Upregulated adenosine 2A receptor accelerates post-infectious irritable bowel syndrome by promoting CD4+ T cells’ T helper 17 polarization
2022-07-11LiWeiDongZhiChaoMaJiaoFuBaiLiHuangFuJinLiuDemingSunChengLan
Li-Wei Dong, Zhi-Chao Ma, Jiao Fu, Bai-Li Huang, Fu-Jin Liu, Deming Sun, Cheng Lan
Abstract
Key Words: Adenosine 2A receptor; CD4+ T cells; T helper 17 polarization; Post-infectious irritable bowel syndrome; Regulation
INTRODUCTION
The predominant clinical features of irritable bowel syndrome (IBS) include abdominal pain, abnormal bowel habit, and stool changes. IBS occurs predominantly in developed countries. The incidence rate is about 10%-60%. During the past three decades, the incidence of IBS has rapidly increased in underdeveloped countries[1]. IBS can occur after an acute gastrointestinal infection, which is defined as post-infectious IBS (PI-IBS)[2]. The patients suffer from refractory discomfort, live with problems, and have to undergo high medical costs for their treatment. The laboratory reports of these patients do not show morphological findings, which leads to a shortage of time in targeting treatment[3]. Low-grade inflammation and immune activation in the colonic mucosa are observed during IBS[4]. PI-IBS might be alleviated due to regulation of the inflammatory response[5]. Upregulated ephrin type-A receptor 2 and activated nuclear factor kappa-light-chain-enhancer of activated B cells signaling pathway can alleviate PI-IBS[6]. Moreover, non-coding RNAs such as microRNA can modulate inflammation and immune response by targeting peroxiredoxin-1 in PI-IBS[7].
Recently, interleukin-17 (IL-17) raised concerns in PI-IBS. For instance, rifaximin protects mice against PI-IBS by suppressing the production of IL-17[8]. Our previous studies reported that heat shock protein 70 alleviated PI-IBS by upregulating T helper 17 (Th17) response in γδ T cells[9,10].
Adenosine originates from ATP in the local tissue during inflammation and regulates tissue blood flow[11]. As an endogenous molecule, adenosine exhibits anti-inflammatory and immunosuppressive effects by binding to the adenosine receptors (ARs), which are located on some immune and tissue cells and function as a G-protein coupled receptor[12-14]. ARs consist of the following four subtypes: A1R,adenosine A2a receptor, adenosine A2b receptor, and A3R, which are expressed at various locations in the intestine[15]. Different receptors induce different pathophysiological roles. A2BR inhibits the release of inflammatory cytokines, tumor necrosis factor-α, IL-6, and IL-12[16-18]. We previously reported that ARs contribute to the modulation of immune response by γ δ T cells[19,20]. In the intestine, adenosine and its receptors regulate the enteric nervous system and visceral hypersensitivity. Endogenous adenosine affects the secretion of hydrocarbon by duodenum, whereas extracellular ATP induces the apoptosis of intestinal epithelial cells[21,22]. Adenosine inhibits inflammatory damage in the intestine,protects the intestine against ischemia, and reduces lesions in chronic septic mice by A1AR, A2AR, and A2BR[23,24]. A3R regulates distal colonic neuro-muscle function in rats with colitis[25]. Adenosine receptors also contribute to the inhibition of visceral pain by electroacupuncture in IBS[26]. The role of ARs in intestinal functions shows that they can be a potential target for intestinal inflammatory diseases.However, ARs are not widely studied in IBS, especially in PI-IBS. A2AR is reported to protect the enteric Glia barrier function against intestinal injury induced by hypoxia[27].
The relationship between A2AR, CD4+ T cells, and IL-17 is unknown. Whether A2AR is expressed in CD4+ T cells is not known. Whether A2AR affects IL-17 production directly or through other indirect pathways is unknown. Furthermore, the relationship between A2AR, IL-17, and CD4+ T cells in PI-IBS is unknown. Therefore, we investigated the role of Th17 polarization of CD4+ T cells regulated by A2AR in PI-IBS.
MATERIALS AND METHODS
Animal grouping
57BL/6 mice (female, mean age: 56 d, average weight: 15 g) were provided by the Hainan Provincial Experimental Animal Center and fed in pathogen-free, room temperature conditions under a normal 12-h light/dark diurnal rhythm. The animals were provided with a standard diet and waterad libitum.Four groups of mice were formed: Control, PI-IBS, PI-IBS+Sch58261, and PI-IBS+CGS21680 (n= 24 in each group). Twelve mice were processed for the measurement of A2AR, cytokines, and signaling molecules protein and mRNA levels. Six mice were sacrificed in order to isolate, purify, and culture their CD4+ T cells. Visceral hypersensitivity and intestinal motility were evaluated in the remaining 6 animals.
PI-IBS modeling
Infection withTrichinella spiralis(Lanzhou Veterinary Research Institute, Lanzhou, China) was performed to establish the PI-IBS model[28]. After 60 d of infection, the parasite larvae were digested from Sprague–Dawley rats with 1.5% gastric pepsin (Invitrogen, Carlsbad, CA, United States) and suspended in 0.2 mL of saline. The mice were fed the larvae (300 larvae/mouse).
