Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
2022-07-11HumayraAfrinChristianelJosephSalazarMohsinKaziSyeRizwanAhamaMajeAlharbiNurunnabi
Humayra Afrin,Christianel Joseph Salazar,Mohsin Kazi,Sye Rizwan Ahama,Maje Alharbi,M Nurunnabi,e,f,∗
a Environmental Science &Engineering,University of Texas at El Paso,El Paso,TX 79965,United States
b Border Biomedical Research Center,University of Texas at El Paso,El Paso,TX 79965,United States
c Department of Pharmaceutics,College of Pharmacy,King Saud University,Riyadh 11451,Kingdom of Saudi Arabia
d Central Laboratory,Department of Pharmaceutical Chemistry,College of Pharmacy,King Saud University,Riyadh 11451,Kingdom of Saudi Arabia
e Department of Biomedical Engineering,University of Texas at El Paso,El Paso,TX 79965,United States
f Department of Pharmaceutical Sciences,School of Pharmacy,University of Texas at El Paso,El Paso,TX 79902,United States
Keywords:Cardiotoxicity Anticancer drug Chemotherapy Screening Biomarker
ABSTRACT Cardiac toxicity is one of the most common side effects of anticancer drugs.Cardiac toxicity results dysfunction of heart including hypotension,heart failure,and even cause death in extreme cases.The potential risk of cardiotoxicity is a huge concern in chemotherapeutics mediated cancer treatment.The individual with any pre-existing cardiac issues are excluded from clinical trials due to the potential risk of cardiotoxicity.Because of the potential cardiotoxicity,there is an emerging need for alternatives of some very potent anticancer drugs (doxorubicin/DOX,5-fluorouracil/5FU,trastuzumab).While a patient is being treated with anticancer drugs,early blood screening,biomarker detection,and careful monitoring of cardiac functions are necessary to be able to avoid any irreversible cardiac damage.Therefore,early detection methodology to monitor cardiotoxicity in real-time,and a drug formulation that prevent interaction between drug and cardiac cell,seemingly have potential to mitigate the risk.In this review,we have summarized the cardiotoxicity of the most used anticancer drugs,their pathophysiology and some of the conventional and newer screening methods available to manage an individual patient in clinic.We have also incorporated our perspective on how a rationale designing of biomolecules can be used to overcome the cardiotoxicity generated chemotherapeutics.
1.Introduction
The human heart is one of the vital organs of the body.Heart pumps the blood throughout the body,supplies oxygen and nutrients to the tissues and removes carbon dioxide and other wastes.Heart is a four-chamber organ which includes many cell types including myocytes,endothelial cells,fibroblasts,and vascular smooth muscle cells.Each cell type play vital role in maintaining regular cardiac function [1].Cardiomyocyte cells are permanent cell that do not regenerate.Therefore,any damage to the cardiomyocytes negatively impact to the cardiac function.Antineoplastic agents have shown cardiac toxicity which is a rising concern among oncologists [2–4].According to the data collected by National Center for Health Statistics in 2018,in 2021,1898,160 new cancer patients will be diagnosed in United State and among them 608,570 patients will die [5].Incidence of cancer increases with the increased age and is more within 40−80 years of age [6].The risk of developing cardiac disease increases with the advanced age with or without exposure the of anticancer agents.It is a huge challenge for oncologists to continue the antineoplastic therapy with minimum or no cardiovascular side effect to minimize the cardiac dysfunction that may result fatality.
Anticancer treatment modalities including chemotherapy,immunotherapy and radiotherapy involving cardiac structure causes cardiac complications [7].Anthracycline (DOX,epirubicin,idarubicin),HER-2 inhibitor (Trastuzumab),anti-VEGF therapy (tyrosine kinase inhibitor,imatinib,dasatinib,nilotinib,bosutinib,sunitinib,sorafenib,axitinib,ponatinib),and immunotherapy have shown cardiac side effects including heart failure (HF),cardiomyopathy,myocarditis,hypertension (HTN),decrease left ventricular ejection fraction (LVEF),atherosclerosis [2,8]as a short term or long term complication.A pre-and post-treatment systematic screening and monitoring for cardiac disease can help in early detection and improve quality of life of both cancer patients and survivors [8,9].Research is being carried out to develop an effective early diagnostic tool,better monitoring system and novel drug delivery system.A metanalysis on tyrosine kinase inhibitor (TKI) cardiotoxicity in 2018 stated that,the early awareness,initiation of appropriate management,and close monitoring can enhance the benefits of TKI therapy [10].
Previously,there have been some extensive reviews published on cardiotoxic anticancer drug [11,12],screening and management[9].However,in this review we will discuss the available strategies and techniques for monitoring cardiac function utilizing biomarkers and modern imaging technologies.We will also discuss how early detection and better diagnosis can help physicians to develop an appropriate dose regimen to reduce the risk of irreversible cardiac damage.Finally,we will discuss the recent advancement in drug delivery science that has shown potential to minimize direct interaction between the anticancer drug and cardiac tissue as delineate the knowledge gap and possible future research direction.
2.Cardiac complication from anticancer therapy
2.1.Pathogenesis of cardiotoxicity
Anticancer mediated cardiac myopathy has been classified into two categories.Type 1: A irreversible myocardial damage due to oxidative stress on cardiac myocyte,and mitochondrial dysfunction causing morphological changes which is caused by anthracyclines,mitoxantrone or cyclophosphamide.Type 2: A reversible cardiac damage caused by trastuzumab or tyrosine kinase inhibitors (TKIs)which blocks the ErbB2 pathway and interrupt growth,repair,and survival of cardiomyocytes without changing the morphology[13–16].
