Two-dimensional covalent organic framework nanosheets:Synthesis and energy-related applications
2022-07-11LingjunChnMinhuHungBoChnChngtoGongNnjunLiHongfiChngChnYongwuPngGuodongXu
Lingjun Chn,Minhu Hung,Bo Chn,Chngto Gong,Nnjun Li,Hongfi Chng,Y Chn,Yongwu Png,∗,Guodong Xu
a College of Materials Science and Engineering,Zhejiang University of Technology,Hangzhou 310014,China
b Department of Chemistry,City University of Hong Kong,Hong Kong,China
c Department of Chemistry,The Chinese University of Hong Kong,Hong Kong,China
d School of Chemistry and Environmental Engineering,Yancheng Teachers University,Yancheng 224007,China
e Institute of Materials Research and Engineering,A∗STAR (Agency for Science Technology and Research),Innovis 138634,Singapore
Keywords:Covalent organic framework nanosheets Top-down method Bottom-up method Batteries Photocatalysts Electrocatalysts
ABSTRACT Two-dimensional (2D) covalent organic framework nanosheets (CONs) are attracting increasing research attention because of their unique properties derived from their ultrathin thickness,high surface-tovolume atomic ratio,and extremely large surface area.2D CONs can provide high transport pathways for charge carriers (e.g.,electrons,holes and ions) through either the conjugated skeletons or the open channels.Therefore,they have shown great potential in energy related applications.In this review,we firstly introduce the recent developments and characteristics of 2D CONs by focusing on the two typical synthetic methods, i.e.,top-down and bottom-up methods.Then,the energy-related applications in energy storage and conversion of 2D CONs are summarized.Finally,we give our personal views on the challenges and perspectives for the future research of 2D CONs and their composites.
1.Introduction
In recent years,two-dimensional (2D) materials have attracted a great deal of research interest due to their large specific surface areas,outstanding optical transparency,excellent electrical and thermal conductivities,and extremely strong light-matter interactions [1–3].Since the discovery of graphene,various kinds of unimolecular thin 2D materials have been synthesized and investigated [4,5],such as 2D metal organic frameworks (MOFs) [6–8],transition metal dichalcogenides (TMDs) [9–13]and graphitic carbon nitride (g-C3N4) [14–17].Due to their special structures and morphologies,these 2D materials show unique properties and have been extensively studied [18–20].
Among these materials,covalent organic frameworks (COFs)have aroused intense research interest owing to their ordered structures with tunable functional groups,programmable pore architectures,and high overall porosities [21–29].Therefore,COFs have been widely used in adsorption and separation [30–34],catalysis [35,36],biosensor [37,38],chemical sensor [2,39]and energy storage [40–42].However,the traditional bulk COF crystals could not meet the full requirements for certain applications.For example,although there are abundant active sites in bulk materials,the long path from outside to inside make the interior sites almost inaccessible.Therefore,controlling the thickness,size,and morphology of COF may sometimes be necessary [43].Compared with bulk COF crystals,2D covalent organic framework nanosheets(CONs) offer unique properties,such as tunable thickness down to 1.15 ± 0.1 nm,large specific surface area,and high surfaceto-volume atomic ratio [44–47].As a result,more and more 2D CONs have been prepared and studied for various applications.
In this review,we provide a brief summary of the recent progress in the preparation and applications of 2D CONs,with emphasis on the critical structure factors of 2D CONs on the transportation of electrons and ions in energy-related applications.Firstly,we introduce two typical synthesis approaches of 2D CONs,i.e.,top-down and bottom-up methods,and discuss their advantages and disadvantages in detail.Then,we briefly showcase some typical applications,including energy storage and conversion.Finally,we give our personal perspectives on the current challenges and the future development directions in this promising research field.
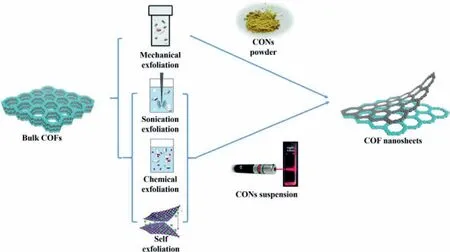
Fig.1.Schematic illustration of the preparation of CONs by top-down method.Reprinted with permission [47].Copyright 2020,Royal Society of Chemistry.
2.Synthetic approaches of 2D CONs
To prepare the CONs,two kinds of approaches have been proposed,both of which possess advantages and disadvantages in different aspects.In bulk layered COFs,each layer is mainly stacked byπ-πinterlayer interactions [48].The strategy of the top-down method is to exfoliate the pre-synthesized bulk COFs by breaking the interlayerπ-πinteractions [49,50].Specific techniques include,but not limited to,solvent-assisted sonication,mechanical grinding delamination,and chemical exfoliation.These techniques usually are straightforward and easy to operate with low manufacturing costs [49].However,they usually suffer from low yield,uncontrollable thickness,and possible damage of nanosheets [51,52].Different from the top-down method,the strategies of the bottom-up method,including gas-liquid interface synthesis,liquid-liquid interface synthesis,molecule-exchange method,and self-exfoliation,are to prevent the CONs from stacking during COF synthesis instead of post-treatment.Compared with top-down method,bottom-up method could produce 2D CONs with more precisely controlled thickness,and can achieve relatively large-scale production.However,it always requires complicated preparation procedures,and sometimes even harsh conditions [49].
The physical properties (morphology and thickness) of the synthesized CONs are usually characterized by scanning electron microscopy (SEM),transmission electron microscopy (TEM),atomic force microscopy (AFM) and others.Among them,SEM technique provides the surface morphology information of materials by collecting secondary electron signals.However,this method could hardly obtain crystallographic information about the CONs,such as lattice fringes and diffraction spots.Therefore,TEM (especially high-resolution TEM (HR-TEM)) is often used to characterize CONs.During the TEM characterization,the electron beam passing through the sample will be partly deflected as a result of the anisotropy in CONs,thus causing the TEM images with different brightness.Consequently,more information such as lattice parameters and electron diffraction patterns need to be acquired.Furthermore,the low-dose TEM has recently been applied in the characterization of CONs,which can overcome the electron-sensitive of CONs and achieve enhanced atomic resolution.AFM is another important technique,which can describe three-dimensional topological information (e.g.,thickness) of CONs through the interaction between CONs and the AFM tip.
In the following sections,more details of these two synthetic methods and characterization strategies will be discussed.
2.1.Top-down method
The intralayer interactions between the atoms in each COF layer are covalent bonds,which are relatively strong and difficult to break.However,the interlayer interactions between the COF layers are often weaker interactions,such as Van der Waals interactions or hydrogen bonding [53].As a result,thin 2D nanosheets can be successfully isolated from their bulk counterparts by breaking the interlayer interactions while keeping the intralayer interaction intact.This strategy is named as top-down method (Fig.1).Although the small grain size of bulk crystal would undoubtedly restrict the production of 2D CONs with a relatively large area,the top-down method is effective,easy to operate,applicable to most COFs,and therefore frequently used [41].
2.1.1.Solvent-assisted sonication exfoliation
The ultrasonic exfoliation has been widely considered as a simply operated,easily controlled,and highly efficient method to fabricate various nanomaterials [54].Since the structure of 2D COFs is similar to graphite,the exfoliation method applied to preparing graphene from graphite is probably effective in producing CONs[55,56].During ultrasonic exfoliation,the high energy shear force or bubbles would be produced,which would easily overcome the weak interactions between layers of COFs [57–62].It was found the efficiency of ultrasonic exfoliation is affected by several key factors,including the type of solvent,sonication time,and sonication power [63–71].
In 2011,Zamoraet al.successfully prepared CONs by solventassisted sonication for the first time (Fig.2).Firstly,the boronic ester-linked COF-8 was synthesizedviathe condensation of 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP) and 1,3,5-tris[4-phenylboronic acid]benzene (BTPB).Then,the obtained bulk COF-8 was sonicated in a series of solvents,including dichloromethane(CH2Cl2),1,4-dioxane,mesitylene,acetone and 2-propanol.When bulk COF crystals were sonicated,the intercalation of solvent into the interlayer space of 2D COFs would be accelerated.With the help of solvent molecules,the interlayer space would be expanded,and thus the interlayer interaction would be weakened.And it has been found that a suitable solvent would not only contribute to the exfoliation and but also stabilization of the nanosheets.Specifically,suitable surface tension between the COFs and solvent could lead to a lower energy input necessary for solvent intercalation and improve the exfoliation efficiency.Among all solvents mentioned above,CH2Cl2is the most effective solvent for COF-8.The final collected CONs consist of 10–25 layers of nanosheets and the thickness of CONs is 4–10 nm.However,CONs prepared from COF-8 are not sufficiently stable since the boronate ester group suffers from poor hydrolytic stability [66].
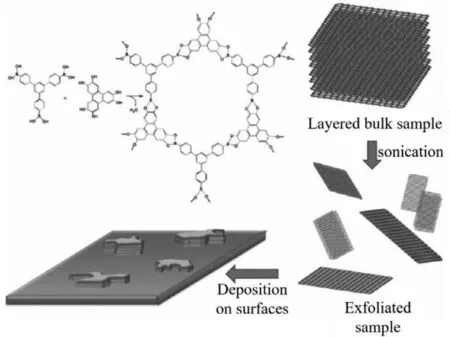
Fig.2.Schematic representation of the synthesis of COF-8 bulk material,micromechanical exfoliation and surface adsorption procedures.Reprinted with permission[66].Copyright 2011,Wiley-VCH.