Histopathological examination
On the 56thday after infection, the animals’ ileum was fixed in 10% formalin and embedded in paraffin wax. The tissues were mounted on slides after creating 5-μm-thick sections, followed by dewaxing and dehydration. The slides were stained with hematoxylin-eosin and used for further evaluation of the inflammatory score following previous scoring standards[29].
Abdominal withdrawal reflex
Visceral hypersensitivity was evaluated with the abdominal withdrawal reflex (AWR)[30]. A gasbag was inserted into the anesthetized animal’s anus, which was distended in volume from 0.25 mL to 0.65 mL for 15 min, thrice, followed by a recording of the animal’s behavior. The AWR score was found to be in accordance with the standard from 0 to 4 points, as described previously[30]. The AWR scoring standard: when stimulated, the animals are in stable mood, 0 point; if the animals are in unstable mood,twisting their heads once in a while, 1 point; slightly contracting their abdominal and back muscles, 2 points; intensively contracting their abdominal muscles and uplifting the abdomen from the ground, 3 points; intensively contracting abdominal muscles, bowing the abdomen and uplifting the abdomen and perineum, 4 points.
Colon transportation test
Intestinal motility was evaluated with the colon transportation test (CTT); 0.4 mL of activated carbon was placed in the animal's stomach, and the animal’s initial time to black stool elimination was recorded. After 8 h, the Bristol stool scoring system was used to assess the total stool’s characteristics[31]. Normal shaped stool, 1 point; soft or deformed stool, 2 points; water-like stool, 3 points.
Cellular immunohistochemistry
Cellular immunohistochemistry was performed as reported elsewhere[32]. The suspended cells were fixed in 4% paraformaldehyde and uniformly placed onto slides. The sections were then incubated with primary antibody rabbit anti-mouse A2AR monoclonal antibody and a secondary antibody goat antirabbit HRP antibody (ab60032 and ab6721; Abcam, Cambridge, United Kingdom). DAB reagent sets(Beijing Sequoia Jinqiao, Beijing, China) were used to determine the reactivity. The quantitative expression of A2AR in the cellular membrane was calculated using a semi-quantitative integral method.The staining intensity was scored on a scale of 0-3. The percentage area of positively stained cells was classified as 0-4 corresponding to the values of < 5% to > 75%. The evaluation with a score of ≥ 6 was expressed as a positive expression. The stain was analyzed by Nikon DR-Si2 cell count software and digital image analysis; two senior pathologists verified the results.
Western blotting
Western blotting (Wuhan Boster Corporation, Wuhan, China) was performed to evaluate the protein level. RIPA (R0278; Sigma-Aldrich, Saint Louis, MO, United States) buffer was used to extract the protein from the ground ileum tissue samples. The Bradford assay was performed to determine the protein concentration. After separation on SDS-PAGE gel electrophoresis, 40 µg of the sample was transferred onto a polyvinylidene fluoride membrane. The following transmembrane conditions were set during the assay: (1) β-actin and A2AR: running at 200 mA for 90 min; (2) Janus kinase (JAK) and p-JAK: running at 200 mA for 120 min, followed by running at 300 mA for 30 min; (3) Signal transducer and activator of transcription (STAT3) and p-STAT3: running at 200 mA for 20 min, followed by running at 300 mA for 15 min; and (4) Receptor-related orphan receptor-γt (RORγt): running at 200 mA for 120 min.
The dilution rate of the primary antibodies rabbit anti-mouse multiple-clone antibodies is shown in Table 1.
Blotted in TBST for 60 min, the membrane was incubated with rabbit anti-mouse protein antibodies(ab2787; Abcam, United States) as the primary antibodies and with goat anti-rabbit antibodies as the secondary antibodies (ab8226; Abcam) at 4 °C for 12 h. ECL chemiluminescent assay was performed to autograph the membrane. The detected gray-scale ratio of protein/β-actin represented the relative protein level.
Real-time quantitative PCR
Total RNA in the ileum tissues was extracted with the Trizol reagent set (Invitrogen). The primers were designed by Invitrogen (Table 2). β-actin served as an internal control.
RT-PCR was performed under the following protocol conditions: The samples were run by a predenaturation program (5 min at 94 °C), followed by a denaturation program (1 min at 94 °C). The amplification and qualification program was repeated for 30 cycles (50 s at 57 °C, 20 s at 60 °C), followed by an extension program (7 min at 72 °C).
The relative expression was expressed as the ratio of the target gene to the control.
Determination of ATP
The ileum tissue sample was treated ultrasonically and centrifuged at 4 °C for 15 min, followed by the addition of double-distilled water (1:9). The 10% homogenate was heated in a boiling water bath for 10 min and then centrifuged at 3000 rpm for 10 min. The supernatant was collected for enumeration.
The concentration of ATP was measured by a colorimetric method. Briefly, the OD of the tube was measured at the wavelength of 636 nm. The ATP concentration in the sample μmol/gprot) = (measured OD - Control OD)/(Standard OD - OD) × standard concentration (1000 μmol/mL)/sample concentration (gprot/L).
Isolation and purification of CD4+ T cells
CD4+ T cells were isolated from the animals' spleen[33]. Briefly, the animals were sacrificed and their spleens were collected and washed with PBS. The red blood cells were rinsed with red blood cell lysis buffer. The lymphocytes liquid overflowing from the cleaned spleen was repeatedly washed with PBS,centrifuged at 1960 rpm, and incubated with CD4 (FITC) 0.25 µg/test; the CD4+ T cells were selected by fluorescence-activated cell sorting (FACS).
