MeOTf-catalyzed formal [4+2]annulations of styrene oxides with alkynes leading to polysubstituted naphthalenes through sequential electrophilic cyclization/ring expansion
2022-07-11SongZouZeyuZhngChoChenChnjunXi
Song Zou,Zeyu Zhng,Cho Chen,∗,Chnjun Xi,b,∗
a MOE Key Laboratory of Bioorganic Phosphorus Chemistry &Chemical Biology,Department of Chemistry,Tsinghua University,Beijing 100084,China
b State Key Laboratory of Elemento-Organic Chemistry,Nankai University,Tianjin 300071,China
Keywords:Methyltriflate Catalytic reaction Annulation Ring expansion Polysubstituted naphthalenes
ABSTRACT MeOTf-catalyzed formal [4+2]annulation of styrene oxides with alkynes to afford polysubstituted naphthalenes has been realized,which undergoes sequential electrophilic cyclization/ring expansion.A range of substrates were tolerated in the formation of naphthalene derivatives with high regioselectivity in satisfactory yields.The reaction could also be carried out on gram scale.
Polysubstituted naphthalenes are found in a wide variety of biologically active molecules [1–7].They have also been employed in a range of optical and electronic materials [8–10].Owing to the important application of the naphthalenes,their synthesis have been extensively studied [11–33].Among them,catalytic construction of the second aromatic ring of the naphthalene frame through the incorporation of two carbons of alkene or alkyne unitviaa formal [4+2]process with aryl compounds is irrefutably straightforward [34–42].For example,gold(I)-catalyzed intramolecular annulations of aryl alkenyl carbonyl compounds (Scheme 1A-a) [34]or aryl enynes (Scheme 1A-b) [35]to afford substituted naphthalenes.Examples on catalytic intermolecular transformations including Pdcatalyzed annulation of 1-phenylalken-2-yl halides or triflates or metals with alkynes (Scheme 1B-c)][38]and/or metal-catalyzed or promoted condensation ofα-aryl substituted carbonyl compounds with alkynes (Scheme 1B-d) [39–41]have been reported.Arylethylene oxides are versatile and valuable synthetic building blocks for the synthesis of organic molecules due to their ready availability and high reactivity to undergo different organic transformations[43–52].For example,Au-or Ga-catalyzed reaction of aryl ethylene oxides with alkynes to afford naphthalenes through Meinwald rearrangement/electrophilic cyclization (Scheme 1C,path a) [51,52].Despite these progresses,developing metal-free reaction for the synthesis of naphthalenes is still highly desirable,especially metalfree reaction in the drug scanning process is of great interest since removing potentially toxic trace metals from the end products are omitted.Inspired by great progress on the annulation of aryl ethylene oxides and our ongoing project on methyltriflate (MeOTf)-promoted cyclization,we herein describe a MeOTf-catalyzed intermolecular formal [4+2]annulation of aryl ethylene oxides with alkynes to afford polysubstituted naphthalenes.This reaction undergoes an electrophilic cyclization of arylepoxides with alkynes followed by ring-expansion to afford polysubstituted naphthalenes with high regioselectivity (Scheme 1C,path b),although few examples on HNTf2-catalyzed benzannulation of epoxides with prop–1-yn-1-ylbenzene to afford the corresponding naphthalene have been reported [41,42].
