusted and TBS-adjusted FRAX scores were calculated.
The measurements of the levels of glycated hemoglobin A1c (HbA1c), total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, uric acid, creatinine, calcium, phosphorus and alkaline phosphatase were performed with a biochemical analyzer AU480 (Beckman Coulter, Minneapolis, MN, United States). eGFR was calculated using the CKD-EPI formula (2009). Albumin concentrations were determined in the morning urine samples by immunoturbidimetric method with a fully automated chemistry analyzer BS-120 (Mindray, Shenzhen, China); the result was adjusted to excreted creatinine. Serum levels of PTH and 25(OH)D were measured by ELISA with the use of Access 2 Immunoassay System analyzer (Beckman Coulter) and Access Intact PHT, Access 25(OH) Vitamin D Total kits (Beckman Coulter).
When you arrive at the troll s abode14, you must perform all kinds of foolish tricks, and see that you break a whole lot of his windows, and do all other damage that you can
Statistical analysis
Dell Statistica 13.0 (Dell Software, Aliso Viejo, CA, United States) was used for most of the applied statistical procedures. The sample size was calculated with a predetermined Type I error rate α = 0.05, power goal 1-β = 80% and standardized size effect 0.5 for clinical characteristics (age, duration of diabetes, age and duration of menopause, height, body weight, body mass index [BMI], waist-to-hip circumference), laboratory parameters (HbA1c, eGFR, calcium, phosphorus, 25(OH)D, PTH) and body composition (fat and lean mass, android and gynoid fat mass and percentage, android/gynoid fat mass ratio). The minimal number of participants in each group was defined as 34 persons. Assuming the prevalence of osteoporosis[19,20] and decreased TBS[21,22] in patients with T2D and using principles described previously[23,24], we estimated the minimal number of study participants as 150 individuals.
Quantitative data are presented as medians (lower quartiles; upper quartiles), frequencies are presented as percentages (%). The Kolmogorov-Smirnov (KS) test was applied to test the normality. As the majority of the quantitative parameters were not distributed normally, the non-parametric Mann-Whitney U-test was used for the group comparisons. The differences in discrete parameters were assessed using the
test.
values below 0.05 were considered as significant.
3. Hansel: In the original manuscript of the story, the brother was referred to as Little Brother. The Grimms chose the name Hansel for the character and included it in the first edition of their tales. Hansel is a common name used for a male character in German folktales. Hansel is essentially53 the same as John Doe representing an anonymous54 or everyman character.Return to place in story.
Spearman rank correlation analysis was applied to test associations between variables. Multiple linear regression analysis with backward elimination was used to reveal factors affecting TBS. The description of the model included beta coefficients with standard errors and
values, adjusted coefficient of determination (
), standard error of estimate and
value of the model.
To date, several imaging modalities, including DXA, radiography, micro-computed tomography, high-resolution peripheral quantitative computed tomography (HR-pQCT), and high-resolution magnetic resonance imaging, have been proposed for bone quality assessment[25]. Among these methods, HR-pQCT and TBS are the most used tools to study the bone microarchitecture in diabetes[26]. HR-pQCT is a non-invasive three-dimensional imaging modality for assessment of bone microarchitecture and bone strength in the appendicular skeleton (
, distal radius and tibia)[27]. In the Framingham-HR-pQCT study a modest deterioration in cortical bone and reductions in bone area in patients with T2D were revealed[28]. At the same time, in another population-based study by Nilsson
[29] more favorable bone microarchitecture was observed in elderly women with T2D compared to non-diabetic subjects. TBS is a gray-level textural metric that can be extracted from the two-dimensional lumbar spine DXA image[30]. This analytical method for bone microarchitecture assessment is more available and less expensive than HRpQCT.
A significant proportion (44.8%) of women in our study showed TBS values less than 1.31. Earlier it was found that T2D women 50 years old and over had lower TBS but higher BMD when compared to non-diabetic women[11]. Postmenopausal women with newly diagnosed T2D showed a decrease in TBS and bone formation markers[34]. A recent study has demonstrated a negative association between TBS and pre-diabetes in subjects aged over 60 years and discordance between TBS and BMD in these subjects[35]. Therefore, the reduction of TBS may reflect an early stage of the impairment of bone health in diabetes. Previously an inverse association between age and TBS was observed in population studies in French and non-Hispanic white US women[36,37]. In this study we were unable to identify age as an independent risk factor for TBS reduction. This can be explained by the relatively small sample size, the upper age limit of 75 years, and the greater influence of other risk factors.
Now the grandparents... I closed my eyes, dreading10 the hopelessness of my situation. I had no grandparent to stand proudly for me. I finally opened my eyes, and there they were, Job and Molly, standing11 proudly with all the other grandparents. Job looked over at me, his eyes beaming like diamonds.
Before she started she restored all the men whom her sister, Latifa, had bewitched, to their own forms, and received their blessings176, and set them forward to their homes
RESULTS
Study participants
Three hundred twelve women were initially screened, 176 of them met the inclusion criteria (1-4). These subjects underwent DXA with BMD and TBS assessment. According to DXA results, 17 women had osteoporosis and 63 had osteopenia; these individuals were excluded. Ultimately, 96 women with normal BMD were included in the final analysis.
The mean age of women was 64 years (range:50-75 years) and mean time since menopause was 16 years (range:1-37 years). Thirteen women were overweight, 79 were obese and four had a normal BMI. The BMI ranged from 19.1 to 50.2 kg/m
(median 33.6 kg/m
). The duration of T2D varied from 1 to 48 years (median 15 years). All patients received antihyperglycemic therapy, including metformin (
= 80), sulfonylurea (
= 34), sodium glucose cotransporter 2 inhibitors (
= 26), dipeptidyl peptidase-4 inhibitors (
= 9), and insulin (
= 70), mostly in combinations. The mean level of HbA1c was 8.76% (72.2 mmol/mol), ranging from 5.61 to 13.64% (37.7 to 125.6 mmol/mol).