Evaluation of CD4+ T cells’ function
The effect of A2AR on CD4 T cell function, including proliferation, apoptosis, ATP production, and Th17 polarization response was evaluated by the CCK8 assay, Annexin V staining assay, and ELISA.Furthermore, the intra-cellular signaling pathway was investigated by Western blotting and RT-PCR.
Proliferation
The CCK8 assay was performed to determine proliferation of the isolated CD4+ T cells. The cell density was set to 5 × 105/mL. The cells were added to a 96 well culture plate at the concentration of 100 µL (5 ×104cells), followed by treatment with 100 nmol/L of CGS-21680 and 1 μmol/L Sch58261, cultured for 0.5 h at 37°C under 5% CO2atmosphere. Then, 20 μL/well of CCK8 was added into the system and continuously cultured for 2 h at 37°C under 5% CO2atmosphere. The OD450of the cultured cells was measured with an enzyme-labeled instrument.
Apoptosis
The rate of apoptosis of CD4+ T cells was measured by Annexin V staining assay. Briefly, the collected CD4+ T cells were incubated in a 6-well plate (5 × 105cells/well), to which 100 nmol/L of CGS-21680and 1 μmol/L of Sch58261 were added and cultured at 37°C under 5% CO2for 0.5 h. Then, 5 μL of Annexin V-APC and 5 μL of 7-AAD staining liquid were added to the wells. FACS was performed to detect the rate of apoptosis.

Table 1 The dilution rate of primary antibodies
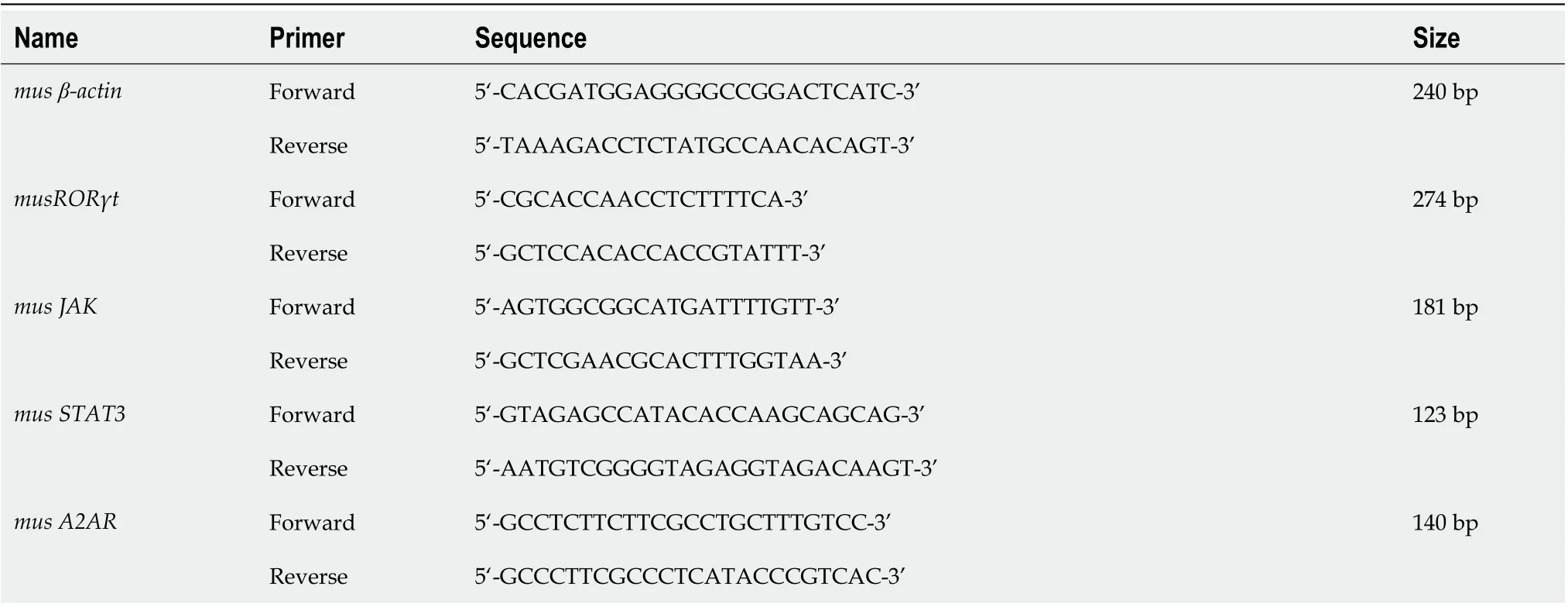
Table 2 Primers used in the present study
Th17 polarization
ELISA was performed to determine the concentration of cytokines in the supernatant of CD4+ T cells. A high-sensitivity (sensitivity range: 0.25-16 pg/mL) ELISA kit (R&D Systems, Minneapolis, MI, United States) was used to analyze the IL-17 and IFNγ protein concentrations according to the manufacturer’s protocol.
Signaling pathway
The protein and mRNA of the signaling-associated molecules were determined by Western blotting and RT-qPCR, as described previously.
Statistical analysis
The Kolmogorov–Smirnov test was performed using SPSS 22.0 software to indicate normal distribution of the data. Data were expressed as the mean ±standard error of the mean. The differences between the two groups were analyzed by the Student’st-test. Values in the same row with different superscripts indicate significance (P< 0.05), otherwise no significance (P> 0.05).