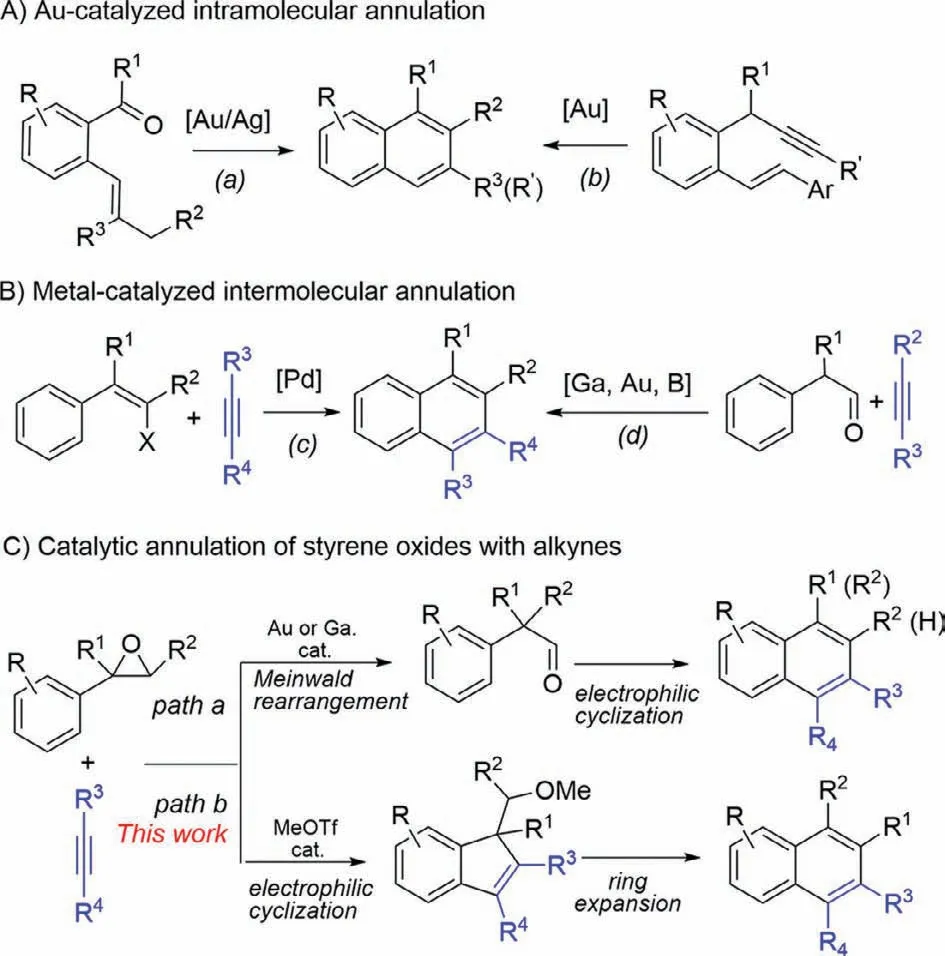
Scheme 1.Catalytic formal [4+2]benzannulation approaches to naphthalenes.
In the preliminary experiment,we chose 2-phenyloxirane 1a and 1,2-diphenylethyne 2a as substrates for model reaction.Inspired by our previous studies on the MeOTf-catalyzed annulation reactions [53–55],we initially investigated the reaction in dichloroethane (DCE) as solvent under different temperature(Table 1,entries 1–4).The results revealed that the best reaction temperature is 110 °C.Then,the ratio of 1a and 2a was adjusted(entries 5–7),the optimal ratio of the 2-phenyloxirane 1a and the diphenylacetylene 2a was 1.2: 1.0,in which product 3aa was obtained in 57% yield (entry 6).Furthermore,the effect of reaction time was examined (entries 6,8–10),the best yield of product was obtained for the reaction proceeding for 24 h (entry 9).Screening the solvent such as dichloromethane (DCM),chloroform (CHCl3),andn-hexane for this reaction (Table 1,entries 6,11–13),DCE is confirmed as a superior solvent in this reaction (entry 9).Based on the structure of the product 3aa,we envisioned that water is another product in this reaction,which may restrain the reaction.Additives such as molecular sieves (4 ˚A),p-toluoyl chloride,and tosyl chloride were employed as water-remover,respectively (entries 14–16).To our delight,the yield of the product 3aa was improved to 96% when 1.1 equiv.of the tosyl chloride used as additive in this reaction (entry 16).In the cases of usingp-toluoyl chloride and tosyl chloride as water-removers in the reaction and 4-methylbenzoic acid 4-methylbenzenesulfonic acid were detected by GC–MS,respectively.Notably,HOTf (5 mol%) was used instead of MeOTf as catalyst in this reaction and the desired product was not observed (entry 17) as well as the starting materials remained.On the basis of the above results,the optimal conditions are shown in entry 16.