Anthracycline (ATN) mediated cardiotoxicity is very much dose dependent.For example,2.2% of the patient receiving DOX results in heart failure (HF) at a cumulative dose of 300 ∼<550 mg/m²and this incidence increases up to 26%−30% with total doses of 500–800 mg/m2[17].Heart failure related mortality rate among the patients dosed with DOX is in range of 27%−60% [18,19].Another study shows 4% patient develops HF receiving DOX at a dose of 500–550 mg/m2whereas dose of 600 mg/m2or more raises the risk to above 36% but epirubicin or idarubicin shows very low degree of HF incidence [20,21].The main cause of cardiac damage due to doxorubicin is inhibition of topoisomerase 2β(TOP2) and reactive oxygen species (ROS) [22–24].TOP2 is overexpressed in cardiomyocyte.Doxorubicin forms a complex with TOP2 and DNA.This complex causes DNA damage and leads to cell death [24–26].DOX stimulates the ROS/RNS (reactive nitrogen species) production and causes free radical injury (Fig.1) and causes DNA damage [27].Other factors for DOX induced cardiac damage includes mitochondrial iron accumulation,calcium overload in sarcoplasmic reticulum,impaired protein synthesis [27–31].Another study stated that doxorubicinol,DOX metabolite,is known to be responsible for cardiotoxicity.Doxorubicinol is also known to be more potent than DOX at inhibiting calcium channel [31,32]
HER-2 is a protooncogene,one of the 4-member family (HER-1,HER-2/erbB2,HER-3,HER-4) of receptor tyrosine kinase,is highly expressed in 20%−30% of breast cancers [33]and associated with poor prognosis,increase chance of metastasis and drug resistance of breast cancer [34].In heart HER-2,HER-4/HER-4 homodimerization,and HER-4/HER-2 heterodimerization induce cell survival and activate protective pathways specially in cardiac stress (Fig.2)[35].Inhibition of neuregulin (which triggers dimerization) in heart causes impairment of HER-4/HER-4 homodimerization and HER-4/HER-2 heterodimerization on cardiomyocyte which leads to mitochondrial dysfunction mediated cell death [25,36,37].
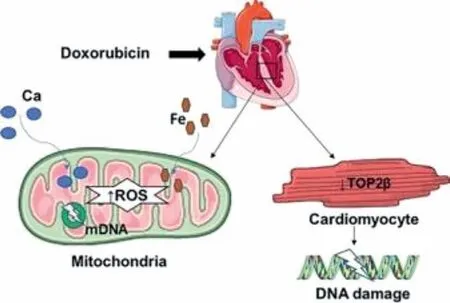
Fig.1.Anthracycline causes cardiac damage by inhibiting TOP2 (topoisomerase 2β),and by producing ROS (reactive oxygen species).TOP2 inhibition in cardiomyocyte causes DNA intercalation and damages myocardial DNA.Excessive production of ROS in mitochondria causes damage of mDNA (mitochondrial DNA).Intracellular accumulation of Ca and Fe also contributes the cardiotoxicity produced by DOX therapy.
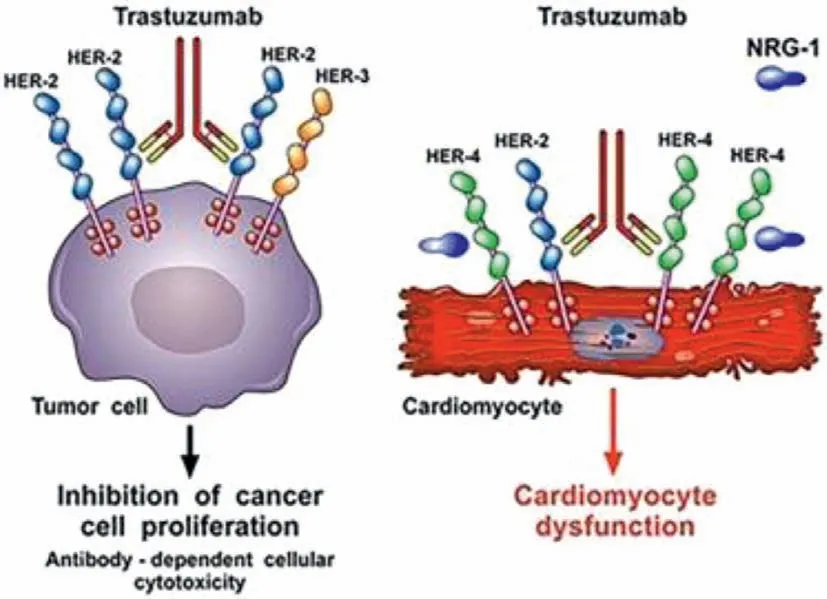
Fig.2.Trastuzumab promotes dimerization of HER-2 which is overexpressed in breast cancer and causes proliferation of cancer cell.HER-2 homodimerization and heterodimerization with HER-3 is inhibited by trastuzumab and halt the cancer cell growth (left side).On the other hand,trastuzumab inhibits the neuregulin which inhibits the protective dimerization of HER-2 and cardiomyocyte and causes mitochondrial dysfunction and cell death.Reproduced with permission from [30].Copyright 2018,Frontiers.
Tyrosine kinase inhibitor (TKI) mediated mechanism of cardiotoxicity has reported earlier in elsewhere [36].Small moleculebased TKI has been shown to have cardiotoxicity like HF and left ventricular dysfunction (LVD) [37,38].Imatinib causes cardiotoxicity by activating Endoplasmic Reticulum (ER) stress response,decreasing the mitochondrial membrane potential,releasing cyt c into the cytoplasm,depleting ATP in cell and cell death[36,37].
Several recently published review paper discussed on the mechanism of action of 5-flurouracil (5-FU) induced cardiotoxicity in detail [39,40].5-FU mediated cardiotoxicity induction mechanism involved thrombosis due to endothelial injury,and increased metabolism which leads to depletion of energy,ischemia,and cellular damage.5-FU also result oxidative stress,coronary spasm and reduction in the ability of transfer O2by red blood cells that lead to myocardial infarction [40].