Ethical considerations
The experimental protocol was approved by the Animal Care and Use Committee of Hainan Province and conducted in accordance with the Chinese guidelines for animal welfare.
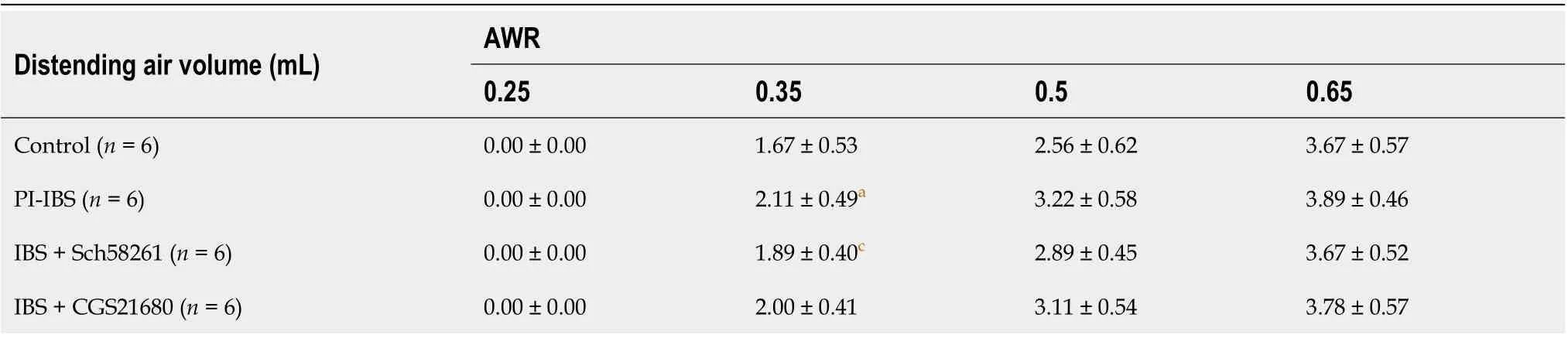
Table 3 The effect of adenosine 2A receptor on the abdominal withdrawal reflex score in the post-infectious irritable bowel syndrome mouse

Table 4 The effect of adenosine 2A receptor on intestinal mobility in the post-infectious irritable bowel syndrome mouse
RESULTS
PI-IBS mouse model and the effect of A2AR on the clinical features of PI-IBS
On the 56thday of infection withT. spiralis, the experimental mice showed no significant colonic inflammation (Figure 1), accompanied by a higher AWR score, longer CTT, and higher Bristol stool grade(Tables 3 and 4), suggesting that the PI-IBS mouse model was successfully established. Injection of the A2AR agonist CGS21680 significantly increased the clinical features and slightly accelerated intestinal inflammation. On the other hand, the A2AR antagonist Sch58261 markedly improved the model animal’s clinical manifestations. These results confirm the important role of A2AR in the development of PI-IBS. However, the underlying mechanism behind this role warrants further investigation.
Changes in intestinal ATP and A2AR in the PI-IBS mouse
When compared with the control group, intestinal ATP significantly increased in the PI-IBS mouse (P<0.05; Figure 2) along with an increase in A2AR protein expression (P< 0.05; Figure 3A and B). The A2AR antagonist Sch58261 significantly decreased intestinal ATP and A2AR protein levels, while the A2AR antagonist CGS21680 significantly increased these protein levels (P< 0.05; Figure 2, Figure 3A and B). These results suggest that adenosine and A2AR are involved in the development of PI-IBS.
CD4+ T cells’ isolation and A2AR expression
CD4+ T cells were isolated and purified from PI-IBS mice spleens by FACS (Figure 3A) for further functional evaluationin vitro. Cellular immunohistochemistry results revealed that the CD4+ T cells from PI-IBS mice obviously increased their A2AR protein and mRNA expression, which could have been regulated by the A2AR antagonist and agonist, respectively (P< 0.05; Figure 3C-E). These results suggest that CD4+ T cells could express A2AR, and their expression was regulated by A2AR.
CD4+ T cells from PI-IBS mice showed obvious proliferation, decreased apoptosis, and increased ATP and IL-17 production, which could have been regulated by the A2AR antagonist and agonist,respectively (P< 0.05; Figure 4A-D). These results suggest that CD4+ T cell function, especially Th17 polarization was regulated by A2AR.
CD4+ T cells signaling pathway
The JAK/p-JAK protein and mRNA expression in CD4+ T cells from PI-IBS mice were detected by Western blotting and RT-PCR. The A2AR antagonist Sch58261 decreased JAK/p-JAK protein and mRNA expression, while the A2AR agonist CGS21680 increased JAK/p-JAK protein and mRNA expression. These results suggest that, during PI-IBS, the JAK pathway participates in CD4+ T cell A2AR-associated biological behavior (Figure 5A and B).