Characteristics of women with T2D depending on TBS values
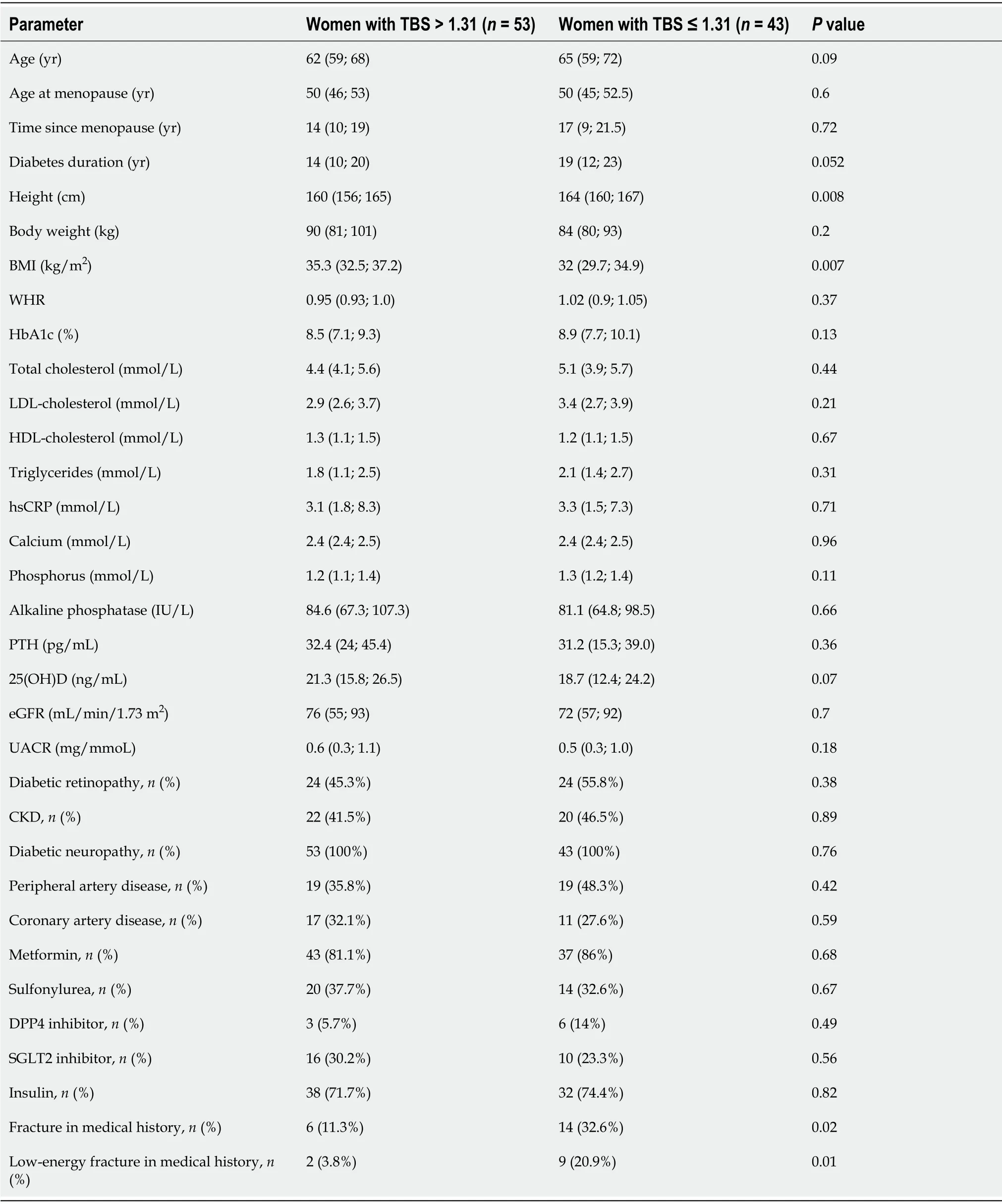
The clinical characteristics of women with preserved and decreased TBS are presented in Table 1. Women with TBS ≤ 1.31 were taller and had a lower BMI when compared to those with normal TBS (
= 0.008 and
= 0.007 respectively). There was a trend towards greater age and longer diabetes duration in women with TBS ≤ 1.31 (
= 0.09 and
= 0.052 respectively). The levels of HbA1c were slightly higher in women with TBS ≤ 1.31, but the difference with women with TBS > 1.31 were not statistically significant (
= 0.13). No differences in HbA1c, eGFR, calcium, phosphorus, alkaline phosphatase, PTH and 25(OH)D levels were found between the groups. Most women, including 45 (84.9%) with TBS > 1.31 and 38 women (88.4%) with TBS ≤ 1.31, had 25(OH)D concentrations < 30 ng/mL. The prevalence of diabetic complications and diabetes-associated conditions, as well as antihyperglycemic therapy, did not differ between the groups.
Night represents the unconscious, the feminine principle, death, evil, germination, potentiality, darkness, the subconscious, the womb, and the precursor of creation (Olderr 1986).Return to place in story.
The following list of exclusion criteria was applied:Endocrine diseases other than T2D (hyperthyroidism, hypothyroidism, hyperparathyroidism, hypopituitarism, acromegaly, and Cushing syndrome); Rheumatic diseases (rheumatoid arthritis, psoriatic arthritis, spondyloarthritis, systemic lupus erythematosus, systemic sclerosis, vasculitis, and crystal-induced arthritis); Inflammatory bowel diseases, celiac disease, malabsorption or bariatric surgery in medical history; Chronic kidney disease with an estimated glomerular filtration rate (eGFR) less than 45 mL/min/1.73 m
; Ever diagnosed with any kind of malignancy; Immobilization for more than one month in medical history; Treatment with thiazolidinediones, glucocorticoids, anticonvulsants or immunosuppressive drugs, postmenopausal hormonal replacement therapy, anti-osteoporotic therapy at the time of the study or in the past.
Ah! we shall soon see that! thought the old Queen-mother;4 however, she said not a word of what she was going to do; but went quietly into the bedroom, took all the bed-clothes off the bed, and put three little peas5 on the bedstead. She then laid twenty mattresses6 one upon another over the three peas, and put twenty feather beds7 over the mattresses4.