Immunotherapy is among the newest treatment modality for cancer that works as a revolutionary in poor prognostic cancer.Most importantly the immunotherapy considers as much safer therapeutic modality compared to the small molecule-based chemotherapeutics.Immune-check point inhibitors (ICI) such as avelumab,pembrolizumab,ipilimumab,and nivolumab causes autoimmune cardiotoxicity [41].Result from a preclinical trial shows programmed cell death 1 (PD 1) and cytotoxic T lymphocyte antigen (CTLA) deficient mice started to die between 3rdand 5thweek due to cardiac complications [42,43].Though the pathogenesis behind cardiotoxicity is still poorly understood,some article referred that PD 1 inhibitor produces autoantibodies against cardiac myosin[44].Pembrolizumab (PD 1 inhibitor) causes cardiotoxicity by accumulating excess intracellular calcium,and reduction in cardiomyocyte viability [45].Other possible causes include targeting an antigen shared by the tumor,skeletal muscle,and the heart by T cells,resulting in low selectivity of the enhanced T cell responses between cardiac and skeletal muscle which leads to myocarditis(Fig.3) [41].
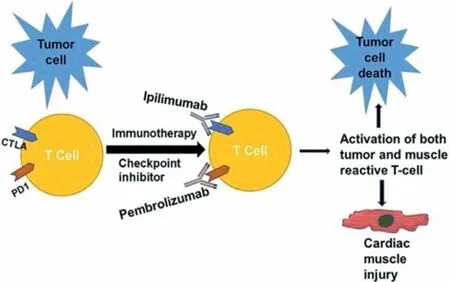
Fig.3.CTLA inhibitor like ipilimumab acts via switching off a negative regulator of T cell activation that prevents antitumor T cell responses,and PDL-1 inhibitor (pembrolizumab) removes a barrier to T cell function once T cells have infiltrated the tumor and its environment.In addition,ipilimumab and nivolumab also enhance T cell responses in cardiac and skeletal muscle,leading to autoimmune cardiotoxicity.Reproduced with permission from [41].Copyright 2017,Cell Press.
2.2.Cardiotoxic chemotherapeutics
The anthracycline antibiotics are arguably one of the most active groups of chemotherapeutic agents in oncology.Commonly used anthracycline antibiotics include DOX,daunorubicin,and epirubicin.They have proven activity against a spectrum of malignancies,including lymphoma,gastric cancer,small cell lung cancer,sarcomas,and breast cancer.Anthracycline causes dose dependent heart failure,and myocarditis for instance,DOX causes dose dependent cardiomyopathy where risk of cardiac failure increases linearly from 2.2% to 7% at a dose above 550 mg/m² [17,18,46].Majority of the patients develop cardiomyopathy within first year of treatment with a mortality rate more than 40% [17].Almost 9.9%of patients treated with daunorubicin experience cardiomyopathy.Trastuzumab and anthracyclines combination treatment found severe (NYHA III–IV) heart failure in 16% of patients.This incidence was much higher than that associated with anthracycline treatment alone [33].In one study 17 out of 172 children treated with daunorubicin showed cardiac complications with 360–1260 mg/m²dose.
5-FU is a very potent anticancer drug used widely in gastrointestinal cancer.Due to its cardiotoxic effect,now a days,alternative medicines like raltitrexed are considered [47].Some review articles thoroughly discussed about the cardiotoxicity profile of 5-FU [48–50].A study conducted in 1997 among the patients treated with 5-FU reported that 1.9% of patients develops symptomatic cardiac complications [51].It has been observed from a meta-analysis from different clinical trial that cumulative 7.9% patients reported cardiac complications.Among that 44.9% patients reported arrhythmia and 1.9% patients developed ischemic event [52].
A large variety of cardiotoxic events with manifestations such as heart failure,cardiomyopathy,heart block,myocardial fibrosis and myocarditis was documented with anti-CTLA-4 and anti-PD 1 (immune-checkpoint inhibitor [ICI]) therapy [53].Several studies reported autoimmune myocarditis and heart failure in patients treated with pembrolizumab [53–56].79% (23 out of 30) reported left ventricular dysfunction from ICI with a 27% mortality rate [57].A case report of 2 patients published in 2016 stated that a combination therapy with ipilimumab and nivolumab caused myocarditis where none of the patient had any cardiac risk factors except hypertension [58].However,another study,in patient receiving combination of 3 mg/kg ipilimumab and 1 mg/kg nivolumab,found patients experiencing myocarditis [59].
Radiation therapy involves damages in the surrounding tissue and causes pericardial disease,restrictive cardiomyopathy,accelerated coronary artery disease,and valvular heart disease [7].Radiation-induced cardiac disease is 10% to 30% by 5 to 10 years post-treatment [60].