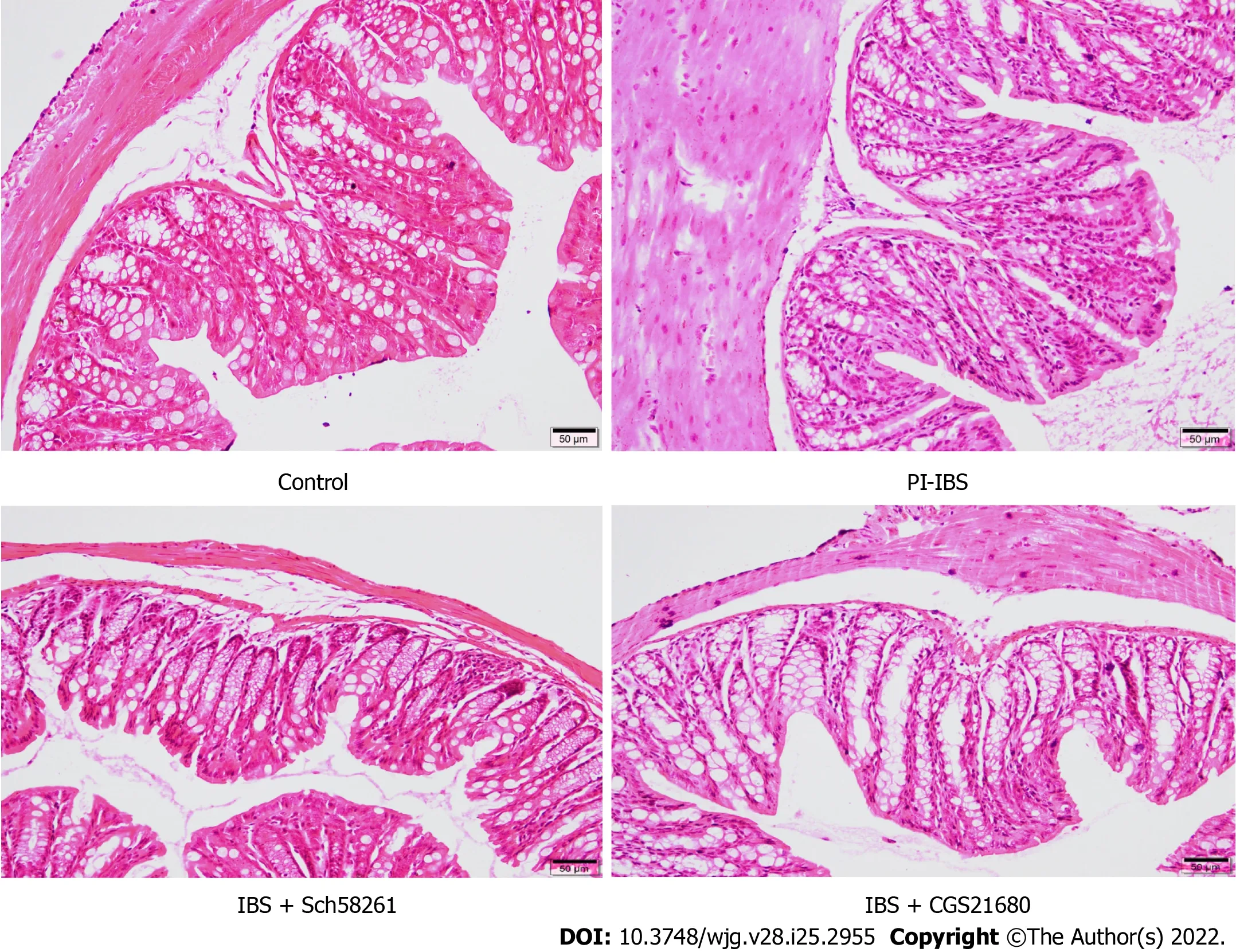
Figure 1 The effect of adenosine 2A receptor on the histopathological changes in the post-infectious irritable bowel syndrome mouse. On the 56th day of infection with T. spiralis, colon tissues from the infected mice were collected, fixed, sectioned, stained with hematoxylin-eosin, and evaluated by light microscopy. Compared with the control mouse, the post-infectious irritable bowel syndrome (PI-IBS) mouse model showed no significant inflammatory changes in colon tissues. CGS21680, an agonist of adenosine 2A receptor, induced subtle inflammatory changes in the colon tissues of the PI-IBS model mouse, such as slight infiltration of inflammatory cells. IBS: Irritable bowel syndrome.