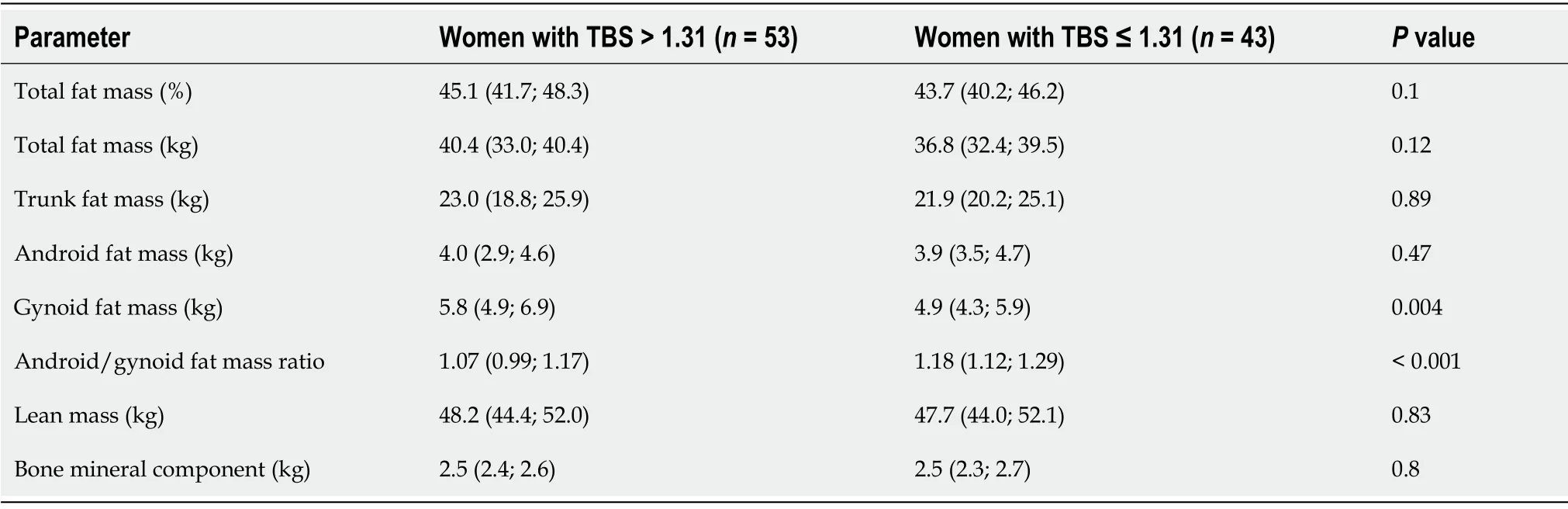
Women with reduced TBS had lower gynoid fat mass and higher android/gynoid fat mass ratio (
= 0.004 and
< 0.0001 respectively). No differences in trunk fat mass, lean mass and BMC were found (Table 3).
Associations of TBS with clinical and laboratory parameters
In observed women, TBS correlated positively with BMI (
= 0.33,
= 0.001), total fat mass (
= 0.26,
= 0.01) and gynoid fat mass (
= 0.39,
= 0.001). Height and android/gynoid fat mass ratio demonstrated inverse correlations with TBS (
= -0.26,
= 0.01 and
= -0.44,
= 0.00001 respectively), meanwhile, all assessed laboratory parameters, with the exception of 25(OH)D, did not show significant relationships. The levels of 25(OH)D demonstrated weak positive correlation with TBS (
= 0.21,
= 0.042). In addition, 25(OH)D correlated negatively with android fat mass (
= -0.20,
= 0.048), waist circumference (
= -0.24,
= 0.024), PTH (
= -0.34,
= 0.006), and alkaline phosphatase (
= -0.28,
= 0.007).
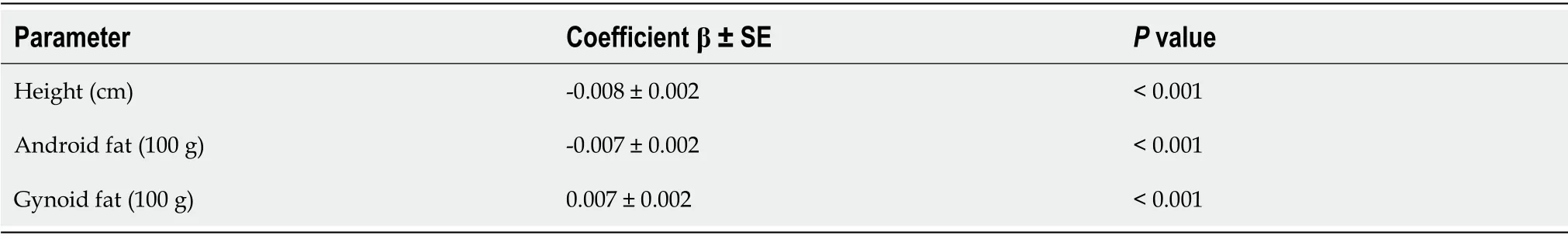
In a model of multivariate linear regression analysis, TBS was positively associated with gynoid fat mass (+0.007 per each 100 g), whereas the influence of height and androgen fat mass was negative (-0.008 per each cm and and -0.007 per each 100 g, respectively, Table 4). The same factors were identified in a multiple logistic regression model (Table 5). Thus, gynoid fat mass turned out to be a protective factor for TBS (-10% per each 100 g), while height and android fat mass were the risk factors for TBS reduction (+13% per each cm and each 100 g). However, the influence of android fat mass became significant only after being adjusted on height and gynoid fat mass. Moreover, the influence of all factors included in the logistic regression model increased after adjustment.
She bowed her head in her hands and cried, Is there no one under heaven who will take pity on me? Suddenly a soft voice replied, Be comforted, my child: I have come to help you
My teammates on the United States Disabled Ski Team used to tease me about the size of my chest, joking that my greatest handicap() wasn t my missing leg but my missing cleavage. Little did they know how true that would become. This past year, I found out that for the second time in my life I had cancer, this time in both breasts. I had bilateral2 mastectomies().
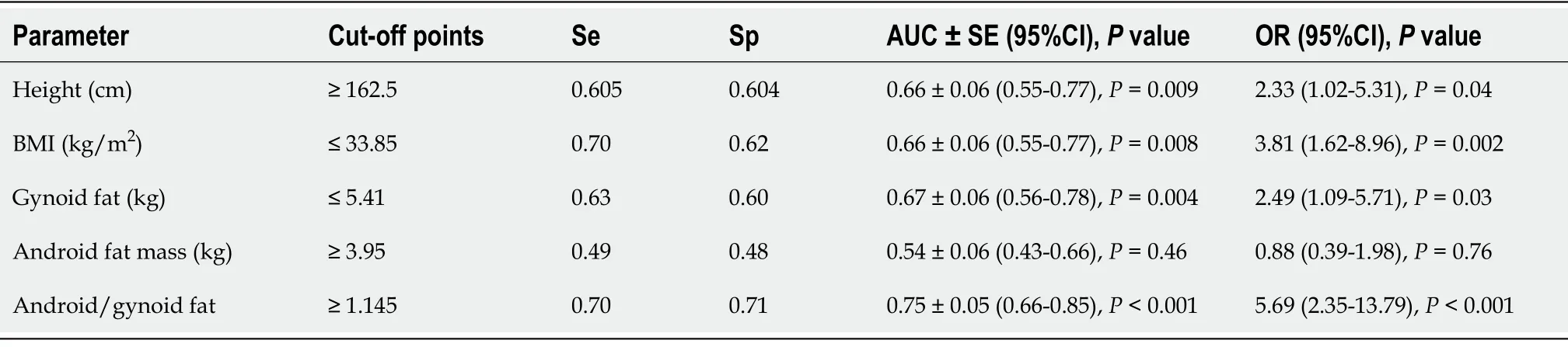
We have used ROC-analysis to estimate the cut-off values of the factors associated with TBS (Table 6). The height ≥ 162.5 cm, BMI ≤ 33.85 kg/m
, gynoid fat mass ≤ 5.4 kg (≤ 43.2%), and android/gynoid fat mass ratio ≥ 1.15 were identified as the risk factors of decreased TBS.