In another work,Dichtelet al.reported the successful fabrication of 2D CONsviasolvent-assisted sonication from COF-43(Fig.3a).Specifically,the starting materials dihydrazide and trialdehyde were added in the mesitylene/dioxane mixed solvents with acetic acid as catalyst.After heating in 120 °C for 72 h,the COF-43 crystals with high purity and crystallinity were obtained.Then,the bulk COFs were immersed in different solvents followed by sonication for 0.5–2 min to produce CONs.The thickness of the obtained COF-43 nanosheet is 1.32 ± 0.47 nm,suggesting a 3–5-layer structure (Fig.3b).Furthermore,the solvent effect on the crystallinity of the resulted CONs was also investigated.It was found that the CONs produced in dioxane,water,and dimethyl formamide possess low crystallinity.In contrast,those produced in tetrahydrofuran,chloroform,and methanol maintains high crystallinity.Suitable solvents can interact with the CONs and thus weaken theπ-πinteraction between layers.Moreover,the interaction between the suitable solvents and CONs can also improve the stability of obtained CONs obviously [67].
In addition to the solvent effect,the skeleton of the bulk COFs also has an important influence on the solvent-assisted sonication.Theπ-πinteractions between layers could be broken more easily for COF materials with flexible building units instead of rigid ones.In 2017,Zhanget al.reported the imine-linked TPA-COF (TPA: trisphenyl amine)viathe co-condensation of two flexible C3vsymmetry building units (i.e.,tris(4-aminophenyl)amine (TAPA) and tris(4-formylphenyl)amine (TFPA)) (Fig.3c).After ultrasonication in ethanol for 3 h,the CONs with a thickness of around 3.5 nm were produced (Fig.3d) [37].Generally,this solvent-assisted sonication exfoliation can be suitable for most bulk layered COFs with suitable solvents,especially those bearing flexible units.Yet,it suffers from low yield and potential structural defects.
2.1.2.Mechanical exfoliation
Apart from the solvent-assisted sonication exfoliation,the mechanical grinding delamination is also an effective method to prepare CONs from bulk COFs [72–76].This method utilizes the mechanical force generated by grinding,ball milling or volumetric change of solvents to destroy the interaction between layers of bulk COF materials.For example,Banerjeeet al.reported the exfoliation of a series of layered COFs,including TpPa-1,TpPa-2,TpPa-F4,TpPa-NO2,TpBD,TpBD-(Me)2,TpBD-(OMe)2and TpBD-NO2using above-mentioned technique (Fig.4).Those 2D layers stack vertically and form the bulk dark red crystals,while few-layered COFs are yellow.In order to destroy the interaction between layers,the COF crystals were dispersed in methanol and mechanically ground or ball-milled at 25 Hz for 30 min,followed by being dispersed in methanol and centrifuged at 8000 rpm for 5 min.Through the above procedures,a clear solution containing CONs was obtained with lengths and widths of several micrometers and thicknesses ranging from 3 nm to 10 nm.The produced CONs show the poly-layer morphology with an interlayer distance of approximately 3.5 ˚A,which can also be proved by the AFM image.In order to further study the crystallinity after grinding,PXRD patterns of the bulk COFs and CONs were both collected.According to the results,the PXRD patterns of bulk COFs and CONs were almost the same,except that the intensity of the first peak of CONs decreased and the last peak of CONS broadened.These results suggest the successful preparation of CONs with the intralayer structure remained intact after grinding [75].
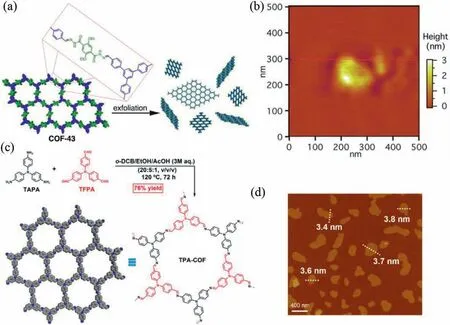
Fig.3.(a) Exfoliation of COF-43 yields a suspension of few-layer 2D polymers.(b) AFM image show platelets corresponding to few-layer structures.Reprinted with permission [67].Copyright 2013,American Chemical Society.(c) Schematic illustration of synthesis and extended hexagonal structure of the bulk TPA-COF material.(d) AFM image of TPA-COF nanosheets with the thickness indicated.Reprinted with permission [37].Copyright 2017,American Chemical Society..
In general,mechanical exfoliation is widely used to prepare CONs from bulk COFs due to its attractive advantages,such as simplicity,eco-friendliness,and energy efficiency.However,the introduction of mechanical force would easily damage the structure of CONs when excessive mechanical force was applied in the course of mechanical exfoliation.Besides,this exfoliation method also encounters a series of challenges,including the low yield,the difficulty of dispersion,and the aggregation of CONs,which would greatly restrict the practical applications of this method.
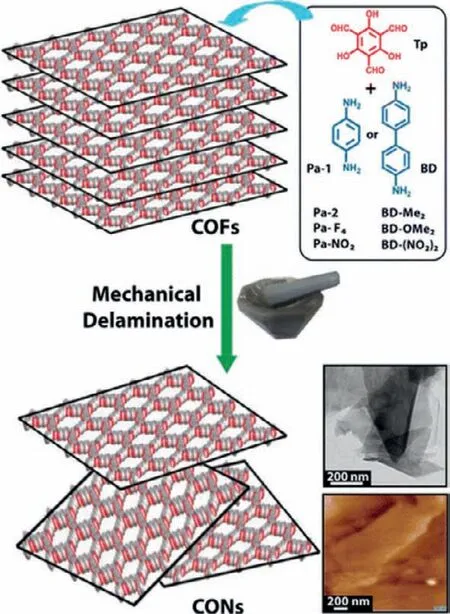
Fig.4.Schematic representation of the formation of CONs from as-synthesized bulk COFs via mechanical grinding.Insets: The structure of monomers at right top;TEM and AFM images of CONs (sheet-like morphology at right bottom).Reprinted with permission [75].Copyright 2013,American Chemical Society.
2.1.3.Chemical exfoliation
Generally,it is difficult to precisely control the thickness of exfoliated 2D CONs by sonication or mechanical exfoliation.In this regard,chemical exfoliation is an ideal solution to fabricate CONs with controllable thickness and excellent stability [76–80].In this method,by virtue ofin-situchemical reactions between intercalators and the layered 2D COFs,the interlayerπ-πinteractions would be weakened or even destroyed.Therefore,the layered bulk COFs were exfoliated to produce CONs with high yield and quality.For instance,Banerjeeet al.introduced theNhexylmaleimide molecules to the anthracene moieties of the COF layers by [4+2]cycloaddition reactions (Fig.5a).With the introduction ofN-hexylmaleimide unit,the change in physical appearance to dark yellow was observed.The introduction ofNhexylmaleimide severely disturbs the interlayerπ-πinteractions due to the interlayer space expansion and the loss of the planarity of anthracene units,resulting in the effective exfoliation.As shown in the TEM image (Fig.5b),the thin nanosheet could be clearly observed.Moreover,the average thickness of CONs was measured to be around 17 nm according to AFM analysis,suggesting a fewlayered structure.Furthermore,the functionalization of CONs endows the obvious improvement of hydrophobicity of the synthesized CONs in organic solvents.As a result,a scalable CON-based thin film could be produced at the air-water interfaceviathe layerby-layer (LBL) assembly technique [76].
Besides,nanoparticles can also be introduced in the CONs as a “spacer” to promote exfoliation [77].For example,Wanget al.successfully exfoliated layered TFPB-COF (TFPB: 1,2,4,5-tetrakis-(4-formylphenyl)benzene) and produced few-layered E-TFPB-COF by chemical exfoliation.Firstly,the imine-based layered TFPB-COF was synthesized with TFPB and 1,4-diaminobenzene by Schiff reaction.Then KMnO4was introduced to produce MnO2nanoparticles,which can weaken the interlayer interactions and increase the interlayer space when sonicated.Lastly,the layered TFPB-COF was successfully exfoliated to a few-layered E-TFPB-CONs.It is worth noticing that the stacking of nanosheets was restricted by the MnO2nanoparticles between layers [77].
2.1.4.Self-exfoliation
Self-exfoliation is also a method to produce CONs,which usually introduces charged unit or non-planar monomer into the bulk COFs.With the introduction of these active species,the interlayerπ-πstacking interaction can be weakened effectively and then the layered CONs would be obtained without extra interference.Meanwhile,sonication can be used to accelerate the exfoliation process.
As mentioned above,one of the self-exfoliation strategies is the introduction of charged unit,such as guanidinium units [81–83],ethidium bromide [84],quaternary ammonium groups [58],positively charged pyridine salts [85],and propidium iodide [86].For example,Banerjeeet al.synthesized three types of self-exfoliated guanidinium halide-based ionic CONs,denoted as TpTGCl,TpTGBr,and TpTGI(Fig.6a).With the introduction of positive charged guanidinium structure,the interlayer repulsion led to the exfoliation of bulk COFs.With the help of sonication,ionic CONs can be easily produced.It should be noticed that the sonication can reduce the exfoliation time from 24 h to 2 min.The thickness of obtained guanidinium halide-based ionic CONs is 2–5 nm,which shows a 3–6-layer structure (Fig.6b).However,the specific surface areas of TpTGCl,TpTGBr,and TpTGIare calculated to be 267,305 and 298 m2/g,respectively,which are much smaller than theoretical values [81].Briefly,the introduction of charged building units can effectively promote the exfoliation of the bulk COFs into CONs.Meanwhile,the pore channels of the as-prepared CONs are always blocked by counter anions due to the electrostatic force between charged building units and counter anions.
The introduction of non-planar monomers or large branched chains is another key factor to prepare CONs through selfexfoliation.For example,Vaidhyanathanet al.built a COF using triazole building units,which can increase the skeletal flexibility and facilitate the self-exfoliation (Fig.6c).IISERP-CON1 (IISERP: Indian Institute of Science Education and Research Pune) featuring with different staking distances (3.47 ˚A and 3.26 ˚A) was fabricatedviathe co-condensation of a trialdehyde and 3,5-diaminotriazole under mild solvothermal conditions.Due to relatively weak stacking energy of the obtained IISERP-CON1,the CONs can be obtained by self-exfoliation.As showed in AFM (Fig.6d),the thickness of obtained CONs is 2–4 nm.Even after standing for 24 h,the Tyndall effect can also be observed when CONs are dispersed in methanol[40].