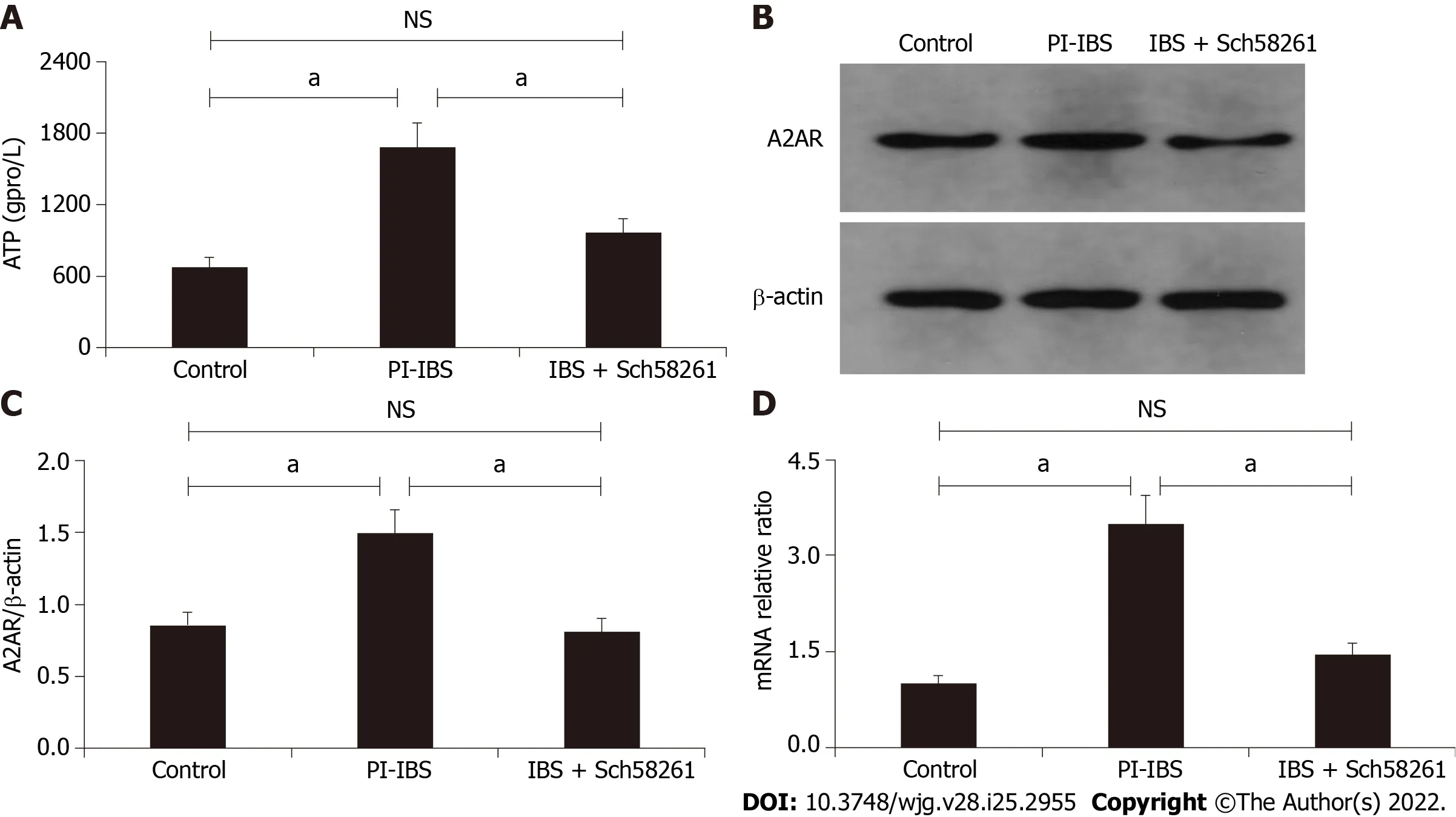
Figure 2 The expression of intestinal ATP and adenosine 2A receptor in the post-infectious irritable bowel syndrome mouse. A: The expression level of ATP in different groups; B and C: Western blot analysis showed the expression level of adenosine 2A receptor (A2AR) in the three different groups; D: Relative intestinal A2AR mRNA level in the three groups. aP < 0.05. A2AR: Adenosine 2A receptor; PI-IBS: Post-infectious irritable bowel syndrome; NS:Not significant.
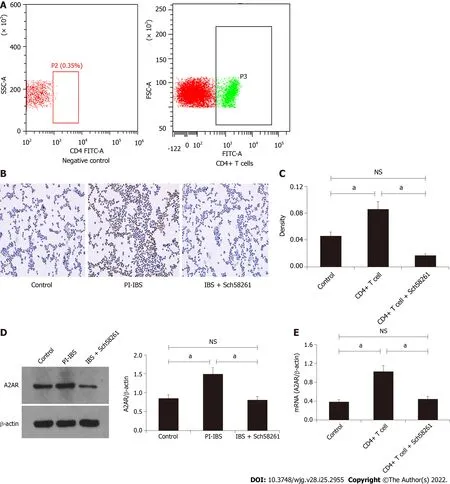
Figure 3 Adenosine 2A receptor expression level in CD4+ T cells isolated and purified from the post-infectious irritable bowel syndrome mouse. A: Fluorescence-activated cell sorting results showed that CD4+ T cells (P3) were successfully isolated and purified; B-C: Immunohistochemistry staining of adenosine 2A receptor (A2AR) in CD4+ T cells; D: Western blot assay evaluated A2AR expression levels in CD4+ T cells isolated from different groups; E: Relative A2AR mRNA levels in the three groups. aP < 0.05. A2AR: Adenosine 2A receptor; PI-IBS: Post-infectious irritable bowel syndrome; NS: Not significant.
The STAT3/P-STAT3 protein and mRNA expression in CD4+ T cells from PI-IBS mice were detected by Western blotting and RT-PCR. The A2AR antagonist Sch58261 decreased STAT3/P-STAT3 protein and mRNA expression, while the A2AR agonist CGS21680 increased STAT3/P-STAT3 protein and mRNA expression. These results suggest that, during PI-IBS, the STAT3 pathway participated in CD4+T-cell A2AR-associated biological behavior (Figure 5A and B).
The RORγt protein and mRNA expression in CD4+ T cells from PI-IBS mice were detected by Western blotting and RT-PCR. The A2AR antagonist Sch58261 decreased RORγt protein and mRNA expression, while the A2AR agonist CGS21680 increased RORγt protein and mRNA expression. These results suggest that, during PI-IBS, the RORγt pathway participated in CD4+ T cell A2AR-associated biological behavior (Figure 5A and B).