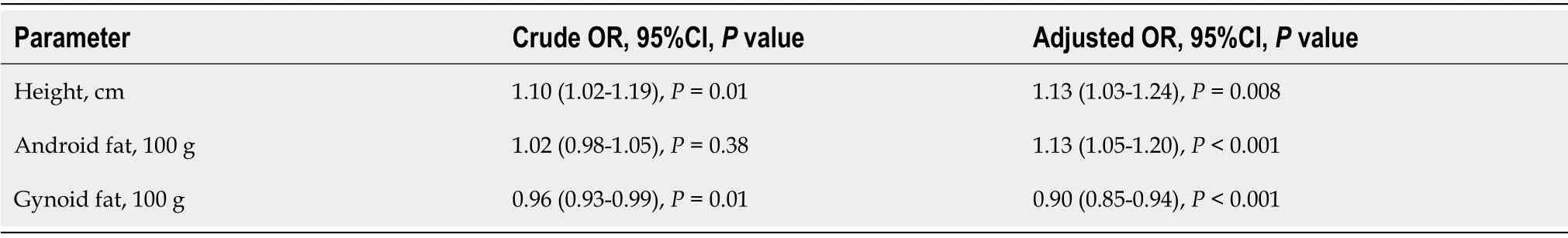
DlSCUSSlON
In this study, we investigated the effects of a number of anthropometric parameters, general and diabetes-related clinical characteristics and body composition on bone microarchitecture, assessed by TBS, in postmenopausal women with T2D and normal BMD.
Multiple logistic regression analysis with backward elimination was used to identify predictors of decreased TBS. The models with lower KS statistics p value and higher area under the curve (AUC), selectivity (Se), and specificity (Sp) were selected. Crude and adjusted odd ratio (OR), 95% confidence interval (CI) and
value were calculated for parameters included in the models.
The normal range for TBS remains a matter of debate. In 2012, an international working group of TBS users proposed the following criteria:TBS ≥ 1.35 is considered to be normal; TBS between 1.20 and 1.35 indicates partially degraded microarchitecture; finally, TBS ≤ 1.20 defines degraded microarchitecture[31]. Later, based on the results of meta-analysis of 14 population cohort studies from North America, Asia, Australia, and Europe (
= 17809) estimated relationship between TBS and fracture risk, slightly different criteria for assessing TBS have been proposed[17]. TBS > 1.31 was attributed to normal microarchitecture, TBS values between 1.23 and 1.31 were associated with partially degraded microarchitecture, and TBS < 1.23 was considered as an indicator of degraded microarchitecture. Taken into account that fractures are the most important clinical events related to the bone health, in this study we also used the cut-off value 1.31 to differentiate women with normal and degrade microarchitecture. This cut-off point has been also applied in recent osteoporosis studies[32,33]. Given the relatively small sample size, we did not distinguish a subgroup of patients with borderline TBS (1.23-1.31).
To assess the parameters associated with decreased TBS, ROC-curve analysis was performed with IBM SPSS Statistics for Windows, Version 26.0 (International Business Machines Corporation, Armonk, NY, USA). The AUC with 95%CI and
value were calculated. The results were considered significant if the AUC with a lower border of 95%CI was above 0.5 and
value was below 0.05. The cut-off values were found with both Se and Sp above 0.55.
The association between abdominal obesity and impaired bone microarchitecture can be mediated
insulin resistance[43]. Increased bone marrow adiposity, the changes in adipokine production and lowgrade inflammation are considered as the relevant mechanisms also[45]. In addition, vitamin D deficiency can worsen bone microarchitecture in women with T2D and abdominal obesity. In our cohort, 25(OH)D demonstrated negative correlation with waist circumference and abdominal fat mass. This data is in agreement with findings from recent meta-analysis of epidemiologic studies indicating an association between vitamin D deficiency and abdominal obesity[46]. Vitamin D deficiency in obese people is attributed to lower dietary intake of vitamin D, lesser skin exposure to sunlight, decreased vitamin absorption, impaired hydroxylation in adipose tissue and 25(OH)D accumulation in fat[47]. At the same time, it is believed that vitamin D deficiency can be associated with insulin resistance and related disorders[48,49].
Our results indicate that greater height, lower BMI and gynoid fat mass, but higher android fat mass and android/gynoid fat mass ratio contribute to TBS decrease in women with T2D. A favorable effect of BMI and fat mass on BMD in postmenopausal women with T2D was documented in previous studies[38,39]. However, data on the effect of obesity on the bone metabolism, TBS and fracture risk are not so optimistic[40-42]. In disagreement with previously reported data[43], we observed a positive association between BMI and TBS. At the same time, we found negative association between android/gynoid fat mass ratio and TBS. Moreover, gynoid fat turned out to be a protective factor and android fat was a risk factor for TBS reduction. These findings provide further support to notion that not only fat mass, but also fat distribution, is important for bone health. Previously, inverse association between android fat and TBS was found in Chinese men[44]. Moon
[40] have shown that TBS increase as visceral fat mass decrease in men and women with T2D. In the Newcastle Thousand Families Study an increase in total and, especially, visceral fat mass was associated with prevalent vertebral fracture irrespective of BMD in women aged about 62 years[41]. It was shown that abdominal fat is related to retarded bone formation and impaired bone quality in premenopausal women[42]. Therefore, central adiposity can be considered as a risk factor of bone fragility in T2D.