Table 1 summarizes the known cardiovascular side effects of commonly used anticancer drugs [18,30,37,50,61-93]
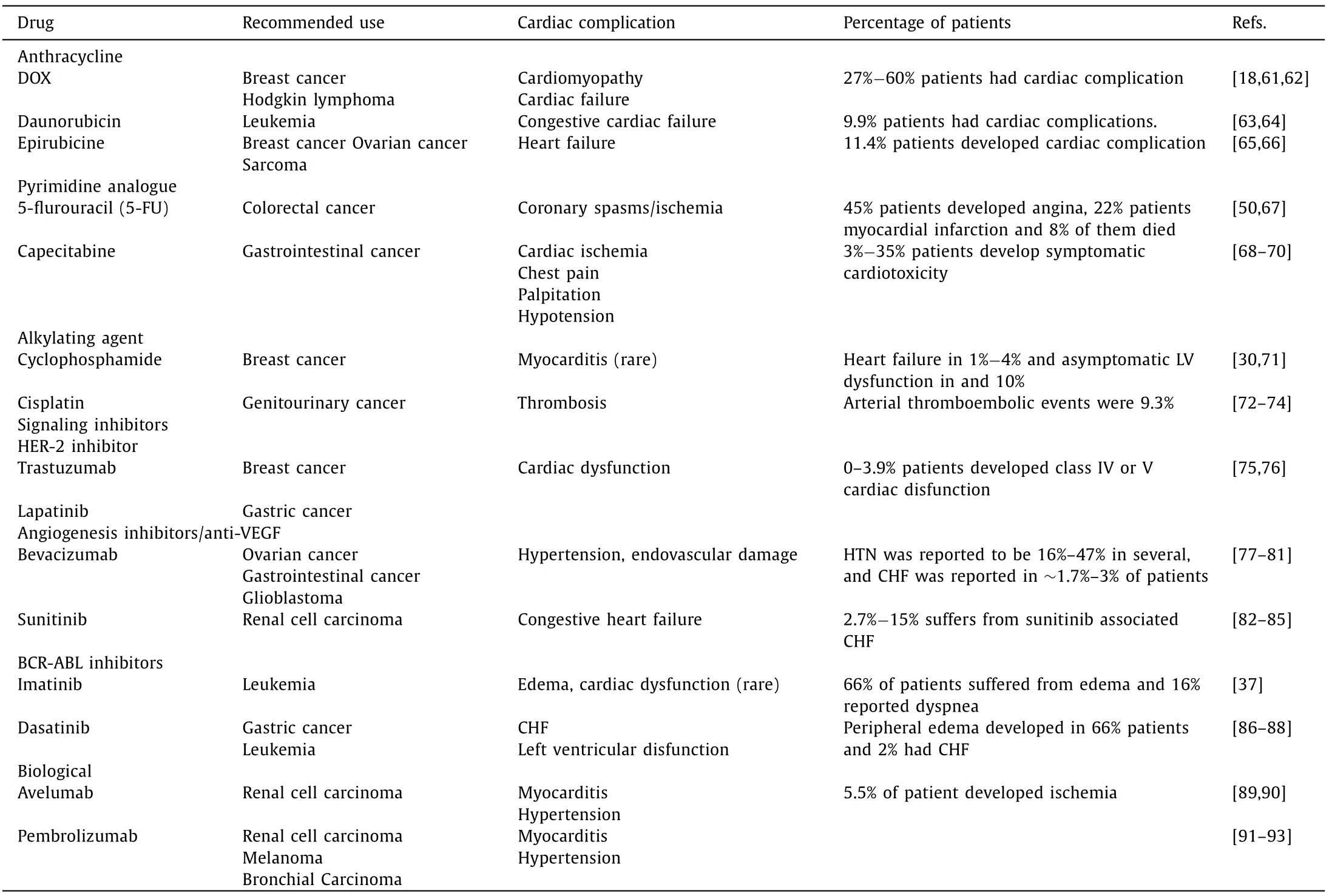
Table 1 This table summarize the known side effect of the commonly used anticancer drugs.
3.Monitoring and screening of cardiotoxicity
3.1.Common monitoring method and its importance
An article published in The U.S.Pharmacist [94]stated that cardiotoxicity ranges from reversible,irreversible,acute,chronic and late onset can simply be classified into two groups: reversible and irreversible.A meta-analysis on childhood cancer survivor stated that the childhood cancer survivors are at 15 times higher risk of developing CHF and 7 times higher risk of early death due to cardiovascular complication [95–97].Severity of cardiotoxicity can be determined from the following markers.For example,40%−49% LV ejection fraction (LVEF) indicates moderate cardiotoxicity,whereas,LVD with LVEF ≤40% indicates severe cardiotoxicity [98].Mortality rate of patients with severe cardiotoxicity was 22.9% [98].Therefore,early diagnosis of cardiovascular side effect is crucial to be able to select appropriate therapy.Early diagnosis can decrease the mortality rate,improve quality of life and halt further development of cardiovascular dysfunction.To mitigate these side effects,researchers proposed some monitoring methods like serial endocardial biopsy,serial B-type natriuretic peptide (BNP) and troponin level testing,radionucleotide MUGA or radionucleotide angiography,exercise testing,and echocardiogram.Currently established guideline for screening chemotherapy related cardiotoxicity includes assessment of left ventricular(LV) ejection fraction (EF),LV blood volume,diastolic function,MRI,computed tomography (CT),and positron emission tomography (PET) [99,100].Other monitoring methods that are used in clinic includes endomyocardial biopsy,radionuclide angiocardiography (RNA),molecular imaging,and biochemical markers such as troponin T and B-natriuretic peptide (BNP) [101,102].Here we will discuss the screening and monitoring methods in 4 different sections: (1) Screening based on clinical manifestation,(2)Analysis from cardiotoxic biomarker,(3) Imaging technologies,(4)Others.
3.2.Physical appearance and clinical manifestation
Cardiotoxicity that usually occurs are angina,arrythmia,tachycardia,bradycardia,atrial fibrillation,cardiomyopathy,congestive heart failure [103,104].Risk factors for developing various degree of toxicity includes dose of chemotherapy,age,sex,preexisting heart disease,high blood pressure [105].Other preexisting conditions include diabetes,renal disease,electrolyte imbalance,high cholesterol,smoking [106,107].The risk factors should be evaluated before starting the treatment.As,risk and degree of cardiotoxicity is worst with pre-existing condition,the patients should be screened for risk factors and conditions should be treated or modified beforehand [107].
Unfortunately,the treatment of cardiotoxicity is still symptomatic and physical appearance or changes in physical activity is visible when the cardiac damage is very significant and in late stage.In most of the cases mild and reversible symptoms are not physically appeared.Coincidingly the physical characteristic of patient undergoing cardiotoxicity involves having shortness of breath,chest pain,fluid retention in the legs,stomach distention,dizziness,and heart palpitations.Though in most of the cases anticancer induced cardiotoxicity had no clinical manifestation or remain asymptomatic as subclinical cardiotoxicity,the treatment of cardiotoxicity is symptomatic [107–109].However,caregivers put the patients under continuous monitoring for heart rate and blood pressure when a patient is being treated with cancer chemotherapeutics to observe cardiac function.The sign and symptoms mediated detection methods are often scientifically invalid and nonquantitative.In many cases abnormal sign and symptoms may lead the oncologist to decide on further diagnosis and determine on dosage amount and frequency.