Figure 4 Functional evaluation of CD4+ T cells in vitro. A: CCK-8 assay indicated that CD4+ T cells from post-infectious irritable bowel syndrome mice showed increased proliferation ability; B: Apoptosis analysis of CD4+ T cells between the three groups; C: ATP expression in CD4+ T cells between the three groups;D: Expression level of IFN-γ and IL-17 in the three groups. aP < 0.05. A2AR: Adenosine 2A receptor; PI-IBS: Post-infectious irritable bowel syndrome; NS: Not significant.
DISCUSSION
Lasting low level inflammation occurs in the intestine in IBS and especially in PI-IBS due to complex immune abnormalities. Therefore, patients show continuous clinical symptoms and not distinct biochemical and pathological changes. However, the precise mechanism underlying immune regulation in PI-IBS is unclear. Therefore, the relationship between ARs and IL-17 response in the inflammation and immune response in PI-IBS should be investigated.
Our results showed that increasing intestinal ATP level in PI-IBS was accompanied by increasing intestinal A2AR level, which suggested that ATP and A2AR could participate in the pathogenesis of PIIBS. The quantitative changes that lead to the development of PI-IBS, resulted from PI-IBS, or increased PI-IBS is not known. The animal’s intestinal tissue remained unchanged following injection of the A2AR antagonist, but the clinical symptoms improved. This result was contradictory to the previous hypotheses which state that adenosine and its receptors play a protective role in inflammation. During the inflammatory process, adenosine and its receptors could trigger another unknown pathway to maintain the balance of inflammation and the immune response, otherwise, a prolonged presence of increased concentrations of adenosine can be harmful and can induce an immunosuppressed environment that is ideal for the onset and development of neoplasia[34]. Our study reports the complex condition in PI-IBS.
We elucidated the mechanism underlying the role of A2AR in PI-IBS. CD4+ T cells act as an important regulator in inflammation and immune response. Th17 polarization is one of the most important pathways of the immune event associated with CD4+ T cells. We first found that the number of CD4+ T cells distinctly increased, accompanied by an increase in the expression of A2AR on these cells. We then found that the agonist of A2AR increased the number of CD4+ T cells accompanied by an increase in the expression of A2AR on these cells. The A2AR agonist increased the proliferation of CD4+T cells and inhibited their apoptosis. On the contrary, the A2AR antagonist decreased the number of CD4+ T cells and inhibited their function. These results suggested that CD4+ T cells participate in A2AR-induced intestinal inflammation.
Th17 polarization was associated with A2AR. IL-17 plays a dual role in inflammation and the immune response. IL-17 can improve inflammation in some conditions. For instance, HSP70 induces γ δ T cells to produce IL-17 that protects mice against PI-IBS[10]. IL-17 protects mice against colitis induced by adherent-invasiveEscherichia coli[35]. In other conditions, IL-17 can increase inflammation. For instance, Th17 cells and IL-17 promotes skin and lung inflammation and fibrosis in the bleomycininduced murine model of systemic sclerosis[36].
Unfortunately, the studies involving animal models have not determined the efficacy of anti-IL-17 antibodies in improving intestinal inflammation[37]. Our results showed that the increase in A2AR expression and chronic inflammation is associated with the Th17 polarization of CD4+ T cells, which suggested that the A2AR-CD4+ T cells-IL-17 polarization pathway is present in PI-IBS.