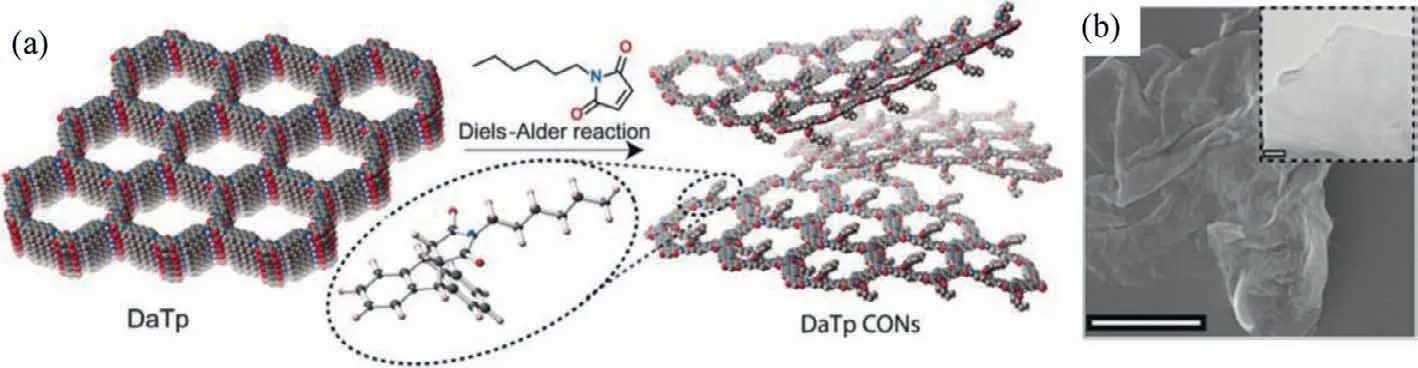
Fig.5.(a) Schematic representation of the exfoliation process.(b) A typical SEM image (Scale bar: 5 μm) of DaTp-CONs.The inset is a TEM image (Scale bar: 100 nm) of DaTp-CONs.Reprinted with permission [76].Copyright 2016,Wiley-VCH.
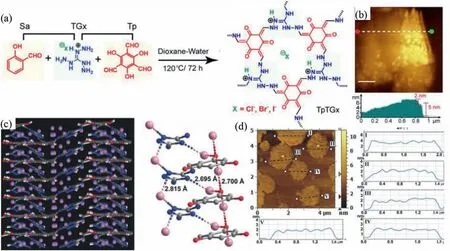
Fig.6.(a) The fabrication of iCONs (TpTGX),X=Cl,Br,I.(b) AFM of TpTGCl and height profile of TpTGCl.Reprinted with permission [81].Copyright 2016,American Chemical Society.(c) Curved and undulating interlayer structure of IISERP-CON1.(d) The AFM image showing the thickness of the drop-casted sample.Reprinted with permission [40].Copyright 2017,Wiley-VCH.
Furthermore,the introduction of charged or non-planar units could lead to self-exfoliation without further treatment.However,the presence of the counter anions along with the charged units usually leads to blockage of the pores,resulting in a small surface area of the collected CONs.
Various CONs synthesized by the above-mentioned top-down method are summarized in Table 1.To sum up,among the topdown method,solvent-assisted sonication exfoliation is the most widely employed technique,which is suitable for most COFs with the help of suitable solvents.Meanwhile,with the help of certain solvent,the sonication efficiency and the stability of obtained CONs can be obviously improved.However,this method usually suffers from low yield,uncontrollable thickness and structural damage.Besides,the mechanical exfoliation also suits the majority of COFs.However,this method may sometime suffer from the structural damage.Compared with these two methods,chemical exfoliation process is gentle and effective,but this method is too complicated to suit for most kinds of COFs.Briefly,top-down method is widely applicable to most kinds of COFs.However,the fabrication of 2D CONsviatop-down method usually suffers from low yield,uncontrollable thickness and relatively low stability,which greatly impedes the practical applications.
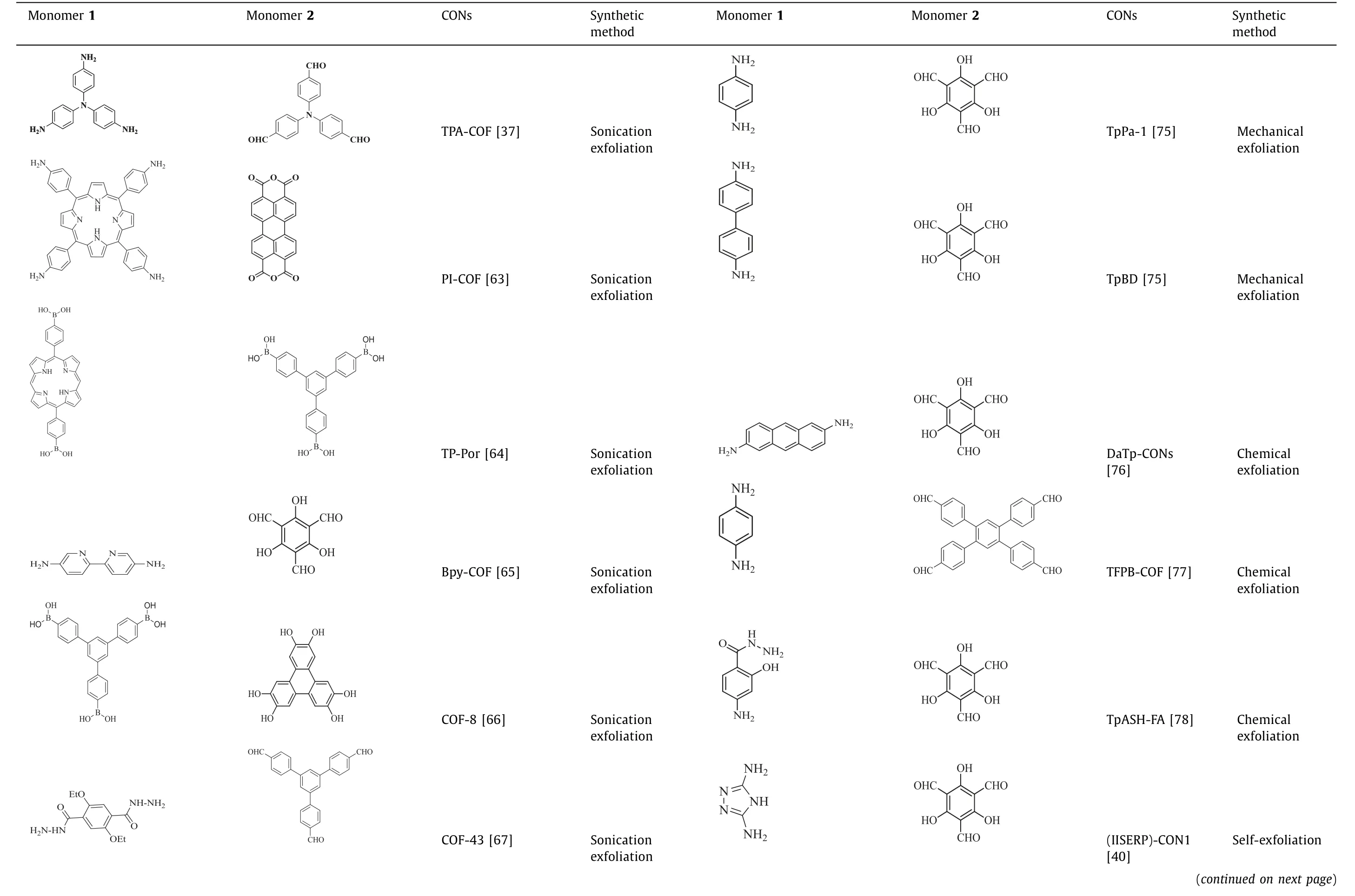
Table 1 Summary of CONs synthesized by the top-down method.