3.3.Utilizing biomarkers as a read out for cardiotoxicity detection
According to the National Institutes of Health,biomarkers are defined such that to objectively measure and evaluated as an indicator of normal biological processes,pathogenic,or pharmacologic responses to therapeutic intervention [110].Therefore,biomarkers are determined by their abilities to have relevance to provide information clinically to health officials and validity to support the effectiveness of using biomarkers as a resource to compare the normal to any anomalies [110].Moazeniet al.has reported that there are several specific biomarkers that upregulate due to induction of cardiotoxicity.These circulating biomarkers such as cardiac troponin and natriuretic peptides could be used for detecting and monitoring cardiac health status.On top of these widely reported biomarkers,galectin-3,soluble ST-2 proteins,myeloperoxidase and fibrocytes are being explored to reliably predict the onset of cardiotoxicity early [111].Refer to Table 2 for a list of various biomarkers that can be considered as read-out to monitor cardiotoxicity [112–149].Therefore,it is imperative to understand transcriptomics,proteomics and metabolomics systems in order to understand anthracycline-induced cardiotoxicity [111].miR-208a has been identified as a heart-specific molecule to be used as an indicator for pre-drug induced cardiotoxicity,regulator of cardiac hypertrophy and biomarker implied cardiac miRNAs are abundantly expressed in the myocardium,that has a vital role in heart function and heart pathology [111,150,151].Decreased level of miR-1–3p,miR-122–5p,miR-127–3p,miR-133a-3p,miR-215–5p,miR-455–3p,and miR-499a-5p,and increased miR-34a-5p level was found in doxorubicin induced cardiotoxic mouse model than the healthy control [152].
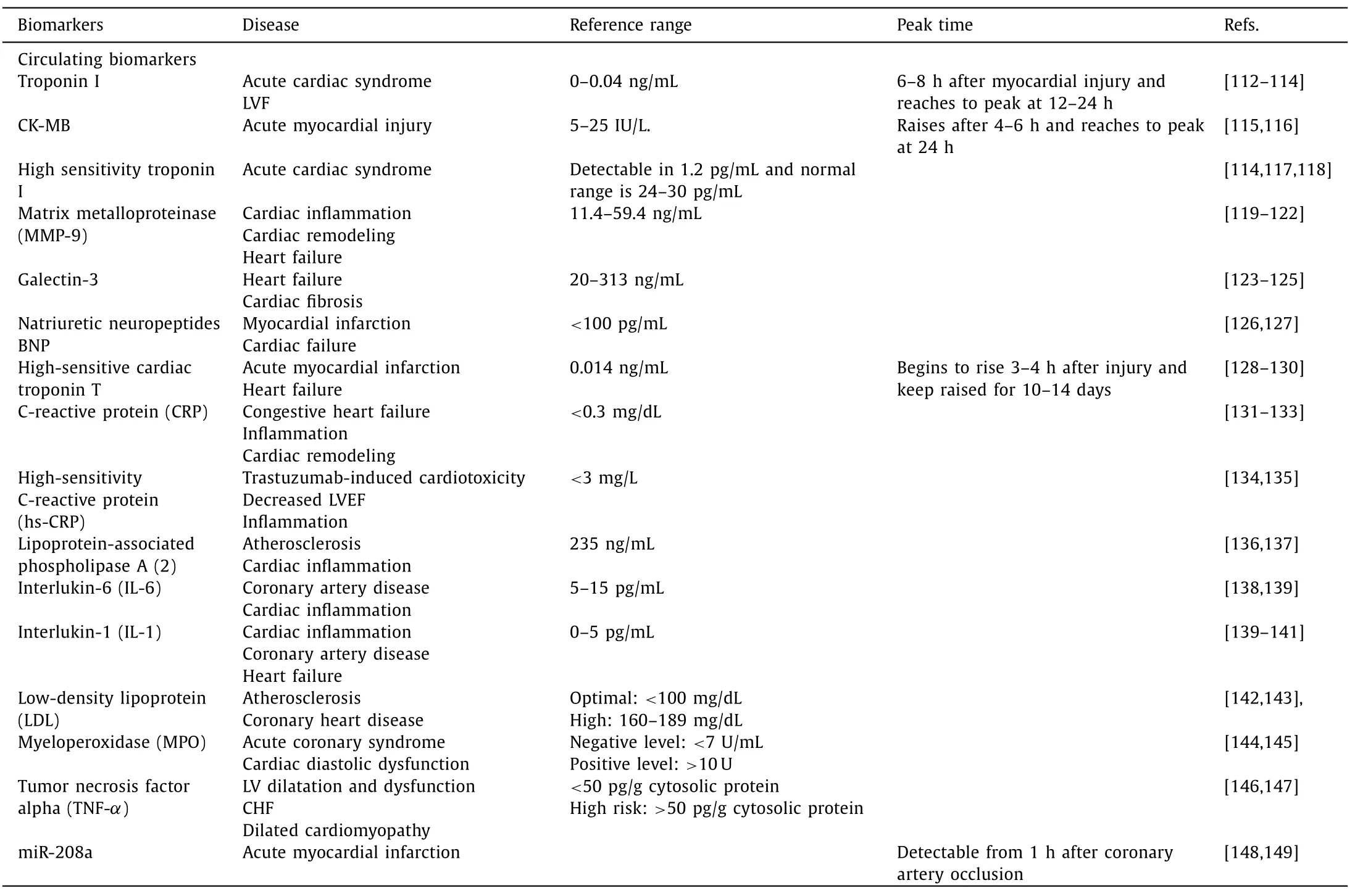
Table 2 List of various biomarkers that can be considered as read-out to monitor cardiotoxicity.