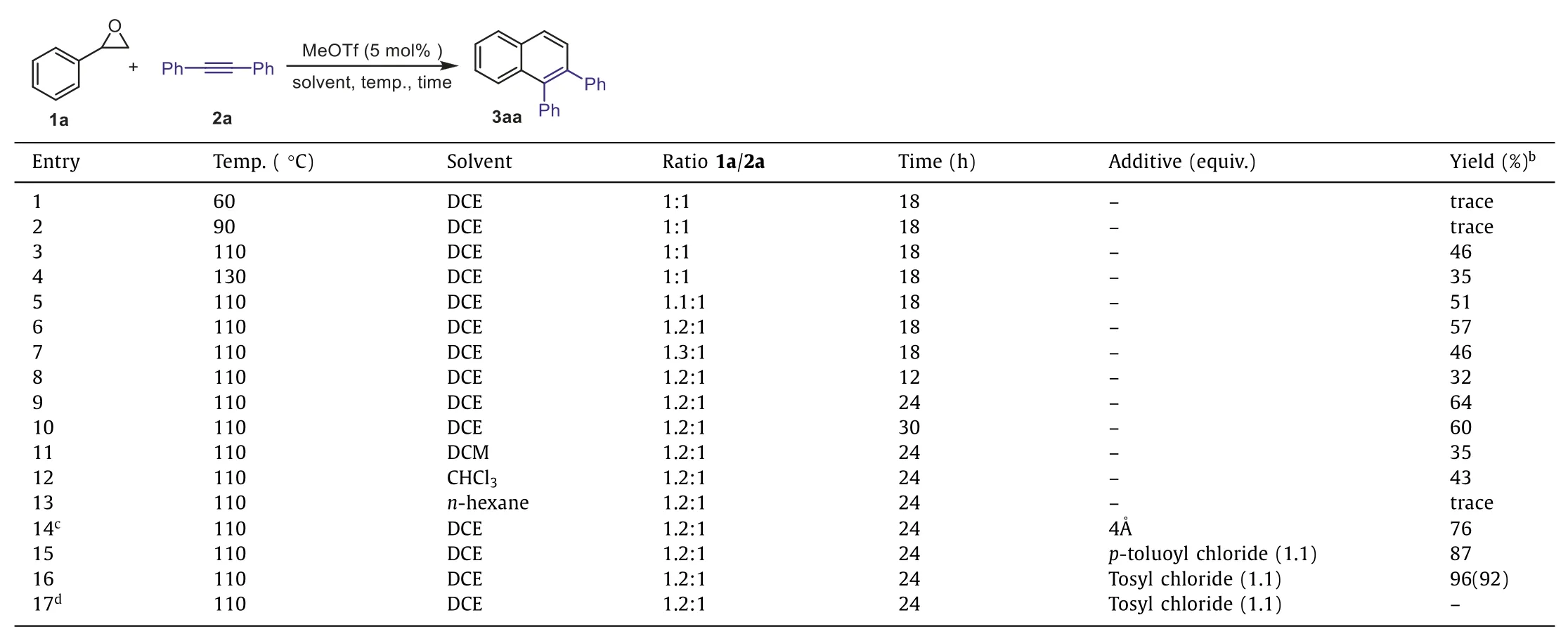
Table 1 Optimization of the reaction condition.a
With the optimized conditions in hand (Table 1,entry 16),a range of aryl ethylene oxides have been firstly employed to look into substrate scope,and the representative results are summarized in Scheme 2.Generally,the substituted aryl ethylene oxides containing electron-donating and electron-withdrawing substituents in the benzene ring could be easily proceed to afford substituted naphthalenes in excellent yields.For instance,arylepoxides containing substituents,such as-Me (for 1a-1c,1n),iPr (for 1d),-OMe (for 1f),Ph (for 1o),and naphthyl (for 1e),-Br (for 1g and 1j),-F (for 1h),and-CF3(for 1i) on the benzene ring displayed greater reactivity and the corresponding products 3 were obtained in high yields.When 1,2-disubstituted aryl ethylene oxides such as 1k,1l,and 1m were employed and the corresponding product 3ka,3la,and 3ma were formed in 88%,83%,and 85% yield,respectively.In addition,when 1,1-disubstituted styrene oxides such as 1n and 1o were used in the reaction and the corresponding product 3na and 3oa,were obtained in 89% and 88% yield,respectively.Notably,when 2-heteroaryl ethylene oxides such as 1-methyl-2-(oxiran-2-yl)−1H-pyrrole,2-(oxiran-2-yl)furan,and 2-(thiophen-2-yl)oxirane were used and the reactions did not proceed as well as the starting materials remained.