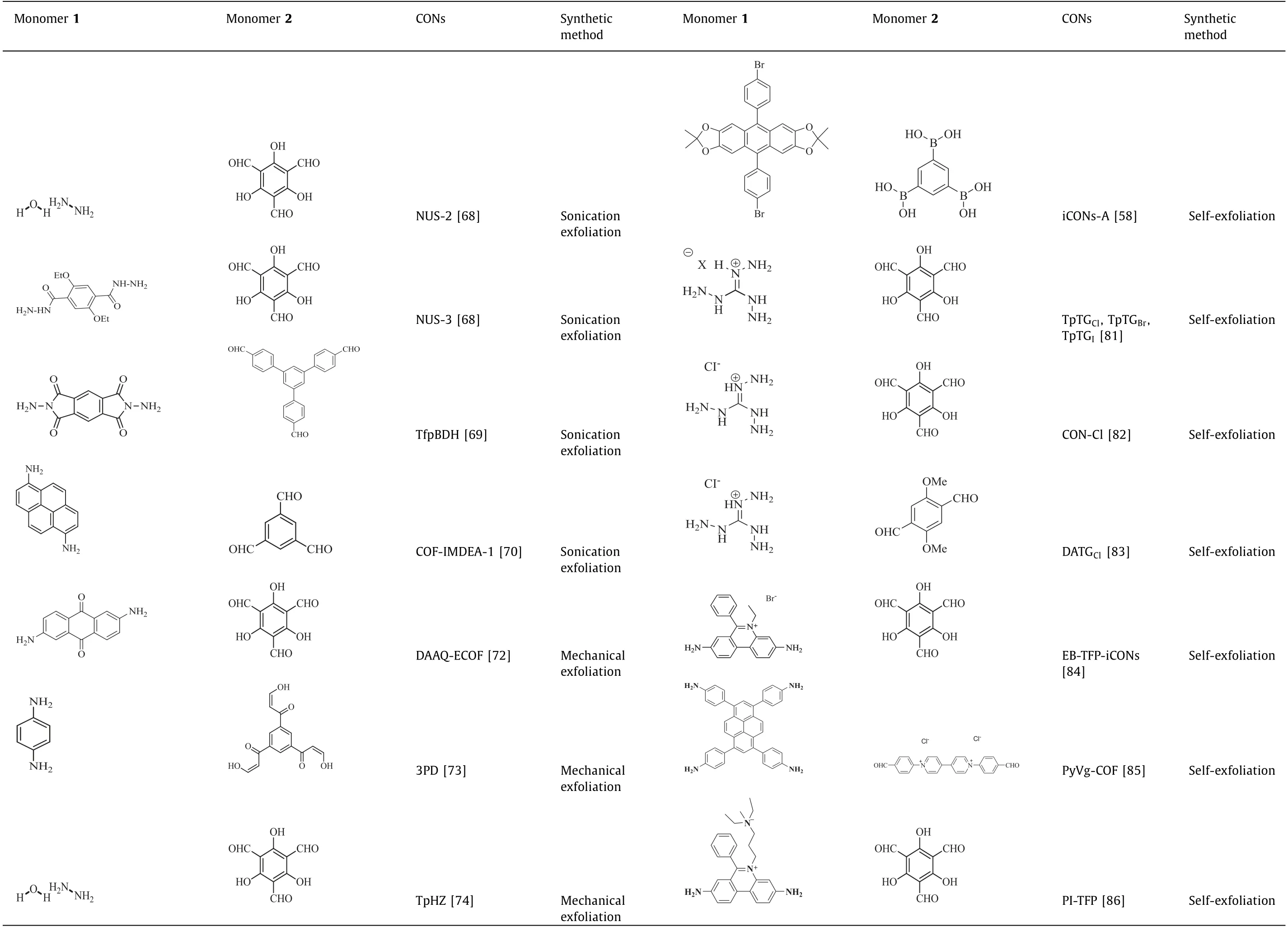
Table 1(continued)
2.2.Bottom-up method
The top-down method usually suffers from low yield and production rate [46].Different from the top-down method,the bottom-up method offers a direct synthesis of 2D CONs from organic ligands (Fig.7).The key point of this strategy is to selectively suppress the COF growth alongside the vertical direction without sacrificing the horizontal COF growth.The most frequently used bottom-up method is interfacial synthesis,including gas-liquid interface synthesis and liquid-liquid interface synthesis.Details will be discussed below.
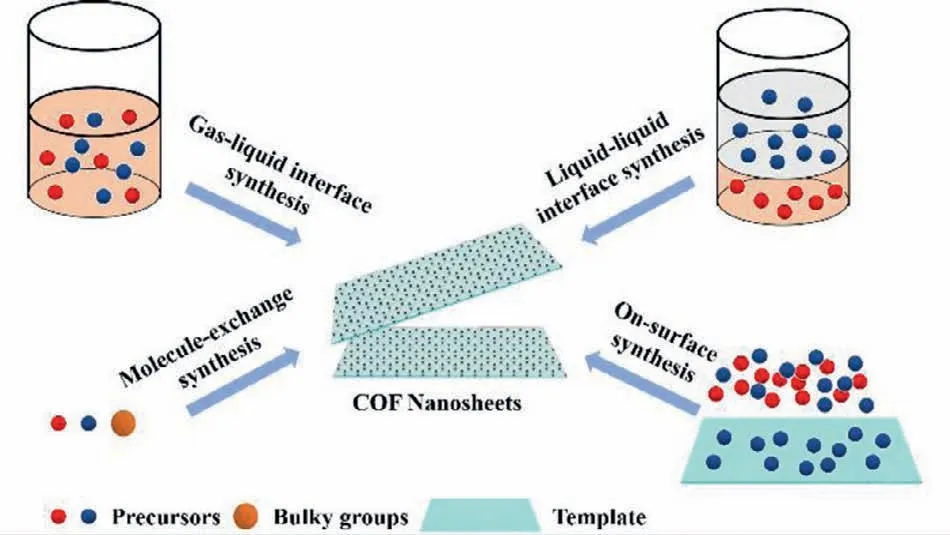
Fig.7.Schematic illustration of the preparation of CONs by the bottom-up method.
2.2.1.Gas-liquid interface synthesis
One of the bottom-up methods to fabricate CONs is gas-liquid interface synthesis,in which CONs can be synthesized on the surface of the gas/liquid interface with the help of volatile solvent or aqueous solution.In gas-liquid interface synthesis,COF growth in the vertical direction could be greatly impeded,since the hydrophobic monomer for COF formation was confined in a Langmuir monolayer interface.Thus,this technique could provide CONs with ultrathin or even single-layer structure [43,87-91].
Zhanget al.firstly reported the synthesis of the imine-linked CONs at the air-water interface [89].The CONs were prepared on Langmuir-Blodgett placed on an anti-vibration table in a dustreduced environment.After measuring the surface pressure with a Wilhelmy balance under the precision of 0.01 mN/m,two kinds of monomers were pre-mixed in chloroform and spread on the water surface.After evaporation of solvent for 30 min,the system was compressed at the rate of 3 mm/min.When the surface pressure was stabilized at 3 mN/m,the trifluoroacetic acid was added into the solution as a catalyst without disturbing the monomers.After 12-h interface condensation,the monolayer CON thin film was successfully prepared with a thickness of 0.7 nm,suggesting the monolayer structure (Fig.8).The tip-enhanced Raman spectroscopy (TERS) and density functional theory (DFT) simulation have been adopted for the structure confirmation.According to the confocal Raman spectra and DFT calculations,the formation of a conjugated system (β-N=C-γ) can be confirmed,indicating the successful formation of Schiff base at the gas-liquid interface [89].The above-mentioned gas-liquid interface synthesis method is a general method for preparing CONs.However,because of the restriction of precursors at the interface,large-scale synthesis of CONs may sometimes be difficult.
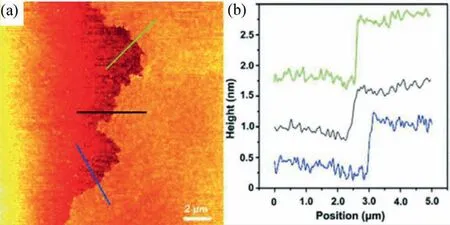
Fig.8.(a) Tapping-mode AFM height image of a polyimine monolayer after vertical transfer onto a 285 nm thick SiO2 on Si substrate.(b) Corresponding line profiles.The lines in (a) indicate where the height profiles were recorded.Reprinted with permission [89].Copyright 2016,Wiley-VCH.

Fig.9.(a) Schematic view and a photograph of the interfacial polymerization of TAPB-PDA COF at the interface of the organic phase and aqueous phase,which contain monomers (TAPB and PDA) and catalyst (Sc(OTf)3),respectively.(b) TEM image of a transferred TAPB-PDA COF film grown with a reduced amount (15.6 mL)of TAPB (1.6 mmol/L) and PDA (2.3 mmol/L) solution over 30 min.Reprinted with permission [92].Copyright 2017,Elsevier.(c) Schematic representation of the preparation process involved in growing NS-CONs.(d) AFM image and height profile of NS-CONs.Reprinted with permission [93].Copyright 2018,Royal Society of Chemistry.
2.2.2.Liquid-liquid interface synthesis
Apart from the gas-liquid interface synthesis,the liquid-liquid interface synthesis could also be employed to synthesize CONs[92–95].Different from gas-liquid interface synthesis,CONs are fabricated at the interface of two immiscible solvents,in which necessary components including monomers and catalysts are dissolved.Compared with the former technique,the liquid-liquid interface synthesis is more flexible to operate.However,it usually provides multi-layer CONs instead of monolayer ones [47].Dichtelet al.prepared the continuous COF films with a large area(up to several cm2) on liquid-liquid interface in the presence of Lewis acid catalyst [92].The monomers were dissolved in organic phase,while the Lewis acid catalysts (scandium triflate) were dissolved in water.Afterward,a COF film could be formed at the liquid interface (Fig.9a).More importantly,the thickness of obtained CONs could be easily tuned from 2.5 nm to 100 μm by varying the initial concentration of the monomers.The macro-and micromorphology of the prepared CONs were investigated.The collected CONs are homogeneous and continuous at both macro and micro levels (Fig.9b).The AFM result shows the presence of two steps(5.5 nm and 2.5 nm),suggesting the formation of folded edge of 2.5 to 3.0 nm-thick film [92].
The liquid-liquid interface synthesis involves two immiscible solvents.Maet al.obtained CONs with high-quality at the interface of two miscible organic solvents with the addition of a buffer solution by this method [93].An aldehyde solution (A)was prepared by dissolving 1,3,5-triformylphloroglucinol (TFP) in dichloromethane (DCM) (Fig.9c).After that,the acetic acid solution (C) was added slowly to fully cover the surface of solution A as a buffer layer.Then,a dihydroxy benzidine (DHBD) solution in the mixed solvent of DCM andN,N-dimethylformamide (DMF) with a volume ratio of 4:1 (solution B) was dropped on the surface of solution C slowly.Since solution B is denser than the buffer layer (solution C),solution B will slowly permeate down,cross the solution C and reach the surface of solution A.Simultaneously,the amine monomer in solution B will react with the aldehyde monomer in solution A in the presence of acetic acid,forming CONs.Moreover,the newly obtained CONs could also function as a buffer layer restricting the permeation rate of amine monomer to aldehyde.As a result,high-quality CONs without unbounded layer stacking were prepared.According to the AFM image shown in Fig.9d,the thickness of prepared CONs is about 0.35 nm.Besides,the HR-TEM image suggests the layer space is 0.32 nm.These results are consistent with theπ-πstacking distance of 0.33 nm according to Materials Studio (MS) simulation [93].