3.4.Using imaging technology for cardiotoxicity detection
Imaging modalities are the mostly used technique to determine cardiotoxicity [153].Abnormal Electrocardiogram (ECG) is widely used to diagnose acute cardiotoxicity.Visualization of cardiotoxicity for qualitative and quantitative analysis of degree of toxicity play vital role in the medical profession.With modern technology,there are various instruments and techniques to detect and monitor degree of cardiotoxicity.In 2019,a review article by Awadallaet al.has demonstrated various techniques that have been using in clinic or have potential to detect cardiotoxicity.The conventional non-invasive imaging procedures for diagnosis of cardiac dysfunction includes echocardiography,cardiac magnetic resonance (CMR)and multigated acquisition (MUGA) scans [8,154-156].2D echocardiography is the most common and available imaging modality though 3D echocardiography provides better accuracy [157].A study on childhood cancer survivor reported that,children treated with anthracycline,measuring of circulating biomarkers coupled with 3D echocardiography and cardiac MRI is more effective in diagnosing late cardiac event [158].Other accessible imaging technique includes stress echo,myocardial strain imaging [159,160].Myocardial strain imaging is recommended every 6 months after completion of chemotherapy for type 1 cardiotoxic drug and for type 2 cardiotoxic drug every 3 months during the therapy.Singlephoton electron computed tomography (SPECT),Positron emission tomography (PET),Computed tomography (CT) are also useful tools to cardiotoxicity detection [161,162].Research has been going on to detect chemotherapy related cardiotoxicity by non-invasive or minimal invasive imaging.68Ga-Galmydar (a PET imaging tracer)could detect the cardiotoxicity in rat using microPET scanning only after 5 days of injecting a single dose of DOX (15 mg/kg)[163].Other PET tracer used to detect cardiac toxicity includes18F-labeled lipophilic phosphonium cation,18F-fluorodeoxyglucose(FDG) [164–167].A report onin vivostudy demonstrated that18FDHMT (a marker for ROS) PET imaging could detect an elevation in cardiac superoxide production in anthracycline treated rat before decreasing the LV ejection fraction [168].Anthracycline induced cardiotoxicity in mice was also detected by molecular imaging of apoptosis using18F-CP18 (a caspase-3 substrate) [169].An increase uptake of18F-CP18 can be observed by microPET,autoradiography and TUNEL staining.In addition increased FAP expression can be detected in a chemotherapy induced cardiotoxic patient by using Ga-68 fibroblast activation protein inhibitor (FAPI) positron emission tomography (PET)/computed tomography (CT) [170].
4.Patient management
4.1.Current clinical guideline and treatment
Due to the severity and vast majority of the patients receiving chemotherapy,end up developing cardiac complications,a new field “cardio-oncology” is emerging for clinical management.Cardio oncology is a multidisciplinary team consisting of medical oncologist,cardiologist,radiation oncologists,hematologists,and specialized nurse [171,172].This team focused on recognizing the risk factors,diagnosis of cardiotoxicity,determining the stage of toxicity,taking proper management to treat the condition and long term monitoring of the surviving patients,and making guidelines [172–174].A report published from a cardio oncology clinic demonstrate among the patients referred (breast cancer patients receiving trastuzumab and developed cardiotoxicity) to their clinic,>90% of them could finish the treatment successfully with trastuzumab despite having existing cardiotoxicity [175].In 2017 and 2018,American Society of Oncology (ASCO) provided a recommendation and guideline for management of cancer survivors with cardiac dysfunction [8,176].ASCO and ESMO (European Society for Medical Oncology) have their own guideline for managing the patients with chemotherapy induced cardiotoxicity [177,178].A cardio-oncology expert panel from the French Working Group of cardio-oncology analyzed both guidelines to harmonized them[174].However,all the guidelines recommended to manage the situation with a multidisciplinary approach,screening before and after starting a cardiotoxic treatment and taking alternative approach(if available) for the high-risk group.
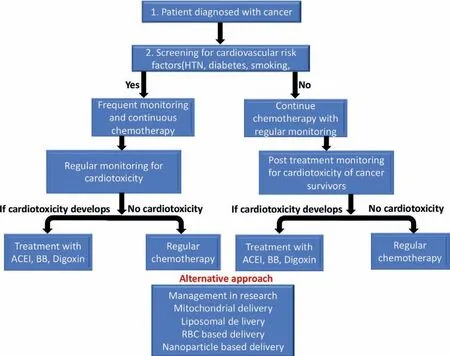
Fig.4.The scheme describes the method of monitoring and management of a cancer patient.A cancer patients should first be screened for the risk factor for cardiovascular diseases before starting chemotherapy.If there is any pre-existing risk factor,they should be monitored frequently then other group with the ongoing chemotherapy.During the chemotherapy monitoring (by clinical manifestation,serum analysis for cardiotoxic biomarker,imaging analysis) should be continued.Appropriate management should be taken if the patient diagnosed with cardiotoxicity in the process.The alternative treatment management might also be considered in future for prevention of cardiotoxicity.
Management for anthracycline and other dose related cardiotoxicity includes careful monitoring (Fig.4) of dose and measurement of systolic function at 6 months after the conclusion of treatment,every year for following 2 or 3 years of post-treatment,and then every 3-to 5-year intervals for lifetime [179].In general,the managements include early detection of anticancer drug induced LVD and treatment.As a treatment for LVD angiotensinconverting enzyme inhibitors (ACE-I) and beta-blocking agents (BB)is recommended to use [180,181].According to the American College of Cardiology and American Heart Association guidelines patients receiving chemotherapy may be considered in the group of stage A HF [182].Use of liposome-encapsulated doxorubicin along with carvedilol,valsartan,dexrazoxane should be considered [168].Patients with HF and cardiomyopathy should be treated according to the established guideline of AHA/ACC in general [183,184].
