Influence of the alkyl side chain length on the room-temperature phosphorescence of organic copolymers
2022-07-11QingyangXuLiangweiMaXiaohanLinQiaochunWangXiangMa
Qingyang Xu,Liangwei Ma,Xiaohan Lin,Qiaochun Wang,Xiang Ma
Key Laboratory for Advanced Materials and Feringa Nobel Prize Scientist Joint Research Center,School of Chemistry and Molecular Engineering,East China University of Science and Technology,Shanghai 200237,China
Keywords:Room-temperature phosphorescence Alkyl chain length Conjugated structure Amorphous polymer
ABSTRACT Pure organic room-temperature phosphorescence (RTP) materials have attracted wide attention owing to their excellent luminescent properties and great potential in various applications.In this work,iminostilbene and its analogues are applied to realize RTP emission by copolymerizing with acrylamide.It can be concluded that the growth of alkane chain in monomers can enhance the lifetime and photoluminescence quantum yield of RTP emission,and polymers with the larger conjugated structure of the monomer show a longer RTP emission wavelength.This work provides a series of new pure organic RTP materials and might provide new thoughts for designing more advanced and superior RTP materials.
Room temperature phosphorescence (RTP) materials have aroused wide attention due to their advantages of long decay lifetime,easy detection,and high responsibility.They showed tremendous potentials in biological imaging [1,2],chemical sensors[3–6],organic light-emitting diodes (OLEDs) [7,8],anticounterfeiting marks [9–11],and data encryption [12–14].Traditionally,RTP materials were inorganic or organometallic complex materials,but the high cost of raw materials and certain cytotoxicity limited their applications in various field.Hence,it is important to develop pure organic compounds with efficient RTP emission.However,pure organic molecules tend to have a large energy gap between their singlet and triplet states (S1and T1) and weak Spin-Orbit Coupling (SOC),which restricts the phosphorescence emission from pure organic RTP materials at room temperature.Moreover,the excited triplet state is very easy to be deactivated via nonradiative transition or quenching by oxygen.Therefore,it is still a big challenge to obtain efficient pure-organic RTP materials.
Recently,some strategies have successfully achieved efficient organic RTP materials by enhancing the intersystem crossing (ISC)or suppressing non-radiative transitions,such as host-guest interaction [2,15,16],crystallization [17–21],heavy-atom effect [22–25],halogen or hydrogen bonding [26–28]and embedded into polymer matrix [29–32].Introducing phosphors into polymer matrix could be a very convenient and effective way to get amorphous RTP materials as it has no strict requirements on the size,structure,or specific state (like crystal state) of the luminescent molecules when compared with other strategies.Some previous reports made by our group have confirmed that polyacrylamide could provide an excellent rigid microenvironment for phosphors to restrict the nonradiative transition by inhibiting the motion and isolating oxygen[22,30].
Iminostilbene (5H-dibenz[b,f]azepine) is an important intermediate for drug synthesis,such as carbamazepine,oxcarbazepine.And it is also an important synthetic raw material in genetic engineering,materials science,and so forth.However,iminostilbene and its analogues have not been used in phosphor materials.Herein,we modified iminostilbene and its analogues with different lengths alkyl chains,and then copolymerized them with acrylamide with the molar ratio of 1:50.The four prepared polymers(P1-P4) exhibited efficient phosphorescence emission at room temperature,in which P1 with longer alkyl chain of the monomers showed the largest RTP emission wavelength at 550 nm,and P4 with shorter alkyl chain of the monomers had the longest lifetime of RTP emission (τ=13.76 ms) and highest photoluminescence quantum yield (ΦPL=43.0%).This study enriched the pure organic RTP systems,and might provide an insight into the design of new RTP materials with various performance.
Four iminostilbene analogues (compounds 1–4) were synthesized and copolymerized with acrylamide (molar ratio=1:50).And the exact structures of the monomers and polymers were shown in Fig.1.All the raw materials were obtained commercially and used for the synthesis directly without further purification.The monomers were synthesizedviaa simple step with a decent yield.The detailed synthesis routes and preparation processes were described in Supporting information.The monomers were characterizedvia1H NMR,13C NMR,and high-resolution mass spectra(Figs.S1-S19 in Supporting information).