The role of hyperglycemia as a factor contributing to the degradation of bone microarchitecture is widely discussed. The mechanisms of bone fragility in hyperglycemia include the accumulation of advanced glycation end products and collagen cross-linking, suppressed osteoid mineralization, reduced osteoblastogenesis, and retarded bone turnover[50]. Ho-Pham
[13] reported that subjects with pre-diabetes have a decrease in TBS when compared with normal individuals. At the same time, Holloway
[14] found no difference in TBS between subjects with normoglycaemia and impaired fasting glucose. A negative association between TBS and HbA1c has been reported in subjects with diabetes[51]. In the Maasticht study a negative association was found between HbA1c and parameters of bone health estimated by HR-pQCT in individuals with well-controlled T2D[52]. In our study, HbA1c was only slightly higher in patients with TBS ≤ 1.31. Even though we did not identify HbA1c as a risk factor for a decrease TBS, we cannot exclude the role of hyperglycemia in the deterioration of bone microarchitecture. Most of the patients had long-term diabetes and non-target glycemic control parameters on combined antidiabetic therapy. These factors could modify the effect of hyperglycemia on TBS. Besides, single HbA1c measurements were included in the analysis. Therefore, the effect of metabolic memory on bone structure cannot be ruled out.
Sunrise on the eastern coast is a special event. I stood at Dolphin s Nose, a spur jutting1 out into the Bay of Bengal, to behold2 the breaking of the sun s upper limb over the horizon of the sea. As the eastern sky started unfolding like the crimson3 petals4 of a gigantic flower, I was overcome by a wave of romantic feelings and nostalgia5(,) -- vivid memorie not diminished by the fact that almost ten years had passed.
The value of TBS as a predictor of low-energy fractures is a matter of increasing interest. It was demonstrated that in postmenopausal women with T2D TBS rather than BMD is associated with vertebral[53] and major osteoporotic fractures[11]. The FRAX score, being unadjusted to TBS, underestimates fracture risk in these women[54]. In our study, women with normal and reduced TBS demonstrated no differences in the unadjusted FRAX scores, although they were different in the prevalent fractures. As expected, incorporation of TBS values into the FRAX algorithm increased probability of the fractures in women with lower TBS. Therefore, TBS can help to improve the assessment of the risk of fractures in women with T2D and normal BMD. However, even after TBS adjustment the risk of fractures may be underestimated. A recent population-based prospective study by Leslie
[55] (the Manitoba BMD Registry) showed that a residual effect of diabetes on major osteoporotic fractures estimated with FRAX persists even after TBS adjustment, though the adjustment attenuated the effect of the disease. Adjustment for diabetes further improves the quality of fracture prediction.
The cross-sectional design and relatively small sample size are the limitations of our study. The recruitment of patients in one clinical center could lead to some sample bias. We could not differentiate visceral and subcutaneous adipose tissue with the applied DXA technique. As the used version of TBS iNsight software does not correct for extremes of BMI, we cannot exclude some underestimation of TBS in patients with obesity class 2 and 3[56].
At the same time, as far as we know, this is the first study estimating the risk factors for impaired bone microarchitecture assessed by TBS in postmenopausal women with T2D and normal BMD. Further studies of a larger size and prospective design are needed to establish the role of the identified factors as predictors of TBS reduction in these women. The value of TBS in the prediction of osteoporosis-related fractures in postmenopausal women with T2D and normal BMD is another challenge for future research.
CONCLUSlON
In this study, we have revealed a decrease in the TBS values in 44.8% of postmenopausal women with T2D and normal BMD. These data indicate that a substantial proportion of postmenopausal women with T2D have impaired bone microarchitecture despite the normal BMD parameters. Greater height and central adiposity turned out to be the risk factors for impaired bone microarchitecture in these women. The results give further support to notion that estimation of TBS should be an essential element of DXA protocol in postmenopausal women with T2D.
ARTlCLE HlGHLlGHTS
Research background
People with type 2 diabetes (T2D) have higher risk of vertebral and some non-vertebral fractures than non-diabetic individuals, regardless of normal or even increased bone mineral density (BMD). As BMD assessment may lead to underestimation of a fracture risk in T2D, additional parameters of bone health should be taken into consideration. The impaired bone microarchitecture is considered as a major contributor to fracture risk in T2D. Trabecular Bone Score (TBS) on lumbar spine dual X-ray absorptiometry(DXA) images is increasingly applied for the assessment of bone microarchitecture. Individuals with diabetes as compared to those without have significantly lower TBS.
Research motivation
At present, data on TBS in postmenopausal women with T2D and normal BMD is limited, and predictors of TBS decrease in these women need to be refined. In particular, the role of body composition and diabetes-related parameters as risk factors for deterioration of bone microarchitecture needs further research.
Research objectives
To identify clinical and body composition parameters that affect TBS in postmenopausal women with T2D and normal BMD.
So she went home, but alas10! she said MORE THAN THREE WORDS; and immediately the iron stove vanished11 and went away over a mountain of glass and sharp swords
Research methods
A non-interventional cross-sectional comparative study was conducted. Postmenopausal women with T2D, aged 50-75 years, with no established risk factors for secondary osteoporosis, were included. BMD,TBS and body composition parameters were assessed by DXA. In women with normal BMD, a wide range of anthropometric, general and diabetes-related clinical and laboratory parameters were evaluated as risk factors for TBS decrease using univariate and multivariate regression analysis and analysis of receiver operating characteristic (ROC) curves.
Research results
Among women with normal BMD, 44.8% showed decreased TBS values (≤ 1.31). Women with decreased TBS were taller and had a lower BMI when compared to those with normal TBS. No significant differences in HbA1c, renal function, calcium, phosphorus, alkaline phosphatase, PTH and 25(OH)D levels were found. In the models of multivariate regression analysis, TBS was positively associated with gynoid fat mass, whereas the height and androgen fat mass were associated negatively.In the ROC-curve analysis, height ≥ 162.5 cm, body mass index < 33.85 kg/m
, gynoid fat mass ≤ 5.41 kg and android/gynoid fat mass ratio ≥ 1.145 were identified as the risk factors for TBS reduction.
Research conclusions
The obtained results indicate that a substantial proportion of postmenopausal women with T2D and normal BMD may have impaired bone microarchitecture. Greater height and central adiposity turned out to be the risk factors for decreased TBS in these women. The results give further support to notion that estimation of TBS should be an essential element of DXA protocol in postmenopausal women with T2D.
Research perspectives
The prognostic value of TBS as a risk factor for fractures in patients with T2D and normal BMD needs further research. Prospective studies should determine the effect of changes in body weight and body composition on bone microarchitecture in these patients. The impact of hyperglycemia, glucose variability and metabolic memory, as well as various antihyperglycemic drugs, also needs to be clarified.