4.2.Potential use of antioxidant drug
Antioxidants collects the free radicals from the body produced by the cells and prevents or reduce the risk of potential damages caused by oxidations.Alone the same line,some antioxidant cardiovascular drugs,such as ACEI (enalapril,captopril,and lisinopril),dexrazoxane,beta blocker (carvedilol,nebivolol) as well as some experimental antioxidant agents such as ranolazine,statins,and phosphodiesterase-5 inhibitors,have shown potential to improve cardiac health insulted by the cardiotoxic drugs [30,185,186].Anticancer drug results in increase production of reactive oxygen species,in other word oxidative stress,within the cardiomyocytes and that lead to cardiac dysfunction.The reactive oxygen species interfere and attack various cellular biomolecules such as lipid,protein and genetic molecules and that resulting in of accelerating tissue damage and cell death.Therefore,antioxidant drugs may have potential to reduce the oxidative stress level by capturing the reactive oxygen species and mitigate the cardiotoxicity of the anticancer drugs.For instance,studies show that carvedilol played vital role in improving the DOX induced mitochondrial cardiotoxicity [185].Dexrazoxane acts as a cardioprotective agent for anthracycline-induced cardiotoxicity by interfering with irondependent redox reactions hence reduces ROS production and inhibits topoisomerase IIβwhich prevents anthracycline binding and DNA double-strand breakage [2,187].A review article by Rodrigoet al.has demonstrated the tole of reactive oxygen species in cardiac damage and how antioxidant vitamins effects on protecting the heart [188].Various studies have reported on the potential cardioprotective effects of vitamin E and C,studies conducted in both pre-clinical and clinical stages [189,190].Vitamin C,an another widely considered antioxidant,has shown potential effects in cardio-protection.However,the plasma concentration of orally administrated vitamin C is as low as 100 μmol/L which is not sufficient to induce enough antioxidant effect.Therefore,an intravenous administration,which can lead to 25–30 mmol/L plasma concentration of vitamin C,is a more preferable route of administration in order for vitamin C to provide enough cardioprotective effects [191].Therefore,we envision that with a proper dose and route of administration antioxidant vitamins can be used as a potential cardioprotective agent while a patient is being treated with cardiotoxic anticancer drug.
4.3.Other cardioprotective modalities
Besides antioxidant drugs,there are other therapeutic modalities that have also shown potential as a cardioprotective agents.For instances,cannabidiol,a major chemical component found in a Cannabis sativa plant,protects the heart from DOX-induced cardiomyopathy by modulating mitochondrial function and biogenesis [192].Alone the same line,Liuet al.has reported that visnagin protects heart from DOX-induced cardiomyopathy through modulation of mitochondrial malate dehydrogenase studies conducted in zebra fish [193].In the same study,Visnagin treatment has shown to improve cardiac contractibility in doxorubicin treated mice.Use of sporopollenin encapsulated imatinib showed controlled release which was 65% within 24 h compared to the control (only imatinib).Only Imatinib released fast and it took only 1 h to release the whole formulation [194].Another study shows PEG polyaspartate bound Epirubicine micelle have more antitumor effects also then only Epirubicin in hepatocellular cancer mice model cardiotoxicity of epirubicin.It also reduced the cardiotoxic effect of Epirubicine [195].Transplantation of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC–CMs) can also reduce the DOX-induced cardiotoxicity [196].
4.4.Targeted drug delivery
Targeted drug delivery to approach as an attempt to reduce cardiotoxicity is still in research.Several of recent studies have reported that targeted delivery of anticancer medications has potential to reduce cardiotoxicity.Degree of cardiotoxicities are mostly dose dependent for most of chemotherapeutic drugs.Disease specific targeted delivery system results higher accumulation of drug within the targeted side of the body and even specific region of the organ [197,198].The higher drug accumulation with the targeted organ also facilitated reduction of non-specific wanted biodistribution and toxicity related to that.For instances,liposomal encapsulation [199–202],mitochondrial delivery [203–206],chemical modification [206],nano-particle encapsulation [207–210],red blood cell loaded [211],and exosome mediated delivery [212]approaches are being considered to increase the therapeutic effect as well as improve cardiac protection.Liposomal vehicle mediated anticancer delivery systems have also been found effective in cardiac protection [213–215].Various preclinical and clinical studies showed that liposomes can predominantly enhance drug accumulate in tumor tissue thus provide improved anticancer activity [216].Studies shows that,liposome sizes ranges between 100 and 200 nm in diameter has better extravasate and accumulate in to tumor site thus better anticancer effect [216].In recent years,several liposomal drug formulation has been clinically approved for anticancer treatment [217].Data from liposome-entrapped Adriamycin(ADM) treated mice model indicated that the levels of ADM were increased several fold in the liver and the spleen with diminished accumulation in heart [218].In another report,Liposomal based-Paclitaxel formulation in metastatic lymphoma mice model showed significantly reduced systemic side effect including cardiac toxicity[219].Alone the same line,co-delivery of liposome of berberine(BER) and DOX significantly inhibited tumor growth in 4T1 murine mammary carcinoma model (P <0.05) and completely overcame the myocardial rupture toxicity compared to free drug [220].PEGylated liposomal DOX showed improved cardiac biopsy in Kaposi Sarcoma patients 0.3vs.3.0 [221].
A very recent study by Wallaceet al.has demonstrated that mitochondrial targeted delivery of cancer therapeutics can improve specificity for cancer cells by acting on targets that only exist in mitochondria of cancer cells thus reduced off targeting mediated side effect [222].A class of HSP90 inhibitors induce rapid mitochondrial membrane permeabilization and cell death in cancer cells while leaving normal cells unaffected [223].Mitochondrial targeted delivery of DOX prevents the nuclear DNA damage and improves myocardial health in H9C2 cell line [203,224].Mitochondrial targeted delivery of resveratrol in DOX-induced cardiomyopathic cell activates cardiac progenitor cells and increase the longevity of mice [225].Another study reported that mitochondrial targeted MitoQ10 significantly reduces the systolic blood pressure (25 mmHg over 8 weeks) and improves cardiac circulation[226].Chemical modification of the parent molecule has also potential in reducing non-specific localization mediated toxicity.For instances,modification of DOX (analogs) and targeted delivery of anthracyclines delivered specifically to the tumor tissue reduces the cardiac side effects [227].
A generation 5 (G5) poly(amidoamine) dendrimers based nanoparticle mediated intertumoral delivery of DOX into hepatic cancer cell has shown significantly reduction the toxic effect of DOX without compromising therapeutic efficacy [228].Applications of human serum albumin conjugated DOX showed accelerate tumor inhibition efficacy;as well as reduced cardiotoxicity[229–231]observed by cardiac imaging and other cardiac biomarkers such as serum creatine kinase-MB,lactate dehydrogenase,superoxide dismutase,and malonaldehyde [232].PLGA loaded imatinib nanoparticle showed great efficacy bothin vivoandin vitrowithout any significant cardiac symptom [233].Resveratrol solid lipid nanoparticles improves DOX induced cardiomyopathy [234].Study also showed that superparamagnetic iron oxide nanoparticles coated with DOX conjugated heparin had more conventional toxicity,antitumor efficacy and less cardiac toxic effect bothin vivoandin vitrostudy [235].Similar studies was done with other anticancer drug also like docetaxel [236],and with different coating like dextran [237],chitosan [238].