2.2.3.Molecule-exchange synthesis
Besides the interfacial synthesis,CONs could also be prepared by molecule-exchange synthesis [96].The key step of this method is to restrict the vertical axialπ-πstacking of CONs layerviaintroducing bulky groups with large steric hindrances into the backbone of COF.Jianget al.developed a scalable general synthetic approach under solvothermal conditions by this strategy.An excessive amount of 2,4,6-trimethylbenzaldehyde (TBA) was added along with 5,10,15,20-tetra(p-aminophenyl)porphyrin (H2TAPP) and 4,4′-biphenyldialdehyde (BPDA) into ethanol/mesitylene/6 mol/L AcOH (Fig.10).Because of the reversible nature of imine bond,the bulky TBA could be successfully introduced to the edge of COF skeleton.As a result,an expanded interlayer space was achieved,which not only effectively hinders the stacking of CON layers but also enables the growth of CONs alongside the plane direction,resulting in the formation of thin CONs with a high yield.According to TEM images,A graphene like structure with a transparent appearance and a lateral size of several micrometers was observed,suggesting the ultrathin nature of COF-367 nanosheets.Due to the ultrathin thickness,the periodic pore lattice of the obtained CONs could be clearly observed by scanning tunneling microscope (STM) technique.The lattice parameters were measured to bea=b=2.7 ± 0.1 nm,α=90° ± 2°,which are consistent with those of the simulated structure of COF-367,indicating the successful fabrication of CONs.The thickness of CONs was further measured by AFM technique,which shows a uniform thickness of 1.15 ± 0.10 nm.These results suggest that the COF-367 nanosheets are composed of three to four monolayers [52].
2.2.4.On-surface synthesis
On-surface synthesis is different from others.In this method,the monomers are firstly deposited on the surface of other solid materials under ultra-high vacuum (UHV) or liquid conditions [97–102].Then the condensation reactions are carried out under different conditions,depending on the physical property of monomers.
In 2017,Talyzinet al.reported a two-step synthesis method,which leads to the vertical growth of COF-1 nanosheets on the graphene oxide’s (GO) surface (v-COF-GO) (Fig.11a).Firstly,graphite oxide and benzene-1,4-diboronic acid (DBA) were dispersed in methanol followed by solvothermal reaction,producing DBA-functionalized GO.Once the reaction was completed,the DBA-functionalized GO was collected and washed in methanol and mesitylene/dioxane solution (1:1,v/v).Then,the DBA-functionalized GO with certain amount of DBA was put in an autoclave for a second solvothermal reaction.This method successfully produced the v-COF-GO for the first time.Furthermore,the micrometer-sized platelets of COF-1 oriented parallel to GO can be produced by using non-functionalized GO.The thickness of the COF-1 nanosheets can be precisely controlled by tuning the amount of DBA,resulting in a thickness of 3–15 nm.It is worth noting that the unique 3D structure can be maintained after carbonization,and the material shows excellent supercapacitor performance because of its unique nanostructure.The specific capacity is 160 F/g at a current density of 1 A/g [103].
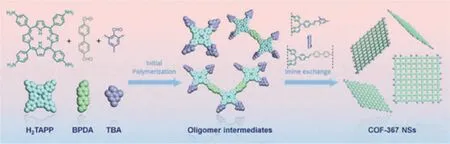
Fig.10.Schematic illustration of the synthesis of the COF-367 NSs.Reprinted with permission [52].Copyright 2019,American Chemical Society.
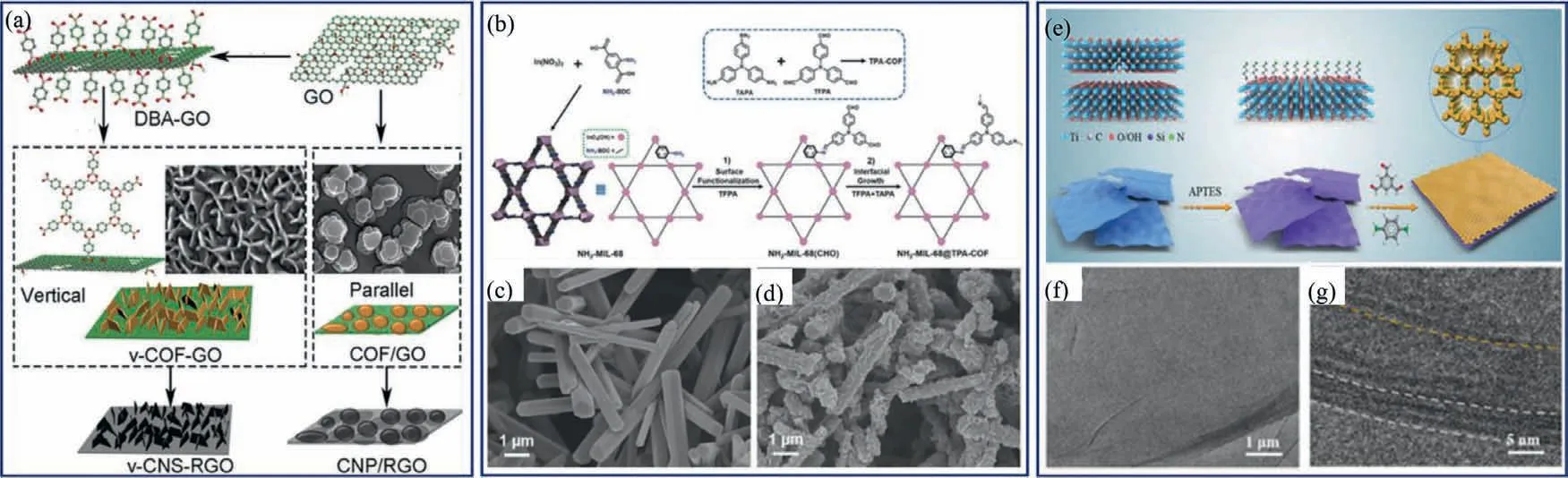
Fig.11.(a) Growth of vertical COF-1 nanosheets on RGO surface.Reprinted with permission [103].Copyright 2018,Wiley-VCH.(b) Synthetic strategy of NH2−MIL-68@TPACOF with core-shell structure.SEM images of (c) NH2−MIL-68 and (d) NH2−MIL-68@TPA-COF.Reprinted with permission [104].Copyright 2018,Wiley-VCH.(e) Schematic illustration of MXene covalent amination,followed by MXene@COF-LZU1 growth.(f) TEM and (g) HR-TEM images of MXene@CONs.Reprinted with permission [105].Copyright 2021,Wiley-VCH.
Similarly,CONs can also be grown on the surface of MOFs.In 2017,Zhanget al.successfully fabricated the MOF@COF core-shell hybrid materials (NH2−MIL-68@TPA-COF) by a twostep procedure for the first time.The amine-functionalized MOF (NH2−MIL-68) was pre-synthesized followed by reacting with tris(4-formylphenyl)amine (TFPA) by solvothermal method(Fig.11b),forming the core-shell structure.The material shows highly ordered pore channels and sheet-like morphology (Figs.11c and d).The obtained NH2−MIL-68@TPA-COF offers excellent catalytic degradation performance of rhodamine B under visible light[104].
Recently,Laiet al.produced ultrathin 2D COF-LZU1 on the surface of aminated Ti3C2Txnanosheets for the first time.Firstly,the Ti3C2TxMXene was aminatedvia in-situhydrolysis of 3-aminopropyl triethoxysilane (APTES) on the surface of Ti3C2TxMXene (Fig.11e).Then,The NH2moieties served as linkage sites and functionalized as a base to produce the ultrathin 2D COF-LZU1.Different from the traditional method to prepare MXene-based compounds by electrostatic interactions or physical mixing,this is the first demonstration of covalent deposition between COFs and MXene.After the growth of COF,a rougher surface was obtained with the thickness increased toca.13 nm (Fig.11f).The MXene lattice spacing of 1.3 nm could be observed (Fig.11g),which suggests the successful composition of COFs and MXene.The obtained composite featuring high stability,high porosity,and excellent electronic conductivity was employed as an effective matrix in Li-metal batteries,leading to dendrite-free Li deposition with long cycle life under high current densities (20 mA/cm2) and enhanced coulombic efficiency (CE) [105].
Various CONs synthesized by above-mentioned bottom-up method are summarized in Table 2.Compared with the top-down method,the bottom-up method could produce 2D CONs with precisely controlled thickness in a relatively high yield.In particular,the gas-liquid and liquid-liquid interfacial synthesis could prepare few-layer,even single-layer CONs with certain initial concentration of precursors.And the thickness of CONs could be effectively tuned ranging from nm to μm by varying the initial precursor concentrations.Molecule-exchange synthesis can also achieve precise control of thickness,but the method is more complicated in comparation with the above-mentioned interfacial synthesis.