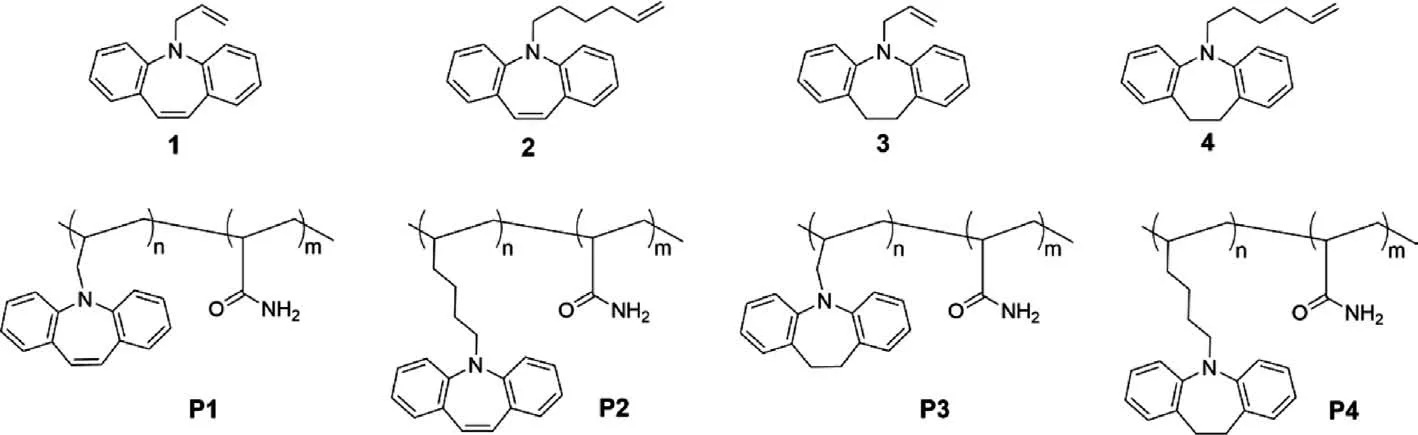
Fig.1.Molecular structures of compounds 1–4 and polymers P1-P4.
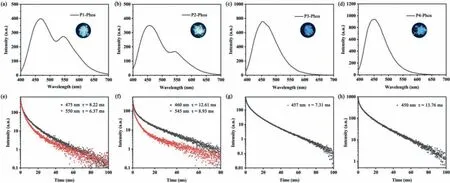
Fig.2.Phosphorescence emission spectra of (a) P1,(b) P2,(c) P3 and (d) P4 and phosphorescent decay lifetime of (e) P1,(f) P2,(g) P3 and (h) P4 in amorphous solid state respectively.
In order to characterize the state of the four polymers,X-ray powder diffraction (XRD) of the powders was conducted and the result was displayed in Fig.S20 (Supporting information).There was no sharp peak in the four curves,which indicated the polymers were all in amorphous state.Aqueous gel permeation chromatography (GPC) has also been used to determine the molecular weight and molecular weight distribution of the polymers,and the specific data was listed in Table S1 (Supporting information).
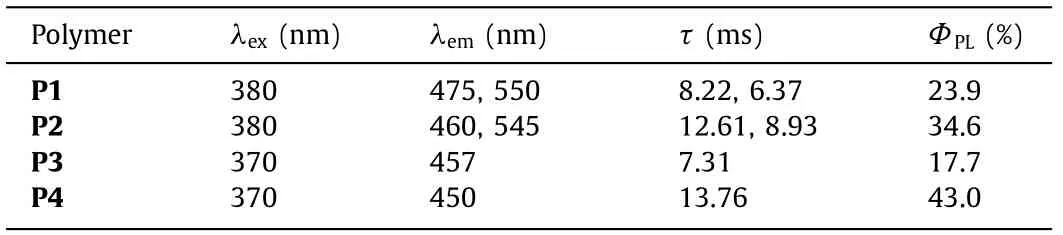
Table 1 Photophysical data of the four polymers in the amorphous solid state at room temperature.
Fig.2 showed the phosphorescence emission and lifetime decay spectra of the four polymers.Obvious phosphorescence emission peaks were recorded for the four polymers in the amorphous state at room temperature.And the lifetime was measured to be about 10 ms for these polymers.Moreover,the excitation spectra of these phosphorescence emissions could match with the corresponding absorption spectra (Fig.S21 in Supporting information).Detailed photophysical data for these polymers was collected in Table 1.Among all the polymers,P4 possessed the longest phosphorescent lifetime (τ=13.76 ms) and highest photoluminescence quantum yield (ΦPL=43.0%).And P1 showed the longest phosphorescence emission wavelength at 550 nm.
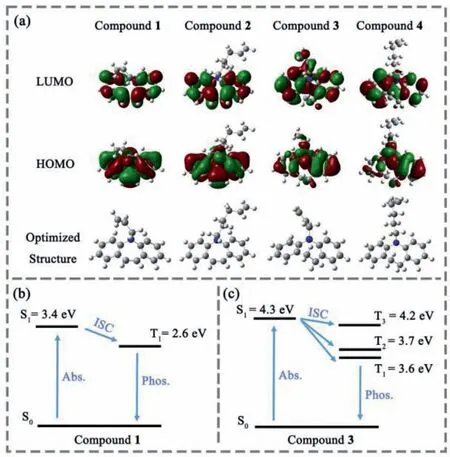
Fig.3.(a) The calculated HOMO,LUMO and optimized structures for the four monomers.Energy level diagrams of compounds 1 (b) and 3 (c) in the gas phase.