Klimontov VV contributed to study conception and design, data analysis and interpretation; Fazullina ON and Korbut AI collected the data and performed data analysis and interpretation; Fazullina ON and Klimontov VV wrote the article; all authors approved the final version of the manuscript.
The study protocol was approved by the Ethical Committee of the RICEL - Branch of IC&G SB RAS (protocol N. 104 from 20 December 2014).
All study participants provided informed written consent prior to study enrollment.
There are no conflicts of interest to report.
No additional data are available.
The fox climbed up stealthily and caught the little creatures with his paws one after the other; and when he had killed them all he put their blood into a little bottle which he wore at his side and returned with it to Grannonia, who was beside herself with joy at the result of the fox s raid
The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See:https://creativecommons.org/Licenses/by-nc/4.0/
Russia
Olga N Fazullina 0000-0002-5868-579X; Anton I Korbut 0000-0003-3502-5892; Vadim V Klimontov 0000-0002-5407-8722.
Chang KL
A
Chang KL
1 Tebé C, Martínez-Laguna D, Carbonell-Abella C, Reyes C, Moreno V, Diez-Perez A, Collins GS, Prieto-Alhambra D. The association between type 2 diabetes mellitus, hip fracture, and post-hip fracture mortality:a multi-state cohort analysis.
2019; 30:2407-2415 [PMID:31444526 DOI:10.1007/s00198-019-05122-3]
2 Sato M, Ye W, Sugihara T, Isaka Y. Fracture risk and healthcare resource utilization and costs among osteoporosis patients with type 2 diabetes mellitus and without diabetes mellitus in Japan:retrospective analysis of a hospital claims database.
2016; 17:489 [PMID:27887655 DOI:10.1186/s12891-016-1344-9]
3 Xu Y, Wu Q. Trends in osteoporosis and mean bone density among type 2 diabetes patients in the US from 2005 to 2014.
2021; 11:3693 [PMID:33580184 DOI:10.1038/s41598-021-83263-4]
4 Moayeri A, Mohamadpour M, Mousavi SF, Shirzadpour E, Mohamadpour S, Amraei M. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors:a systematic review and meta-analysis.
2017; 13:455-468 [PMID:28442913 DOI:10.2147/TCRM.S131945]
5 Koromani F, Oei L, Shevroja E, Trajanoska K, Schoufour J, Muka T, Franco OH, Ikram MA, Zillikens MC, Uitterlinden AG, Krestin GP, Anastassiades T, Josse R, Kaiser SM, Goltzman D, Lentle BC, Prior JC, Leslie WD, McCloskey E, Lamy O, Hans D, Oei EH, Rivadeneira F. Vertebral Fractures in Individuals With Type 2 Diabetes:More Than Skeletal Complications Alone.
2020; 43:137-144 [PMID:31658976 DOI:10.2337/dc19-0925]
6 Oei L, Zillikens MC, Dehghan A, Buitendijk GH, Castaño-Betancourt MC, Estrada K, Stolk L, Oei EH, van Meurs JB, Janssen JA, Hofman A, van Leeuwen JP, Witteman JC, Pols HA, Uitterlinden AG, Klaver CC, Franco OH, Rivadeneira F. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control:the Rotterdam Study.
2013; 36:1619-1628 [PMID:23315602 DOI:10.2337/dc12-1188]
7 Goldshtein I, Nguyen AM, dePapp AE, Ish-Shalom S, Chandler JM, Chodick G, Shalev V. Epidemiology and correlates of osteoporotic fractures among type 2 diabetic patients.
2018; 13:15 [PMID:29502187 DOI:10.1007/s11657-018-0432-x]
8 Botella Martínez S, Varo Cenarruzabeitia N, Escalada San Martin J, Calleja Canelas A. The diabetic paradox:Bone mineral density and fracture in type 2 diabetes.
2016; 63:495-501 [PMID:27481443 DOI:10.1016/j.endonu.2016.06.004]
9 Harvey NC, Glüer CC, Binkley N, McCloskey EV, Brandi ML, Cooper C, Kendler D, Lamy O, Laslop A, Camargos BM, Reginster JY, Rizzoli R, Kanis JA. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice.
2015; 78:216-224 [PMID:25988660 DOI:10.1016/j.bone.2015.05.016]
10 Palui R, Pramanik S, Mondal S, Ray S. Critical review of bone health, fracture risk and management of bone fragility in diabetes mellitus.
2021; 12:706-729 [PMID:34168723 DOI:10.4239/wjd.v12.i6.706]
11 Leslie WD, Aubry-Rozier B, Lamy O, Hans D; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk.
2013; 98:602-609 [PMID:23341489 DOI:10.1210/jc.2012-3118]
12 Lin YC, Wu J, Kuo SF, Cheung YC, Sung CM, Fan CM, Chen FP, Mhuircheartaigh JN. Vertebral Fractures in Type 2 Diabetes Patients:Utility of Trabecular Bone Score and Relationship With Serum Bone Turnover Biomarkers.
2020; 23:37-43 [PMID:30773275 DOI:10.1016/j.jocd.2019.01.003]
13 Ho-Pham LT, Nguyen TV. Association between trabecular bone score and type 2 diabetes:a quantitative update of evidence.
2019; 30:2079-2085 [PMID:31214749 DOI:10.1007/s00198-019-05053-z]
14 Holloway KL, De Abreu LLF, Hans D, Kotowicz MA, Sajjad MA, Hyde NK, Pasco JA. Trabecular Bone Score in Men and Women with Impaired Fasting Glucose and Diabetes.
2018; 102:32-40 [PMID:28965154 DOI:10.1007/s00223-017-0330-z]
15 Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL; IOF Bone and Diabetes Working Group. Mechanisms of diabetes mellitus-induced bone fragility.
2017; 13:208-219 [PMID:27658727 DOI:10.1038/nrendo.2016.153]
16 Costantini S, Conte C. Bone health in diabetes and prediabetes.
2019; 10:421-445 [PMID:31523379 DOI:10.4239/wjd.v10.i8.421]
17 McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, Barkmann R, Boutroy S, Brown J, Chapurlat R, Elders PJM, Fujita Y, Glüer CC, Goltzman D, Iki M, Karlsson M, Kindmark A, Kotowicz M, Kurumatani N, Kwok T, Lamy O, Leung J, Lippuner K, Ljunggren Ö, Lorentzon M, Mellström D, Merlijn T, Oei L, Ohlsson C, Pasco JA, Rivadeneira F, Rosengren B, Sornay-Rendu E, Szulc P, Tamaki J, Kanis JA. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX.