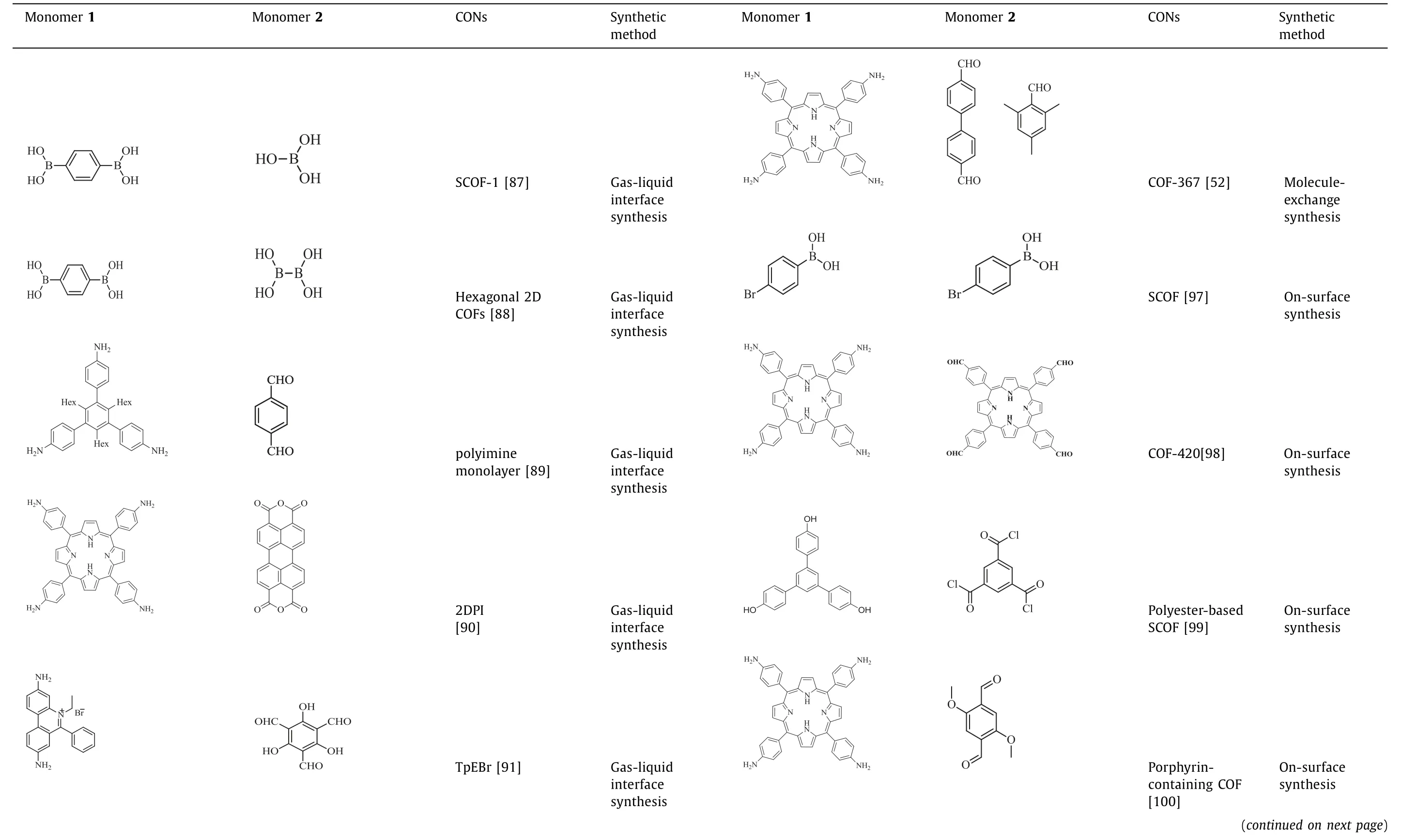
Table 2 Summary of CONs synthesized by the bottom-up method.
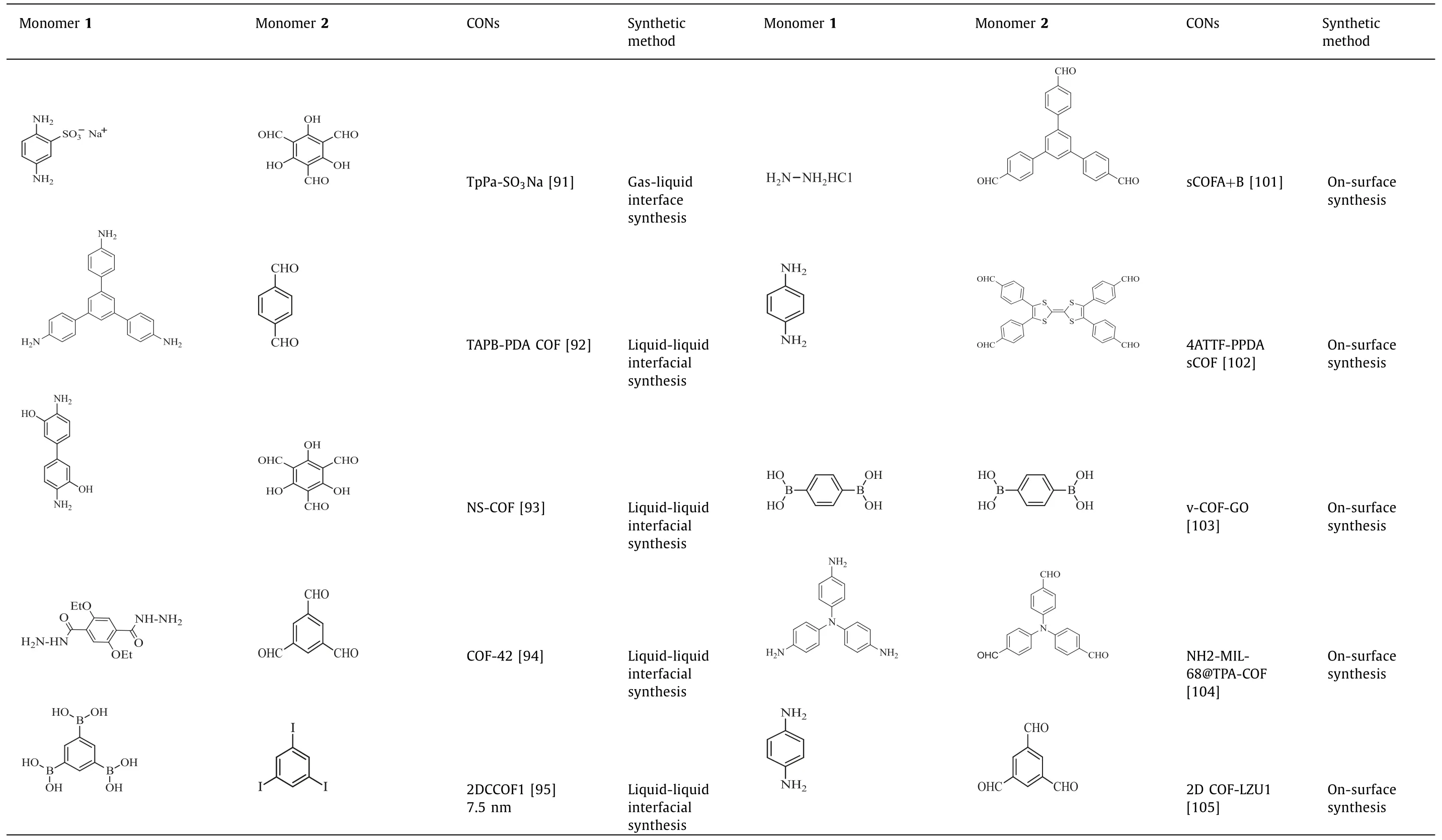
Table 2(continued)
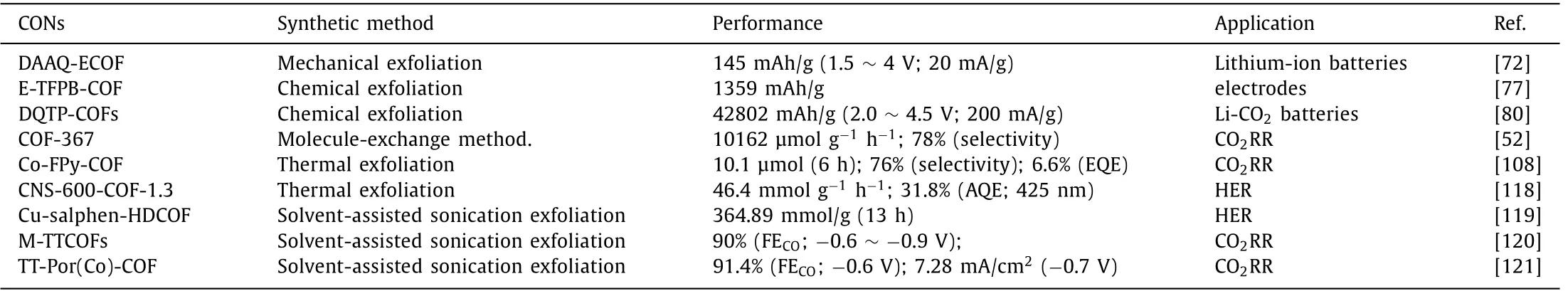
Table 3 Summary of CONs’performances in energy-related applications.
3.Applications of 2D CONs
Owing to their ultrathin thickness,highly exposed active sites,and wide structure tunability,the CONs are promising candidates in many fields.For example,the ultrathin thickness endows the CONs with ultrahigh molecules/electrons/protons transfer rate and highly exposed active sites favor high catalytic activity.In this section,we will summarize the recent development in energy-related applications of CONs.
3.1.Energy storage
In the past decades,2D COFs have been explored in energy storage applications (e.g.,batteries) due to their prominent advantages,such as wide chemical tunability and well-defined porous structure [106].It is possible to precisely attach redox-active group to the skeleton of COFs or introduce redox-active molecules into the pore volume [107,108].Besides,the ordered pore channels of 2D COFs could enable the rapid migration of charge carriers.However,the close stacking along the vertical direction through π-π interactions in 2D COFs would greatly lengthen the migration pathway of ions/electrons,making the interior active sites almost inaccessible,especially at high current densities,thus leading to inferior capacity and rate capability.Therefore,2D CONs enable maximal exposure of active sites and could greatly reduce the distance of charge carrier’s migration pathway,making them promising candidates for energy store and conversion-related applications [109].
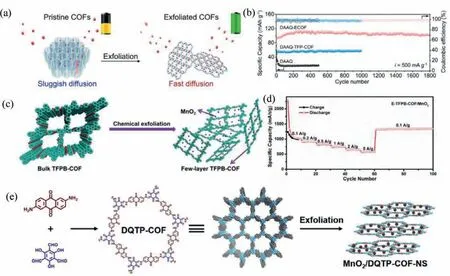
Fig.12.(a) Schematic illustration for the exfoliation of 2D redox–active COFs into exfoliated COFs as cathodes for Lithium-ion battery.(b) Cycle performance of DAAQ ECOF and DAAQ-TFP-COF at 20 mA/g.Reprinted with permission [72].Copyright 2017,American Chemical Society.(c) Chemically exfoliated 2D COFs with high affinity to lithium.(d) Rate capability tests at various current densities (0.1–5 A/g) of the E-TFPB-COF/MnO2.Reprinted with permission [77].Copyright 2019,Wiley-VCH (e) The chemical exfoliation of DQTP-COF.Reprinted with permission [80].Copyright 2021,Elsevier.