Furthermore,the substrate scope of alkynes for the annulation was investigated.The representative results are shown in Scheme 3.First,we tested some asymmetrical disubstituted arylacetylenes.For example,prop–1-yn-1-ylbenzene 2b,hex–1-yn-1-ylbenzene 2d,trimethyl(phenylethynyl)silane 2e,and (bromoethynyl)benzene 2f were employed in this reaction and the corresponding products were afforded in excellent yield with a high regioselectivity.Then,terminal arylalkynes bearing electrondonating groups such as methyl (for 2i) and methoxyl (for 2j) as well as electron-withdrawing groups such as chloro (for 2k) and fluoro (for 2l) at thepara-position were used and the reactions also proceeded smoothly to give the corresponding product 3ai,3aj,3ak and 3al in satisfied yields.When 1-ethynylnaphthalene 2n was applied in the reaction and 1,1′-binaphthalene 3an was isolated in 93% yield.Oct-1–yne 2o was employed and the corresponding product 3ao was also obtained in 84% yield.Nevertheless,the electron-withdrawing alkynes such as methyl 3-phenylpropiolate and 4-phenylbut-3-yn-2-one were employed and the reactions did not proceed and the starting materials remained.In addition,oct–4–yne was used in the reaction and desired product was not observed along with the starting materials remained.
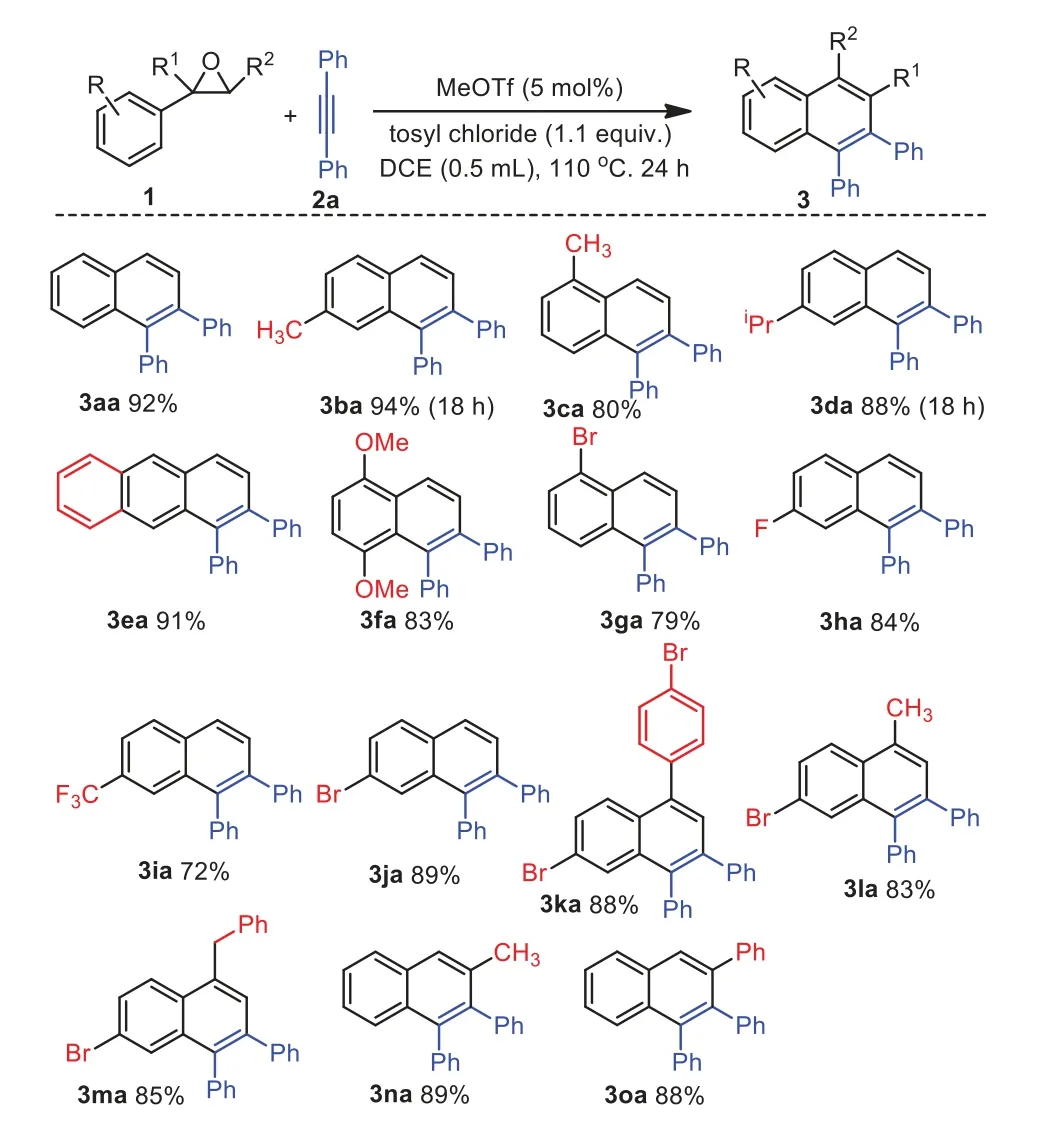
Scheme 2.The scope of aryl ethylene oxides.Reaction conditions: aryl ethylene oxides 1 (0.36 mmol,1.2 equiv.),1,2-diphenylethyne 2a (0.3 mmol,1.0 equiv.),MeOTf(0.015 mmol,5 mol%),tosyl chloride (0.33 mmol,1.1 equiv.) in 0.5 mL DCE under N2;all yields are of isolated product 3.