The effect of the conjugation change of the monomer on the luminescence properties of the polymers was explored in this system.As showed in Fig.2,a clear blue-shift could be observed for P3 and P4 when compared with P1 or P2.This clear blue-shift in the phosphorescence emission spectra was resulted from the decrease of conjugation when the carbon-carbon double bond bridge in monomers 1 and 2 changed to carbon-carbon bond bridge.Interestingly,there was only a single phosphorescence peak around 450 nm observed in P3 and P4,while P1 and P2 exhibited two distinct phosphorescent emission peaks around 470 nm and 550 nm.
Besides the conjugation,the length of the alkyl chain seemed have a slight effect on the photophysical properties of these polymers.With the prolongation of the alkyl chain of monomer,the RTP emission wavelength showed a slightly blue-shift.Although P2 equipped with similar luminous cores with P1,the phosphorescence of P2 with longer alkyl chain blue-shifted slightly compared to P1.And the lifetime for P2 was increased.Meanwhile,the photoluminescence quantum yield of P2 was also increased from 23.9% to 34.9%.Similarly,the influence of alkyl chain length on the photophysical properties of polymers could also be embodied in P3 and P4.Compared to P3,P4 which was equipped with longer alkyl chain had a little bit shorter emission wavelength (450 nm) but higher quantum yield and longer lifetime.Generally,the change of the alkyl chain length has no obvious effect on the absorption and emission spectra because the alkyl chain could only alter the conjugation of molecule slightly.And the absorption and emission spectra for these polymers with same luminous cores (Fig.S21 in Supporting information) certainly confirmed that.The variation of the lifetime and photoluminescence quantum yield might testify that the alkyl chain length of phosphor might have influence on the radiative transition process [33].
In order to explore the influence of the conjugated structure and carbon chain length on the RTP emission in this system,density functional theory (DFT) and time-dependent density functional theory calculations (TDDFT) were carried out by the G09 program at B3LYP/6–31G∗.Similar electron cloud distribution could be observed for compounds 1,2 and compounds 3,4 because of the similar molecular skeleton,while compounds 3 and 4 exhibited a more twisted structure than 1 or 2.Besides,the HOMO and LUMO for compounds 1 and 2 were distributed on the whole molecular skeleton,indicated a well conductivity of the molecule,while the conductivity decreased when the carbon-carbon double bone changed to single bond because the HOMO of compounds 3 and 4 was located on the partial of the molecular skeleton.The decrease of the conductivity should result from the break of the conjugation of compounds 3 and 4 and the twisted structure of them.Moreover,the decrease of the conductivity enhanced the energy level of the S1state of compounds 3 and 4 when compared to compounds 1 or 2.And the enhancement of energy level could well match with the blue-shift of the absorption spectra of these compounds in THF solution (Fig.S22 in Supporting information).And there was no obvious difference on the excited state energy level calculated for compounds 1 and 2,or compounds 3 and 4,whose molecules had different lengths alkyl chains but same cores.Besides,it could be clearly seen in Fig.3 that the carbon chain almost did not take part in the electron transfer process,which was consistent with the experimental result that the change of carbon chain length had almost no effect on the emission wavelength of polymers.
It was known that an efficient intersystem crossing (ISC) was crucial to harness triplet excitons.A larger spin-orbit coupling(SOC) strength and smaller energy gap between the lowest singlet excited state (S1) and its closest triplet excited state (Tn) could promote the ISC process between the singlet and triplet state.The calculated energies for the lowest excited singlet state (S1),the lowest excited triplet state (T1) and Tnfor the four compounds in gas state were all listed in Table S2 (Supporting information).All these four molecules had very small energy gap between S1and its closest triplet state,indicated the certainly possible ISC process.Figs.3b and c showed the energy level diagram of compounds 1 and 3.And the SOC matrix elements (ξ) illustrated in Table S2 (Supporting information) could also prove that.The decent SOC between the S1state and triplet state for these monomers might be the reason for the phosphorescence emission of these polymers.
In this work,four polymers,containing iminostilbene derivatives with different alkyl chain lengths,have been successfully prepared though simple synthesis steps.All of them showed efficient phosphorescent emission at room temperature.Compared with P3 and P4,the RTP wavelengths of P1 and P2 which had larger conjugate structure were much longer.And P1 and P2 both had two phosphorescent emission peaks.In addition,the emission wavelength of P1 with shorter carbon chain length was similar to P2 due to their similar structure.While the lifetime for the phosphorescence of P1 was shorter and photoluminescence quantum yield was lower compared to P2.And such variation could also be observed for P3 and P4.It could be concluded that polymers with longer carbon chain of the monomers would have longer lifetime and higher PL efficiency.This study provided a serious of new polymers with efficient phosphorescence emission,which could enrich the library of pure organic RTP systems and provide new thoughts for designing various kinds of efficient RTP materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos.21788102,22125803,22020102006 and 21871083),Program of Shanghai Academic/Technology Research Leader (No.20XD1421300),‘Shu Guang’Project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (No.19SG26),the Innovation Program of Shanghai Municipal Education Commission (No.2017–01–07–00–02-E00010),and the Fundamental Research Funds for the Central Universities.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi: 10.1016/j.cclet.2021.12.097.
杂志排行
Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