2016; 31:940-948 [PMID:26498132 DOI:10.1002/jbmr.2734]
18 Klimontov VV, Bulumbaeva DM, Fazullina ON, Lykov AP, Bgatova NP, Orlov NB, Konenkov VI, Pfeiffer AFH, Pivovarova-Ramich O, Rudovich N. Circulating Wnt1-inducible signaling pathway protein-1 (WISP-1/CCN4) is a novel biomarker of adiposity in subjects with type 2 diabetes.
2020; 14:101-109 [PMID:31782053 DOI:10.1007/s12079-019-00536-4]
19 DeShields SC, Cunningham TD. Comparison of osteoporosis in US adults with type 1 and type 2 diabetes mellitus.
2018; 41:1051-1060 [PMID:29353395 DOI:10.1007/s40618-018-0828-x]
20 Schacter GI, Leslie WD. Diabetes and Osteoporosis:Part I, Epidemiology and Pathophysiology.
2021; 50:275-285 [PMID:34023043 DOI:10.1016/j.ecl.2021.03.005]
21 Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM. Bone quality assessment in type 2 diabetes mellitus.
2014; 25:1969-1973 [PMID:24718377 DOI:10.1007/s00198-014-2704-7]
22 Nikfarjam M, Heshmat R, Gharibzadeh S, Ostovar A, Maleki V, Moludi J, Nabipour I, Shafiee G, Larijani B. The association between muscle indicators and bone mass density and related risk factors in the diabetic elderly population:Bushehr Elderly Health (BEH) Program.
2021; 20:1429-1438 [PMID:34900794 DOI:10.1007/s40200-021-00881-5]
23 Fritz CO, Morris PE, Richler JJ. Effect size estimates:current use, calculations, and interpretation.
2012; 141:2-18 [PMID:21823805 DOI:10.1037/a0024338]
24 Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited:simplified and practical approaches in pre-clinical, clinical and laboratory studies.
2021; 31:010502 [PMID:33380887 DOI:10.11613/BM.2021.010502]
25 Wang F, Zheng L, Theopold J, Schleifenbaum S, Heyde CE, Osterhoff G. Methods for bone quality assessment in human bone tissue:a systematic review.
2022; 17:174 [PMID:35313901 DOI:10.1186/s13018-022-03041-4]
26 Martínez-Montoro JI, García-Fontana B, García-Fontana C, Muñoz-Torres M. Evaluation of Quality and Bone Microstructure Alterations in Patients with Type 2 Diabetes:A Narrative Review.
2022; 11 [PMID:35456299 DOI:10.3390/jcm11082206]
27 Nishiyama KK, Shane E. Clinical imaging of bone microarchitecture with HR-pQCT.
2013; 11:147-155 [PMID:23504496 DOI:10.1007/s11914-013-0142-7]
28 Samelson EJ, Demissie S, Cupples LA, Zhang X, Xu H, Liu CT, Boyd SK, McLean RR, Broe KE, Kiel DP, Bouxsein ML. Diabetes and Deficits in Cortical Bone Density, Microarchitecture, and Bone Size:Framingham HR-pQCT Study.
2018; 33:54-62 [PMID:28929525 DOI:10.1002/jbmr.3240]
29 Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellström D, Rudäng R, Zoulakis M, Wallander M, Darelid A, Lorentzon M. Type 2 Diabetes Mellitus Is Associated With Better Bone Microarchitecture But Lower Bone Material Strength and Poorer Physical Function in Elderly Women:A Population-Based Study.
2017; 32:1062-1071 [PMID:27943408 DOI:10.1002/jbmr.3057]
30 Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP. Trabecular bone score:a noninvasive analytical method based upon the DXA image.
2014; 29:518-530 [PMID:24443324 DOI:10.1002/jbmr.2176]
31 Cormier C, Lamy O, Poriau S. TBS in routine clinial practice:proposals of use. Switzerland:Medimaps Group, 2012. [cited 1 February 2022]. Available from:https://sanova.at/wp-content/uploads/2020/08/Atlas_TBS.pdf
32 Binkley N, Morin SN, Martineau P, Lix LM, Hans D, Leslie WD. Frequency of normal bone measurement in postmenopausal women with fracture:a registry-based cohort study.
2020; 31:2337-2344 [PMID:32778934 DOI:10.1007/s00198-020-05576-w]
33 Panahi N, Ostovar A, Fahimfar N, Aghaei Meybodi HR, Gharibzadeh S, Arjmand B, Sanjari M, Khalagi K, Heshmat R, Nabipour I, Soltani A, Larijani B. Factors associated with TBS worse than BMD in non-osteoporotic elderly population:Bushehr elderly health program.
2021; 21:444 [PMID:34315430 DOI:10.1186/s12877-021-02375-8]
34 Li H, Wen Y, Liu P, Zhang L, Zhang X, Liu Y, Ma B, Kuang H, Wang J, Song L. Characteristics of bone metabolism in postmenopausal women with newly diagnosed type 2 diabetes mellitus.
2021; 95:430-438 [PMID:34008210 DOI:10.1111/cen.14501]
35 Ebrahimpur M, Sharifi F, Nezhad FA, Bagherzadeh M, Ostovar A, Shafiee G, Heshmat R, Mehrdad N, Razi F, Khashayar P, Nabipour I, Larijani B. Effect of diabetes on BMD and TBS values as determinants of bone health in the elderly:Bushehr Elderly Health program.
2019; 18:99-106 [PMID:31275880 DOI:10.1007/s40200-019-00395-1]
36 Dufour R, Winzenrieth R, Heraud A, Hans D, Mehsen N. Generation and validation of a normative, age-specific reference curve for lumbar spine trabecular bone score (TBS) in French women.
2013; 24:2837-2846 [PMID:23681084 DOI:10.1007/s00198-013-2384-8]
37 Simonelli C, Leib E, Mossman N, Winzenrieth R, Hans D, McClung M. Creation of an age-adjusted, dual-energy x-ray absorptiometry-derived trabecular bone score curve for the lumbar spine in non-Hispanic US White women.
2014; 17:314-319 [PMID:24582086 DOI:10.1016/j.jocd.2013.09.002]
38 Raška I Jr, Rašková M, Zikán V, Škrha J. Body composition is associated with bone and glucose metabolism in postmenopausal women with type 2 diabetes mellitus.