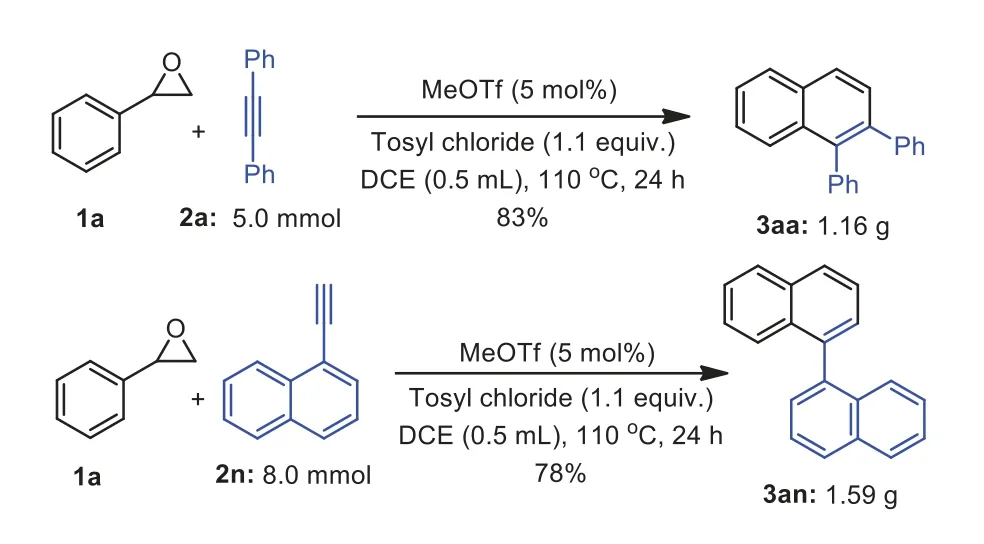
Scheme 4.Gram-scale reactions.
In order to certify the practicability of this reaction,two examples have been selectively amplified to 5.0 mmol and 8 mmol scale of alkynes to afford 1,2-diphenylnaphthalene 3aa in 83% yield(1.16 g) and the 1,1′-binaphthalene 3an in 78% yield (1.59 g),respectively (Scheme 4).
To understand the reaction pathway,we performed control experiments.First,MeOH (5 mol%) and HOTf (5 mol%) were used as co-catalyst instead of MeOTf (5 mol%) under the optimized conditions.The product 3aa was obtained in 62% yield (Scheme 5a).This result indicates that the MeOH and HOTf werein situgenerated and followed formation of MeOTf.As reported that metal could catalyze epoxides into carbonylsviarearrangement to result carbonyl compounds [56],which could be reacted with alkynes to afford naphthalene in the presence of catalysts.Thus,we conducted a reaction of 2-phenylacetaldehyde 4a with 2a under the optimized conditions and the desired product was not obtained and starting materials remained (Scheme 5b).Furthermore,reaction of (E)-(2-methoxyvinyl)benzene 5a isolated by reported literature [54]with 2a in the presence of HOTf (1.2 equiv.) did not afford the desired product (Scheme 5c).These results indicate that the formation of naphthalene skeleton did not undergo aldehydealkyne coupling.During the scope of alkynes,a trace of 3-hexyl-1-(methoxymethyl)-1H-indene 6ao was observed by GC–MS when the reaction of 1a with oct–1–yne 2o was conducted under optimal conditions in 24 h.We envisioned that 6ao maybe the intermediate in our reaction.Thus,the 3-hexyl-1-(methoxymethyl)-1Hindene 6ao,which was isolated in the reaction of 1a (1.2 equiv.),2o (1 equiv.),and MeOTf (1.2 equiv.) at 110 °C for 24 h was treated with 5 mol% of HOTf under the standard conditions and a quantitative yield of 3ao was obtained (Scheme 5d).This result indicates that 6ao is likely the intermediate in the reaction for naphthalene formation.