These theragnostic agents,a combination of imaging and therapeutic modalities,could be used for noninvasive early detection as well as treatment of cardiotoxicity.Other nanoparticles like silica [239],gold [240],PLGA [241]were used,in regard to increase therapeutic effect and reduce potential side effect.Colloidal mesoporous silica nanoparticles (MSNs) functionalized with DOX,and incorporated into Pluronic F127 hydrogels for prolonged release generated great anti-tumor effect in rat with reduction in cardiac side effect [242].Other chemicals has been used with MSNs like galactosylated chitosan [243],succinylatedε-polylysine [244],MUC1 aptamer conjugation [245–247].
4.5.Advancement in cardiotoxic drug delivery
Most of the anticancer drugs,such as DOX,that commonly target caspase 3 and caspase 7 to trigger apoptosis,induce [248].At the same time,using a certain concentration,especially for DOX at 15 mg/kg may cause acute cardiotoxicity [249,250].Studies suggest that polymeric micelles derived from resveratrol (RES) and quercetin (QUE) can reduce cardiotoxicity bothin vitroandin vivo.On this study they produced DOX induced cardiotoxicity on ovarian cancer cell line and found that the delivery of DOX with a combination of RES and QUE in Pluronic® F127 micelles increase the efficacy of DOX [250].
It has been found in the study that overexpression of miR-132 and miR-212 induce cardiomyocyte hypertrophy [251].Result from another study stated that overexpression of miR132/212 inhibited the doxorubicin-induced atrophy on their rat model cardiomyocytes [252].Various drug deliveryvehicles including coencapsulated ICGOx@IO (iron oxide (IO,indocyanine green (ICG)and glucose oxidase (GOx)) with doxorubicin (DOX),and an EGCG((-)-epigallocatechin-3-gallate) loaded PLGA have been found as comparatively safer approaches that has significantly less cardiotoxicity.On top of that near infrared laser mediated dug release and tissue dissociation at the tumor site has been shown to complete ablation of tumor in mice including significantly less cardiotoxicity [253].Near infrared laser mediated release of drug using ultrasound targeted microbubble destruction (UTMD) assisted exosomal delivery of miR21 could also decrease the cardiac cell death and restore the function in DOX induced cardiotoxic mice model [212].In another study,PHPMA35-b-PDPA75 a pH responsive polymerase based delivery of DOX has been shown to have a reduced cardiac side effect while having full potential of treating lymphoma in mice [254].Along the same line,codelivery of formaldehyde and DOX,calycosin-PEG-PPG-PEG copolymer nanomicelles mediated DOX delivery has also been shown to have the antitumor efficacy with less cardiotoxic effect [255,256].Codelivery of DOX with digoxin also showed to improve cardiotoxicity [257].To conclude this segment,ongoing projects are seeking new avenues to treat the oxidative damages that DOX causes and approaching it by either polymeric micelles delivery or targeting expression to inhibit oxidative damage.Therefore,it may be possible to apply anti-cancer treatment at their full potential to patients with less cardiotoxic effects.
5.Conclusion and perspectives
Recent progress in anticancer drug development has significantly improved cancer treatment and management in clinic that has resulted saving millions of lives.However,anticancer therapy induced cardiotoxicity is responsible for many cardiac morbidity and mortality.To reduce the cardiotoxic effect of anticancer drug we need a holistic approach.These includes identifying cardiotoxic drug,understanding mechanism of action in tumor cell and cardiac cell,early diagnosis of toxicity by utilizing conventional and newer biomarkers and technologies,and managing the toxicity clinically.Alternative medicine and modified drug delivery approach may consider as an emerging field for preventing cardiotoxicity.
Though pathogenesis of anticancer drugs is not clearly and completely understood,rationale designing of further research is needed to better understand molecule specific mechanism of cardiotoxicity and associated biomarkers.Further understanding of structure of circulating biomarkers,if any,will guide us in developing imaging technologies.Moving forward,structural modification of the parent drug can potentially contribute to reduce side-effects but there is a high risk of compromising their efficacy in term of therapeutic profile [258,259].Nanotechnology based formulation and drug delivery approach has shown significant progress over the last decades and many of formulations,for instances ABRAXANE® a formulation of paclitaxel and albumin show reduced side effects compared to that of the free form of paclitaxel [260–262].Alone the same line,various polymeric vehicles such micelles,and liposome also have shown potential for cellular and organ specific drug delivery feasibility as well as facilitate less interaction between the biological molecules and drug molecules.Such strategies may have effectiveness in reducing the unwanted effect of the drug molecules without compromising their therapeutic efficacy.Various biocompatible polymeric materials such as polyethylene glycol,and poly(D,L-lactic acid) have great potential of carrying adequate amount of drug payload from site of administration to the site of action [263–266]Such carrier molecule can prevent any possible interaction of the drug molecules with the biomolecules during the systemic circulation,hence reduce toxicity of the anticancer drug.
In addition to the developing formulation to reduce non-specific localization mediated toxicity,a feasible monitoring modality that can facilitate real-time and quantitively detection of cardiotoxicity can improve cancer patient management in clinic.As demonstrated earlier,though there are several cardiotoxicity monitoring modalities available and used in clinic but none of the modalities are good enough to detect the degree of toxicity at early stage and with a quantitative manner.Therefore,development of a more feasible and sensitive tools that can detect the upregulated circulatory proteins and quantify the cardiotoxicity biomarker can be used for real-time and at early-stage toxicity monitoring.With development and implementation of such detection technology,a cancer patient management can be improved,and irreversible cardiotoxicity can be mitigated.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Md Nurunnabi acknowledges the research grant supported by Lizanell and Colbert Coldwell Foundation.The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work,through their research group (No.RG-1435–017).
杂志排行
Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
- Recent progress on the smart membranes based on two-dimensional materials