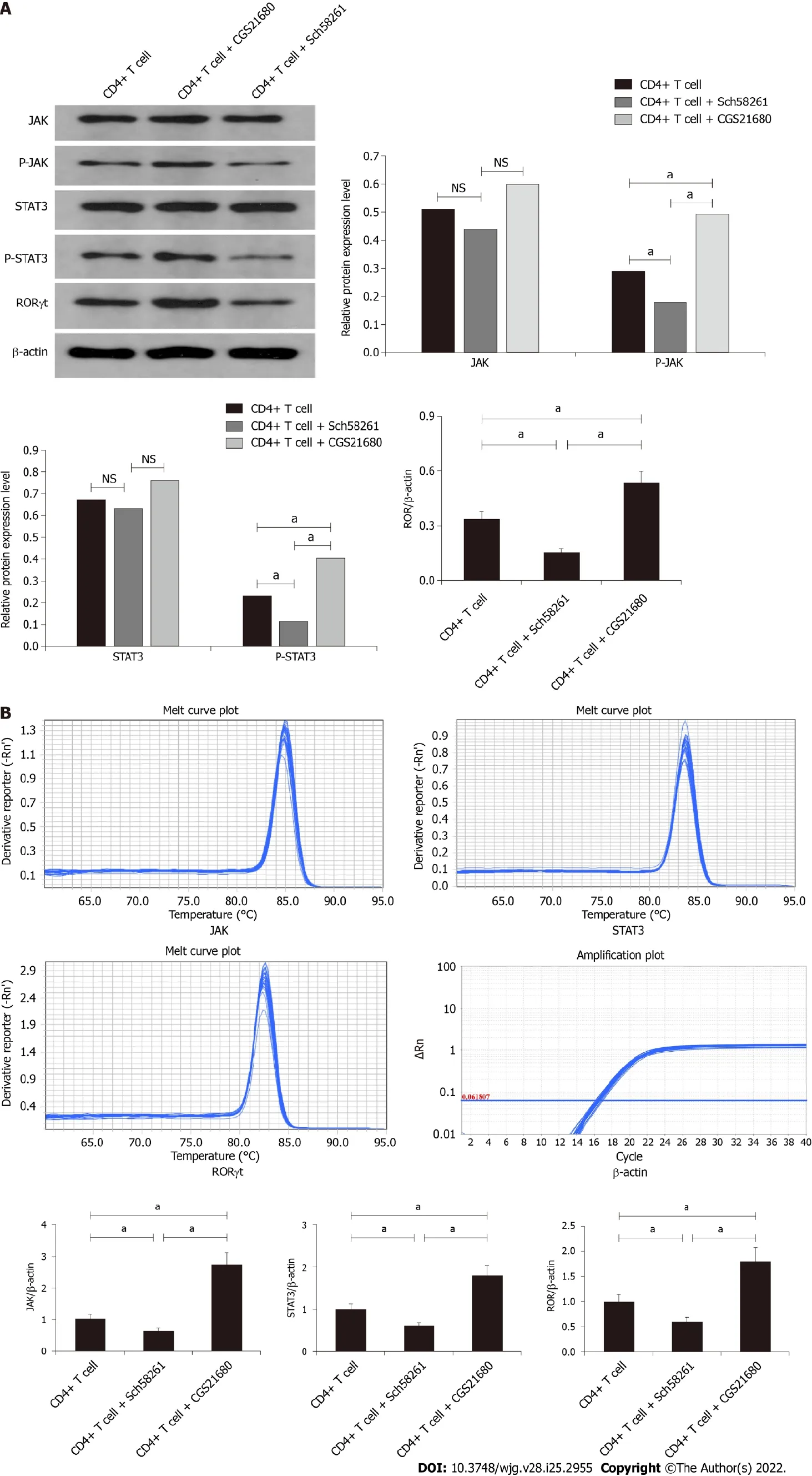
Figure 5 Signaling pathway analysis by Western blot assay. A: Western blot results indicate that the expression level of p-JAK, p-STAT3, and receptorrelated orphan receptor (ROR) were significantly increased in the CD4+ T cell + CGS21680 group and decreased in CD4+ T cell + Sch58261 group; B: The mRNA expression level of p-JAK, p-STAT3, and ROR between the different groups. aP < 0.05. A2AR: Adenosine 2A receptor; PI-IBS: Post-infectious irritable bowel syndrome; NS: Not significant.
We investigated the intracellular signaling pathway of the Th17 polarization of CD4+ T cells induced by A2AR. We found that JAK and STAT3 protein and their phosphorylated products were significantly increased in CD4+ T cells from the PI-IBS mouse model. The A2AR antagonist reduced the protein and mRNA level of JAK and STAT3, which was increased by the A2AR agonist. Our results suggested that the JAK-STAT3 pathway is an important intracellular signaling pathway in the A2AR-induced Th17 polarization of CD4+ T cells in PI-IBS.
The present study has some limitations. The type of visceral hypersensitivity in the PI-IBS mouse model was not investigated. The water content in the Bristol stool grade was not analyzed. The direct isolation of CD4+ T cells from the intestine is difficult. Therefore, we used CD4+ T cells isolated from the spleen, which might have resulted in some variation compared with CD4+ T cells from the intestine.
CONCLUSION
In the present study, we established a mouse model of PI-IBS and the mice were administered an A2AR agonist and antagonist. Interestingly, we found that A2AR increased in PI-IBS accompanied by CD4+ T cell activation, proliferation and production of IL-17. A2AR antagonist improved the clinical symptoms and the cellular and molecular events during PI-IBS. This study showed that the upregulation of A2AR increases PI-IBS by promoting the Th17 polarization of CD4+ T cells.
ARTICLE HIGHLIGHTS
FOOTNOTES
Author contributions:Dong LW and Lan C designed the study and interpreted the findings; Dong LW and Ma ZC performed the experiments, analyzed the data, and drafted the manuscript; Fu J, Huang BL and Liu FJ helped collect and analyze the data; Sun D and Lan C critically revised the manuscript for important intellectual content and reviewed the article; all authors have read and approved the final manuscript.
Supported byNational Natural Science Foundation of China, No. 81160057, No. 81860102, and No. 82060102.
Institutional animal care and use committee statement:The experimental protocol was approved by the Animal Care and Use Committee of Hainan General Hospital.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
ARRIVE guidelines statement:The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Cheng Lan 0000-0002-1435-5510.
S-Editor:Gong ZM
L-Editor:Webster JR
P-Editor:Gong ZM
杂志排行
World Journal of Gastroenterology的其它文章
- Correction to “Gut microbiota dysbiosis in Chinese children with type 1 diabetes mellitus: An observational study”
- Acupuncture and moxibustion for treatment of Crohn’s disease: A brief review
- Correlation of molecular alterations with pathological features in hepatocellular carcinoma: Literature review and experience of an Italian center
- Mapping the global research landscape on nutrition and the gut microbiota: Visualization and bibliometric analysis
- Fecal gene detection based on next generation sequencing for colorectal cancer diagnosis
- Incretin based therapy and pancreatic cancer: Realising the reality