2017; 66:99-111 [PMID:27782739 DOI:10.33549/physiolres.933310]
39 Chain A, Crivelli M, Faerstein E, Bezerra FF. Association between fat mass and bone mineral density among Brazilian women differs by menopausal status:The Pró-Saúde Study.
2017; 33:14-19 [PMID:27908545 DOI:10.1016/j.nut.2016.08.001]
40 Moon HU, Lee N, Chung YS, Choi YJ. Reduction of visceral fat could be related to the improvement of TBS in diabetes mellitus.
2020; 38:702-709 [PMID:32399674 DOI:10.1007/s00774-020-01107-z]
41 Hind K, Pearce M, Birrell F. Total and Visceral Adiposity Are Associated With Prevalent Vertebral Fracture in Women but Not Men at Age 62 Years:The Newcastle Thousand Families Study.
2017; 32:1109-1115 [PMID:28261864 DOI:10.1002/jbmr.3085]
42 Cohen A, Dempster DW, Recker RR, Lappe JM, Zhou H, Zwahlen A, Müller R, Zhao B, Guo X, Lang T, Saeed I, Liu XS, Guo XE, Cremers S, Rosen CJ, Stein EM, Nickolas TL, McMahon DJ, Young P, Shane E. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women:a transiliac bone biopsy study.
2013; 98:2562-2572 [PMID:23515452 DOI:10.1210/jc.2013-1047]
43 Hayón-Ponce M, García-Fontana B, Avilés-Pérez MD, González-Salvatierra S, Andújar-Vera F, Moratalla-Aranda E, Muñoz-Torres M. Lower trabecular bone score in type 2 diabetes mellitus:A role for fat mass and insulin resistance beyond hyperglycaemia.
2021; 47:101276 [PMID:34517124 DOI:10.1016/j.diabet.2021.101276]
44 Lv S, Zhang A, Di W, Sheng Y, Cheng P, Qi H, Liu J, Yu J, Ding G, Cai J, Lai B. Assessment of Fat distribution and Bone quality with Trabecular Bone Score (TBS) in Healthy Chinese Men.
2016; 6:24935 [PMID:27112305 DOI:10.1038/srep24935]
45 Gkastaris K, Goulis DG, Potoupnis M, Anastasilakis AD, Kapetanos G. Obesity, osteoporosis and bone metabolism.
2020; 20:372-381 [PMID:32877973]
46 Hajhashemy Z, Foshati S, Saneei P. Relationship between abdominal obesity (based on waist circumference) and serum vitamin D levels:a systematic review and meta-analysis of epidemiologic studies.
2022; 80:1105-1117 [PMID:34537844 DOI:10.1093/nutrit/nuab070]
47 Migliaccio S, Di Nisio A, Mele C, Scappaticcio L, Savastano S, Colao A; Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. Obesity and hypovitaminosis D:causality or casualty?
2019; 9:20-31 [PMID:31391922 DOI:10.1038/s41367-019-0010-8]
48 Szymczak-Pajor I, Drzewoski J, Śliwińska A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders.
2020; 21 [PMID:32932777 DOI:10.3390/ijms21186644]
49 Theik NWY, Raji OE, Shenwai P, Shah R, Kalluri SR, Bhutta TH, Hannoodee H, Al Khalili M, Khan S. Relationship and Effects of Vitamin D on Metabolic Syndrome:A Systematic Review.
2021; 13:e17419 [PMID:34589329 DOI:10.7759/cureus.17419]
50 Romero-Díaz C, Duarte-Montero D, Gutiérrez-Romero SA, Mendivil CO. Diabetes and Bone Fragility.
2021; 12:71-86 [PMID:33185853 DOI:10.1007/s13300-020-00964-1]
51 Kim JH, Choi HJ, Ku EJ, Kim KM, Kim SW, Cho NH, Shin CS. Trabecular bone score as an indicator for skeletal deterioration in diabetes.
2015; 100:475-482 [PMID:25368976 DOI:10.1210/jc.2014-2047]
52 de Waard EAC, de Jong JJA, Koster A, Savelberg HHCM, van Geel TA, Houben AJHM, Schram MT, Dagnelie PC, van der Kallen CJ, Sep SJS, Stehouwer CDA, Schaper NC, Berendschot TTJM, Schouten JSAG, Geusens PPMM, van den Bergh JPW. The association between diabetes status, HbA1c, diabetes duration, microvascular disease, and bone quality of the distal radius and tibia as measured with high-resolution peripheral quantitative computed tomography-The Maastricht Study.
2018; 29:2725-2738 [PMID:30209523 DOI:10.1007/s00198-018-4678-3]
53 Yamamoto M, Yamauchi M, Sugimoto T. Prevalent vertebral fracture is dominantly associated with spinal microstructural deterioration rather than bone mineral density in patients with type 2 diabetes mellitus.
2019; 14:e0222571 [PMID:31525243 DOI:10.1371/journal.pone.0222571]
54 Majumdar SR, Leslie WD, Lix LM, Morin SN, Johansson H, Oden A, McCloskey EV, Kanis JA. Longer Duration of Diabetes Strongly Impacts Fracture Risk Assessment:The Manitoba BMD Cohort.
2016; 101:4489-4496 [PMID:27603908 DOI:10.1210/jc.2016-2569]
55 Leslie WD, Johansson H, McCloskey EV, Harvey NC, Kanis JA, Hans D. Comparison of Methods for Improving Fracture Risk Assessment in Diabetes:The Manitoba BMD Registry.
2018; 33:1923-1930 [PMID:29953670 DOI:10.1002/jbmr.3538]
56 Amnuaywattakorn S, Sritara C, Utamakul C, Chamroonrat W, Kositwattanarerk A, Thamnirat K, Ongphiphadhanakul B. Simulated increased soft tissue thickness artefactually decreases trabecular bone score:a phantom study.
2016; 17:17 [PMID:26757709 DOI:10.1186/s12891-016-0886-1]
杂志排行
World Journal of Diabetes的其它文章
- More studies are necessary to establish the effectiveness of Jinhuang powder in the treatment of diabetic foot
- Epidemiology for public health practice:The application of spatial epidemiology
- Relationship between quality of life and adolescent glycolipid metabolism disorder:A cohort study
- Elevated levels of fructosamine are independently associated with SARS-CoV-2 reinfection:A 12-mo follow-up study
- Efficacy and mechanism of anti-vascular endothelial growth factor drugs for diabetic macular edema patients
- Association between urinary concentrations of bisphenol A substitutes and diabetes in adults