In 2017,Wanget al.successfully exfoliated the bulk COFs into CONs for Lithium-ion batteries by ball-milling delamination method (Fig.12a).According to the AFM image,the thickness of the obtained CONs is roughly 5 nm.TEM images indicate the obtained CONs are ultrathin,transparent and slightly wrinkled.To evaluate the electrochemical performance,DQQ-ECOF was mixed with conductive agent Super P and binder PVDF to form cathode,followed being fabricated in coin cells.DAAQ-TFP-COF and DAAQ were also assembled in coin cells in a similar manner.The cycle performance of the batteries was evaluated in the range of 1.5–4 V at a current density of 20 mA/g (Fig.12b).It can be seen that the discharge capacity of DAAQ-ECOF remains 145 mAh/g after 70 cycles,which isca.96% of the theoretical value.In contrast,the discharge capacity of DAAQ-TFP-COF is only 73% (110 mAh/g) of its theoretical value.This is probably due to the large mass transfer resistance in 2D COFs,leading to incomplete utilization of active sites.The discharge capacity of DAAQ decreases sharply from an initial value of 197 mAh/g to 75 mAh/g within only 20 cycles as a result of dissolution in the electrolyte.Cycle performance at a high current density of 500 mA/g was also evaluated.Surprisingly,the discharge capacity of DAAQ-ECOF retains 104 mAh/g after 1800 cycles,suggesting the fast charge carrier migration and high structural stability of DAAQ-ECOF [72].
Besides the pure CONs,CONs composited with other materials can also be used in the field of battery.Wanget al.fabricated fewlayered E-TFPB-COF by exfoliating layered TFPB-COFviachemical exfoliation (Fig.12c).Then,the obtained E-TFPB-COF was composited with MnO2particlesviathe introduction of KMnO4and used as electrode material.It could be found in Fig.12d that the capacity does not decrease drastically with increasing current density.In fact,the capacity of the composite even increases to some extent when current density returns back to 0.1 A/g,after cycling at various current densities.This outstanding performance could be attributed to the large surface area and ultrathin thickness of CONs,endowing more exposed active sites and shortening the diffusion distance of charge carriers,which can effectively improve the Li+ion diffusion kinetics.At the same time,the MnO2nanoparticles could prevent the aggregation of CONs and keep CONs stable [77].
Li-CO2batteries have also attracted extensive attention due to their higher theoretical energy density (1876 Wh/kg) than Li-ion batteries (265 Wh/kg) [110].More importantly,Li-CO2batteries may be used in environments with high CO2concentrations,such as factories,power plants,and even Mars exploration (96 wt% of CO2in the atmosphere) [111,112].Recently,Lanet al.successfully fabricated the MnO2-supported CON hybrid material by chemical exfoliation based on KMnO4and 2,6-diaminoanthraquinone-2,4,6-triformylphloroglucinol (DQTP)-COFs (Fig.12e).The obtained nanosheet (as thin as 1.87 nm) was composited with the quinone-CONs and MnO2nanoparticles,which can effectively prohibit the coagulation of CONs.The prepared composites were used as cathode catalysts in Li-CO2batteries and exhibited a high discharge capacity of 42,802 mAh/g within 2.0–4.5 V at 200 mA/g.Furthermore,the cathode catalysts exhibit high stability during the higher-stress tests as well as high-rate capability [80].
CONs provide excellent stability as organic electrode materials compared with small molecules.Moreover,the large amount of exposed active sites of CONs results in excellent performance in the field of battery.Great progress of CONs in the field of energy storage has been achieved.However,the CONs would easily aggregate during cycling,which would lead to unsatisfying cycle performance.Therefore,more efforts must be made to overcome above-mentioned problems and achieve better electrochemical performance.Additionally,bulk COFs have shown their promising applications in supercapacitors [113,114],the applications scope of CONs in the field of supercapacitors also needs to be further exploited.
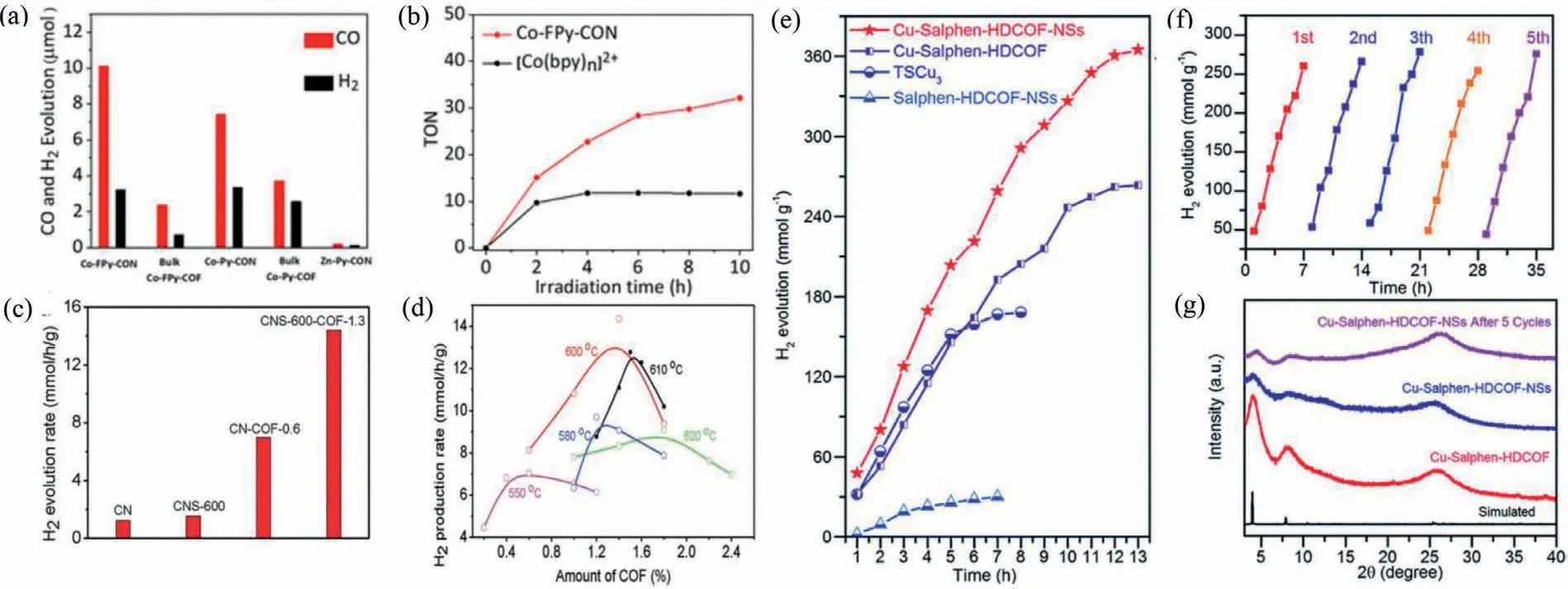
Fig.13.(a) CO and H2 production by CON and bulk COF of Co-FPy-COF,Co-Py-COF and Co-Bp-COF,over 6 h under visible light irradiation (λ > 420 nm,300 W Xe light source),with (Ir[dF(CF3)ppy]2(dtbpy))PF6 as the photosensitizer.(b) TONs of CO production by Co-FPy-CON and [Co(Bpy)n]2+ under visible light irradiation (λ > 420 nm,300 W Xe light source),with (Ir[dF(CF3)ppy]2(dtbpy))PF6 as the photosensitizer.Reprinted with permission [108].Copyright 2020,American Chemical Society.(c) Photocatalytic H2 evolution rate over bulk CN,CNS-600,CN–COF-0.6,and CNS-600-COF-1.3.For all samples,0.5 wt% Pt was loaded as co-catalyst,and 0.1 g of photocatalyst was used.(d) Photocatalytic activity of CNS-x-COF-y.For all samples,0.5 wt% Pt was loaded as co-catalyst,and 0.1 g photocatalyst was used.Reprinted with permission [118].Copyright 2020,Wiley-VCH.(e) Comparison of the hydrogen production of Cu-salphen-HDCOF-NSs,Cu-salphen-HDCOF,TSCu3 and salphen-HDCOF-NSs.(f) Photocatalytic cycles of hydrogen generation of Cu-salphen-HDCOF-NSs.(g) PXRD patterns of Cu-salphen-HDCOF and Cu-salphen-HDCOF-NSs before and after 5 cycles.Reprinted with permission[119].Copyright 2020,Royal Society of Chemistry.
3.2.Energy conversion
As known,porous materials including amorphous porous organic polymers,MOFs,COFs and zeolites are promising catalysts due to their large surface areas and high porosities [115–117].Among them,COFs provide prominent advantages: (1) The active site can be accurately introduced to the highly ordered skeleton;(2) The chemical and physical environment near the active site can be designed,which can be used to accurately tune the catalytic performance;(3) The chemical stability can be improved by suitable bonding.Therefore,COFs demonstrate remarkable performance in the field of catalysis.In this section,the recent development of catalyst materials based on COFs will be discussed.