Scheme 3.The scope of alkynes.Reaction conditions: phenyl ethylene oxide 1a (0.36 mmol,1.2 equiv.),alkynes 2 (0.3 mmol,1.0 equiv.),MeOTf (0.015 mmol,5 mol%),tosyl chloride (0.33 mmol,1.1 equiv.) in 0.5 mL DCE under N2,all yields are of isolated product 3.
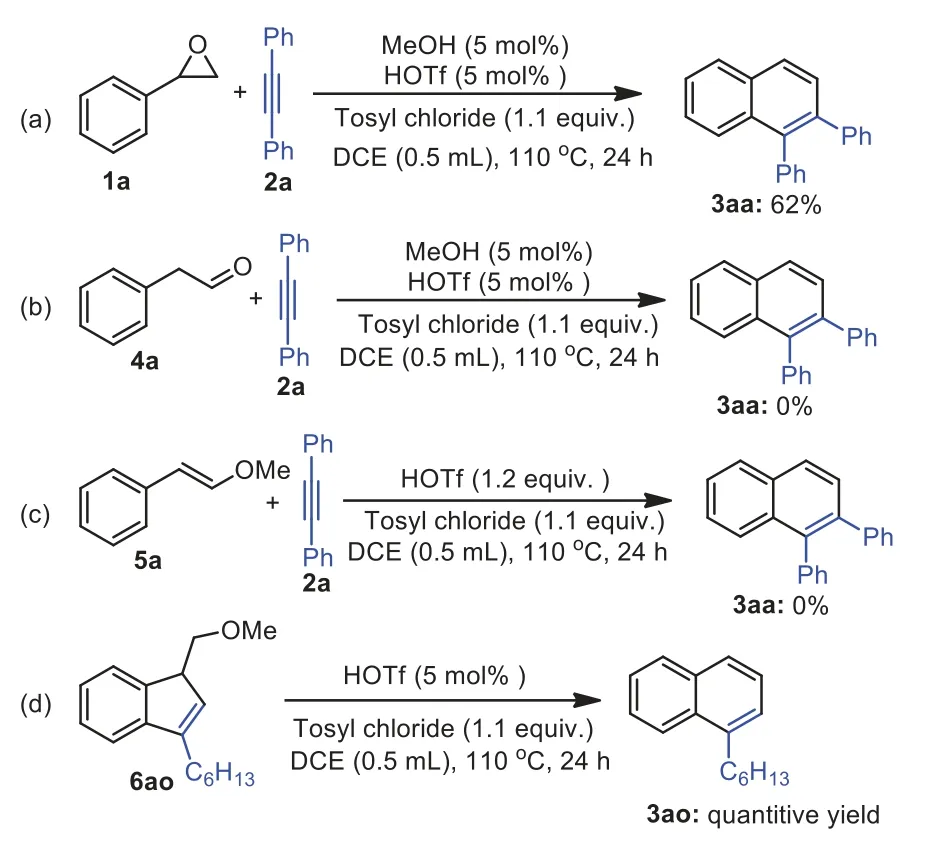
Scheme 5.Control experiments.