Jianget al.successfully developed a scalable bottom-up synthetic approach toward CONs by molecule-exchange strategy.Through this method,gram-scale CONs can be easily produced.Most importantly,the obtained CONs show excellent photoreduction performance of CO2to CO with a rate of 10,162 μmol g−1h−1and a selectivity of 78% [52].In another work,Cooperet al.synthesized the 2D COFs,named FPy-COF,by the Schiff base condensation reaction between 5,5′,5′′,5′′′-(pyrene-1,3,6,8-tetrayl)-tetrapicolinaldehyde and 4,4′-diamino-2,2′-difluorobiphenyl.In order to further tune the photoreduction CO2performance of FPy-COF,single cobalt atom was introduced into the ordered channels of the COF material to prepare the COF composite,which was further sonicated to yield the COF nanosheets,denoted as Co-FPy-CON.The obtained Co-FPy-CON was further applied in photocatalytic CO2reduction.Under the irradiation of visible light with iridium dye,the cobalt-loaded CONs produce 10.1 μmol CO within 6 h with a selectivity of 76% and achieve extremely high external quantum efficiency (EQE) of 6.6% (Fig.13a).And it has been found that the CON skeleton could promote the electron transfer from photosensitive dye to cobalt atom.After a total of 10 h irradiation under the visible light,the CON showed a TON (turnover numbers)of 32.1,which is 2.8 times higher than that of the corresponding homogeneous counterpart [Co(bpy)n]2+(Fig.13b).With the presence of Co single site,the charge carrier transfer was also enhanced.Furthermore,compared with their bulk counterparts,the ultrathin CONs show superior photocatalytic activities,confirming the advantage of CONs [108].
Besides,the CONs can also be used in the field of photocatalytic hydrogen evolution reaction (HER) [43].For example,Yanet al.successfully modified the graphitic carbon nitride nanosheet (CNS)with trace CON through chemical imine bonding.Firstly,the CNS with improved crystallinity and reduced defects was synthesized by delicately tuning the thermal exfoliation conditions of bulk carbon nitride (CN).Then the ultrathin CON was introduced on the surface of CNSviaimine bonding by a solvothermal reaction.The integration of CONs with CNS effectively improves the charge separation because of the strong electronic coupling between these two components.As a result,the hydrogen evolution rate of the obtained material has been greatly improved (Fig.13c).Furthermore,the exfoliation temperature and the number of CONs in composite materials also have been well studied in this work.As shown,the suitable exfoliation temperature and the quality fraction of CONs are 600 °C and 1.3 wt%,respectively (Fig.13d).Moreover,under the optimum condition,the hydrogen evolution rate of the composite material reaches 46.4 mmol g−1h−1with a quantum effi-ciency (AQE) of 31.8% [118].
Recently,Thomaset al.have successfully fabricated the catalyst (named Cu-salphen-HDCOF) with a well-designed 2D salphen COF structure by the co-condensation of 2,3,6,7,10,11-hexaiminotriphenylene (HATP·6HCl) with 2,6-diformylphenol (DFP)under solvothermal conditions.After the successful synthesis,Copper (Cu) ions were integrated into the COF backbone.As reported previously,the Cu ions are extremely effective in the field of water splitting because of their diverse redox properties.Therefore,the obtained catalyst with high loading (15.63 wt%) and homogeneously distributed Cu were used in HER.In order to solve the low utilization of active sites and low ion diffusion rate,the obtained bulk COFs were exfoliated to CONs.The prepared catalyst(Cu-salphen-HDCOF-NSs) displays extremely high hydrogen evolution rate of 36.99 mmol g−1h−1in the first 7-h and the total amount of evolved H2reaches 364.89 mmol/g within 13-h.(Fig.13e).Moreover,no obvious performance decay was observed under cycle test,indicating the excellent stability of the catalyst(Fig.13f).The stability of the catalyst can also be verified by PXRD patterns,which show that the high crystallinity remains after cycle test (Fig.13g) [119].
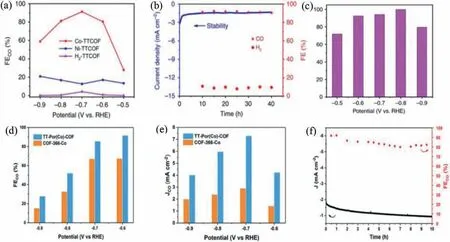
Fig.14.(a) The Faradic efficiency of CO calculated overpotential range from −0.5 V to −0.9 V.(b) Cycling stability test of Co-TTCOF at the potential of −0.7 V vs. RHE.(c)FECO of Co-TT CONs over a potential range from −0.5 V to −0.9 V.Reprinted with permission [120].Copyright 2020,Nature Publishing Group.(d) FECO and (e) JCO from−0.6 V to −0.9 V vs. RHE of TT-Por(Co)-COF and COF-366-Co.(f) Stability test of TT-Por(Co)-COF in CO2-saturated 0.5 mol/L KHCO3 electrolyte at a potential of −0.6 V vs.RHE for 10 h.Reprinted with permission [121].Copyright 2020,Wiley-VCH.
In addition of the excellent photocatalytic performance,CONs are also promising electrocatalysts for converting CO2to CO.Lanet al.designed and prepared a series of metalloporphyrintetrathiafulvalene-based covalent organic frameworks (M-TTCOFs).In this system,the tetrathiafulvalene was designed as the electron donator or carrier,forming an oriented electron transmission pathway when connecting with metalloporphyrin.After embedding certain metals like Co,the crystalline bulk Co-TTCOFs can be used as the electrocatalysts in CO2reduction with high Faradaic efficiency of CO (FECO) (91.3%,−0.7 V) and excellent cycling stability(>40 h) (Figs.14a and b).Moreover,in order to further improve the catalytic activity,the bulk M-TTCOF was exfoliated by sonication.The as-resulted Co-TTCOF nanosheet (as thin as 5 nm) shows the FECOvalue higher than 90% at the potential range of −0.6 V to−0.9 V.Particularly,the FECOcould approach 100% at the potential of −0.8 V (Fig.14c) [120].
In another work,Caoet al.also constructed the donor–acceptor heterojunctions in porphyrin-based CONs (TT-Por(Co)-COF) by thieno[3,2-b]thiophene-2,5-dicarbaldehyde (TT) and 5,10,15,20-tetrakis(4-aminophenyl)-porphinatocobalt (Co-TAPP).Compared with COF-366-Co without TT,TT-Por(Co)-COF offers superior electrocatalytic performance of CO2to CO with a high FECO (91.4%,at−0.6 V) (Fig.14d) and larger partial current density (7.28 mA/cm2at −0.7 V) (Fig.14e).Furthermore,the obtained TT-Por(Co)-COF also shows long-term stability with above 80% of FECOmaintained(Fig.14f) [121].
3.3.Other applications
Due to their large aspect ratio,high processability,and ultrahigh sensitivity to environment change,2D CONs have also been considered as promising candidates for photosensors [2]and gas separation [68].
Choiet al.fabricated the scalable 2D CONs on the surface of water by the photon-assisted imine condensation reaction.As a result,the thickness of CONs was measured to be 0.7 nm,corresponding to a single layer thickness.Furthermore,the thickness of the CONs or the number of layers can be linearly tuned by varying the initial concentration of precursors.According to the UV–vis absorption spectra and photoluminescence (PL) spectra,the prepared CONs show the potential response to light.This has been validated by testing the resistance change of CONs-based electrical devices under light irradiation.The obvious resistance change was observed,demonstrating the outstanding photo response property of CONs,implying the great potential in the photo sensor field.Moreover,the well-ordered and uniform porous structure endows the CONs with excellent gas-or vapor-adsorbent ability,which is the foundation of gas or vapor sensors.The appearance of water vapor would lead to an obvious current change.Although the details of resistance change mechanism still need to be further researched,the result suggests the CONs could be used as a humid sensor with high sensitivity [2].
In addition,CONs can also be applied in the field of gas separation.For example,Zhaoet al.fabricated the functional ultrathin COFs film for CO2separation.In this study,two CONs named NUS-2 and NUS-3 were prepared by solvent-assisted sonicated exfoliating from the corresponding bulk COFs.The obtained two CONs with different pore sizes have been subsequently mixed with commercial polymers poly(ether imide) (Ultem) or polybenzimidazole(PBI) to form the mixed matrix membranes (MMMs).Due to the appropriate pore size (0.8 nm),highly selective absorption of CO2to H2was achieved by NUS-2 based MMM,exceeding the 2008 Robeson upper bound [68].The performances of CONs in energyrelated applications discussed in this review are summarized in Table 3.
4.Conclusion and perspectives
In summary,we introduced the strategies used in the preparation of 2D CONs,i.e.,top-down (e.g.,solvent-assisted sonication exfoliation,mechanical exfoliation,chemical exfoliation,and selfexfoliation) and bottom-up methods (e.g.,gas-liquid interface synthesis,liquid-liquid interface synthesis,molecule-exchange synthesis,and on-surface synthesis).Then we focused on the energyrelated applications of 2D CONs,including batteries,photocatalysis,and electrocatalysis.Although 2D CONs possess many inherent structural advantages for certain applications,and great progress in the preparations and energy-related applications of 2D CONs has been achieved.However,improving device performance and exploring the relationship between structure and performance of CONs still remain challenging.Besides,there are many issues that need to be addressed to apply 2D CONs in a wider range of applications.Firstly,more simple and sufficient methods for preparing COF nanosheets and their composites need to be further developed.Secondly,characterization techniques with more information need to be developed,such as low-dose TEM [122],and aberrationcorrected TEM [123].Thirdly,the overall performance of 2D CONs in electric devices needs to be exploited.To achieve these goals,the poor conductivity of ultra-thin 2D CONs,which severely limits their applications in functional electronic devices,should be improved.Fourthly,the applications of 2D CONs should be further broadened in addition to Li-battery,CO2reduction,and H2evolution,such as the applications in the biological field.Last but not least,the relationship between structure and performance should be further studied to promote the design of highly efficient catalysts.This might be a promising direction for the development of 2D CONs in energy-related studies.
Declaration of competing interest
The manuscript does not include any conflict of interest.
Acknowledgments
Y.Peng thanks the financial support from the Class D of Qianjiang Talent Program (No.ZD20011250001) and the start-up grant(No.2019125016829) in Zhejiang University of Technology.The authors gratefully acknowledge the financial support from the funding of "Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang" (No.2020R01002).G.Xu thanks the financial support from the National Natural Science Foundation of China(No.51907173).
杂志排行
Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