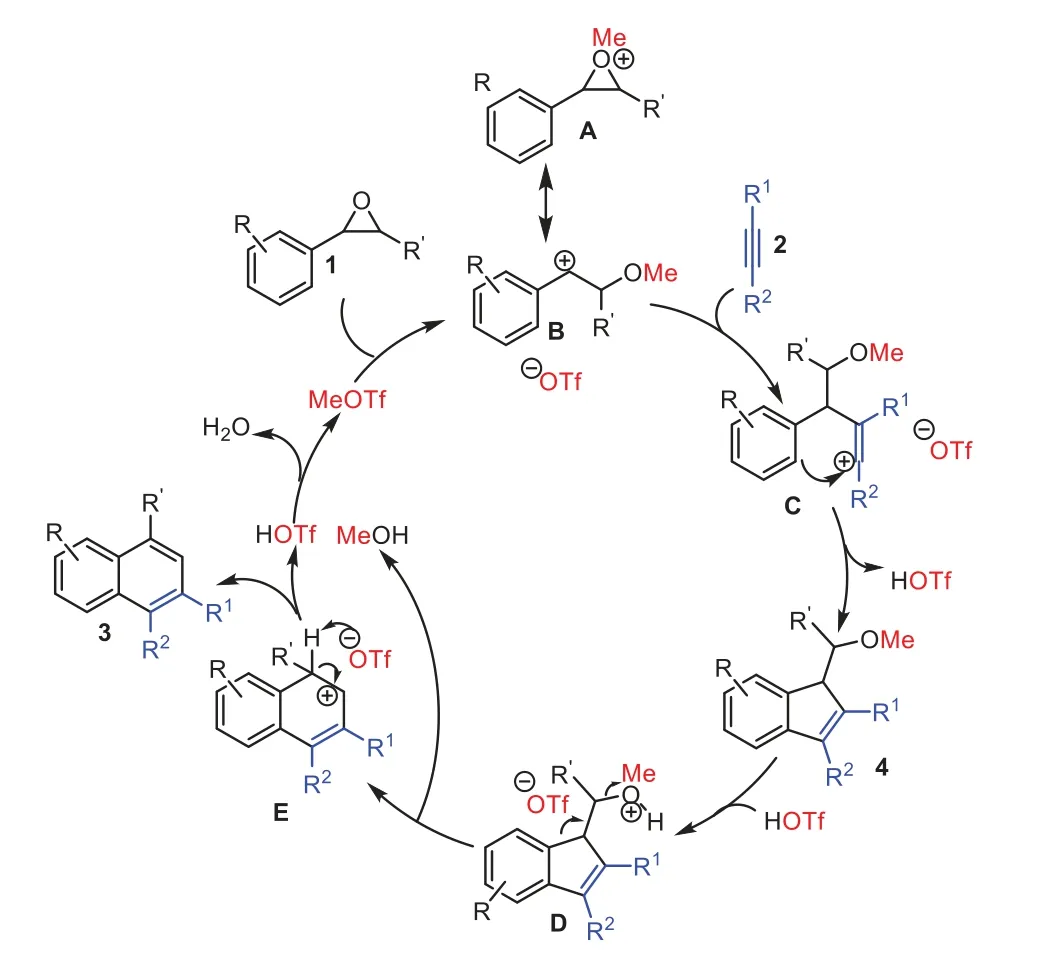
Scheme 6.A plausible mechanism of the reaction.
In summary,we have developed a MeOTf-catalyzed intermolecular formal [4+2]cyclization of 2-aryl ethylene oxide and alkynes to afford polysubstituted naphthalenes through sequential electrophilic cyclization/ring expansion.The reaction proceeds with high regioselectivity.A study of the scope of this reaction has been realized with a wide variety of 2-aryl ethylene oxides and alkynes in satisfied yields.
Based on the above results and previous reports [53–61],a plausible mechanism is shown in Scheme 6.First,methylation of aryl ethylene oxide 1 using MeOTf forms oxonium ion A or its resonance carbocation B,whose high electrophilicity would initiate the regioselective nucleophilic attack of the alkyne 2.The resulting vinylic carbocation C would then undergo an intramolecular electrophilic cyclization with the proximal phenyl ring leading to a 1-(methoxymethyl)-1H-indene 4.Thein situgenerated HOTf mediated a rearrangement of the compound 4 to form intermediate Eviaprotonated intermediate D with releasing MeOH.The intermediate E undergoes elimination of proton to afford naphthalene product 3 together with the regeneration of HOTf.The HOTf reacts with MeOH to regenerate catalyst MeOTf and release H2O as only byproduct.The resulting H2O be moved away by the tosyl chloride to promote the reaction.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No.2016YFB0401400) and the National Natural Science Foundation of China (Nos.21871163 and 22071134).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.12.012.
杂志排行
Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
